Abstract
Increased renal expression of periostin, a protein normally involved in embryonic and dental development, correlates with the decline of renal function in experimental models and patient biopsies. Because periostin has been reported to induce cell differentiation, we investigated whether it is also involved in the development of renal disease and whether blocking its abnormal expression improves renal function and/or structure. After unilateral ureteral obstruction in wild-type mice, we observed a progressive increase in the expression and synthesis of periostin in the obstructed kidney that associated with the progression of renal lesions. In contrast, mice lacking the periostin gene showed less injury-induced interstitial fibrosis and inflammation and were protected against structural alterations. This protection was associated with a preservation of the renal epithelial phenotype. In vitro, administration of TGF-β to renal epithelial cells increased the expression of periostin several-fold, leading to subsequent loss of the epithelial phenotype. Furthermore, treatment of these cells with periostin increased the expression of collagen I and stimulated the phosphorylation of FAK, p38, and ERK 42/44. In vivo delivery of antisense oligonucleotides to inhibit periostin expression protected animals from L-NAME–induced renal injury. These data strongly suggest that periostin mediates renal disease in response to TGF-β and that blocking periostin may be a promising therapeutic strategy against the development of CKD.
Keywords: progression of renal failure, obstructive uropathy, hypertension, fibrosis, extracellular matrix, Pathophysiology of Renal Disease and Progression
CKD is currently a burden for the public health system because of its continual increase of incidence and the lack of efficient therapeutic treatment. Although CKD can result from different causes, such as hypertension, diabetes, or immune or toxic aggressions, the pathologic pathway is common. It is characterized by chronic inflammation and abnormal accumulation of extracellular matrix (ECM) within the renal parenchyma, leading to a decrease of functional nephrons and the subsequent decline of renal function. Activation of the TGF-β pathway is considered a key event in the development of renal fibrosis.1 For example, administration of decorin, a TGF-β scavenger, has been shown to decrease renal fibrosis.2 Similarly, angiotensin II receptor antagonism resulted in renal function recovery by inhibiting TGF-β expression.3 In line with this finding, restoration of renal function and structure have been observed after administration of BMP7, an agent that directly antagonizes the TGF-β pathway.4
Periostin, also called osteoblast-specific factor 2, is a 90 kDa extracellular protein expressed during development and very early in postnatal tissue5,6; its expression in healthy adult tissues is very low but increases considerably after injury. Periostin expression has previously been shown to be significantly increased in vitro by both TGF-β and BMP2 treatment in MC3T3-E1 osteoblatic mouse embryonic fibroblasts and chick embryonic heart endocardial cushion cells.6–8 Many studies in the heart have shown that periostin is secreted by fibroblasts to regulate collagen deposition, thereby altering the mechanical properties of connective tissues.9 Animals lacking periostin expression exhibit reduced fibrosis after myocardial infarction.10 Periostin also has the ability to associate with other ECM components, such as tenascin and fibronectin, and can interact with integrins, such as avb3 or avbv, resulting in activation of the Akt or phosphatidylinositol 3-kinase pathways.11
Presently, little is known about the role of periostin in renal diseases. It has been shown to be expressed de novo in cysts of epithelial cells in human autosomal dominant polycystic kidneys.12 A recent study describing gene expression profiles of biopsies from patients with glomerulopathies found that periostin was highly expressed in the tubulointerstitial and fibrotic compartments and that its expression was inversely correlated with renal function.13 Other investigators observed increased concentrations of urinary periostin in a small cohort of CKD patients.14 We reported that periostin is highly upregulated during disease progression and inversely downregulated during regression in a model of hypertensive renal disease.15
Although the above results indicate that periostin can be a new biomarker of renal disease progression, whether periostin participates in the development of CKD has not been examined. This hypothesis was investigated in the present study using two complementary approaches: mice lacking periostin (Postn null mice) and in vivo administration of antisense (AS) oligonucleotides (ODNs) in hypertensive rats. We found that lack or inhibition of periostin expression was associated with a better preservation of renal structure and function. The proposed mechanism is that periostin mediates and amplifies renal inflammation and fibrosis in the renal epithelium. These data show the proof of concept for targeting periostin as a promising therapy against the progression of CKD.
Results
Periostin Expression Is Strongly Induced in Kidneys after Unilateral Ureteral Obstruction
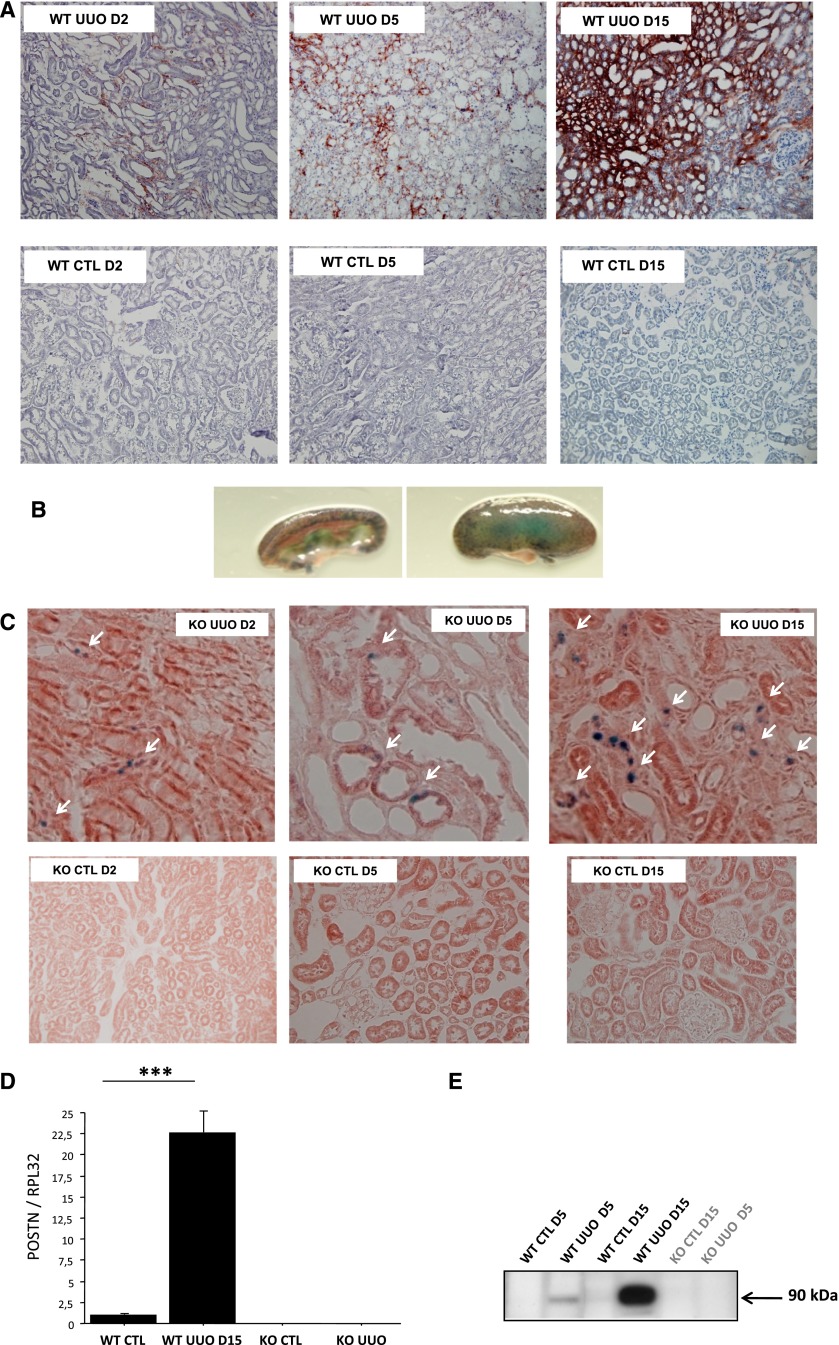
As early as 2 days after ureteral obstruction, periostin expression was detected around the first structural alterations in the renal medulla; it increased at 5 days after unilateral ureteral obstruction (UUO) and became very pronounced at day 15, extending to important areas of the renal cortex (Figure 1A). This upregulation was specific to the injured kidneys, because the contralateral (nonobstructed) or control kidneys exhibited limited expression of periostin (Figure 1A). Because the endogenous periostin protein is secreted, whereas the β-galactosidase reporter remains within the cells of its synthesis, we used X-gal staining in Postn null/X-gal mice to localize the renal cells synthesizing β-galactosidase. As shown in Figure 1, B and C, upper left panel, X-gal staining appears in the collecting duct cells at day 2 post-UUO. These cells are the first to be injured in the model of UUO.16 Thereafter, on days 5 and 15, X-gal staining was found at the distal and proximal tubular cells (Figure 1C, upper center and right panels), depicting the evolution of the disease. Quantification of mRNA (quantitative RT-PCR) and protein (Western blot) expressions confirmed the continuous increase of periostin expression with time after UUO (Figure 1, D and E). Periostin staining and mRNA and protein expressions were negligible in control kidneys (Figure 1).
Figure 1.
Periostin is highly induced in the obstructed kidney during the progression of renal disease. (A) Immunocytochemistry shows a progressive expansion of periostin expression in the renal parenchyma that follows the development of renal injury, whereas controlateral kidneys do not show periostin expression. (B and C, upper panel) Localization of β-galactosidase activity indicates cells synthesizing periostin: collecting duct cells at D2, tubular epithelial cells at D5, and interstitial cells at D15 (arrows). Controlateral kidneys did not show any β-galactosidase activity at any time point (C, lower panel). Quantification of (D) mRNA and (E) protein expressions confirmed the UUO-induced synthesis of periostin. Nine mice per strain and time point were used. CTL, control; D, day; KO, knockout; ***P<0.001.
Genetic Deletion of Periostin Prevents the Development of Renal Fibrosis
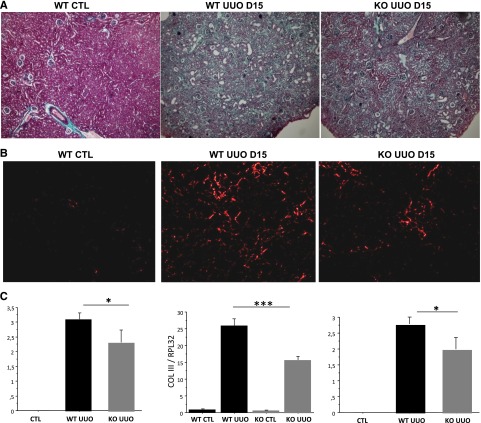
To evaluate whether this exaggerated local expression of periostin is associated with disease progression, histologic comparisons were performed comparing the development of renal lesions between the Postn null and wild-type (wt) littermate mice. Fifteen days after UUO, Postn null mice exhibited reduced tubular dilation and less fibrosis (Figure 2A). To evaluate renal fibrosis, renal sections were stained with Sirius Red. Morphometric analysis revealed that the kidneys of Postn null mice had less fibrillar collagen compared with their wt littermates (Figure 2B). Because renal fibrosis is produced mainly by the accumulation of collagen III, the expression of this gene was measured. As seen in Figure 2C, UUO strongly induced collagen III expression, but this increase was not as marked in the kidneys of Postn null mice.
Figure 2.
Postn null mice show less renal fibrosis and structural damage compared with wt mice after 15 days of UUO. Representative renal sections stained by (A) Masson’s Trichromic and (B) Sirius Red solutions showing Postn null mice with less fibrosis and tubular dilation compared with wt. (C) Quantification of tubulointerstitial fibrosis, collagen III mRNA (Col III), and tubular dilation shows that the UUO-induced increase in these indexes is blunted in Postn null mice. Nine mice per strain were used. *P<0.05; ***P<0.001.
Genetic Deletion of Periostin Lowers Inflammation and Preserves Renal Tubular Structure
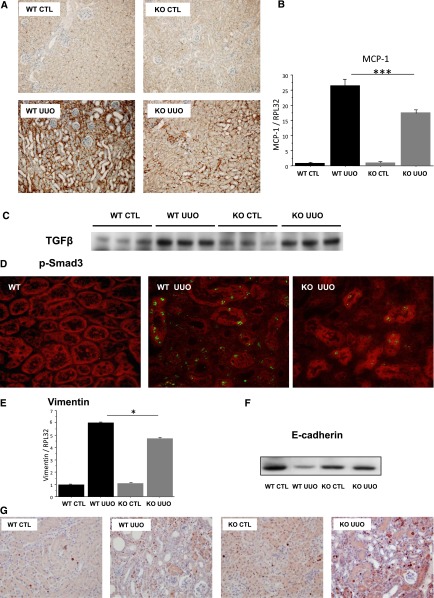
Obstructive nephropathy is characterized by a pronounced tubular inflammatory response and macrophage infiltration, which contribute to the development of renal lesions and interstitial fibrosis. As depicted in Figure 3A, macrophage infiltration, tracked by anti-F4/80 immunostaining, was clearly lower in the interstitium of Postn null mice; this protection from macrophage infiltration was probably caused by decreased activation of monocyte chemoattractant protein-1 (MCP-1) in the kidneys of Postn null mice (Figure 3B).
Figure 3.
Genetic deletion of periostin lowers inflammation and preserves renal tubular structure during UUO. (A) Macrophage infiltration evaluated by F4/80 immunostaining indicates a decreased infiltration of macrophages in Postn null mice. (B) This finding is in agreement with a lesser activation of MCP-1. (C) TGF-β expression (Western blot) increased to a similar degree in wt and Postn null mice after UUO. (D) Representative examples of p-Smad3 staining in wt controls as well as wt and Postn null mice after UUO. In addition, (E) the UUO-induced increase of mRNA expression of vimentin was blunted, and (F) the E-cadherin expression was preserved in the obstructed kidneys of Postn null mice. (G) Representative example of cell proliferation revealed by Ki 67 immunostaining. Nine mice per strain were used. *P<0.05; ***P<0.001.
The subsequent development of renal fibrosis is mainly mediated by TGF-β activation. In line with this finding, we found that TGF-β expression was strongly induced 15 days after UUO in wt mice (Figure 3C) and associated with a strong induction of p-Smad3 expression in renal tubular cells (Figure 3D, center panel). This increase was inhibited in Postn null mice (Figure 3D, right panel). One of the typical characteristics of the phenotype change of tubular epithelial cells during UUO is the loss of E-cadherin expression and the simultaneous induction of vimentin. As expected, vimentin expression increased by several-fold (Figure 3E), and E-cadherin expression profoundly decreased in the obstructed kidneys of wt mice (Figure 3F). In contrast, E-cadherin expression did not decrease, and the upregulation of vimentin was blunted in Postn null mice (Figure 3, E and F).
Tubular dilation in the UUO model is a complex mechanism involving high rates of epithelial cell proliferation counteracted by overincreased apoptosis. In our case, apoptosis rates seemed to be similar between the two strains; however, Postn null mice had an increased proliferation rate compared with wt (Figure 3G).
Effects of TGF-β and Periostin in Renal Collecting Duct Cells
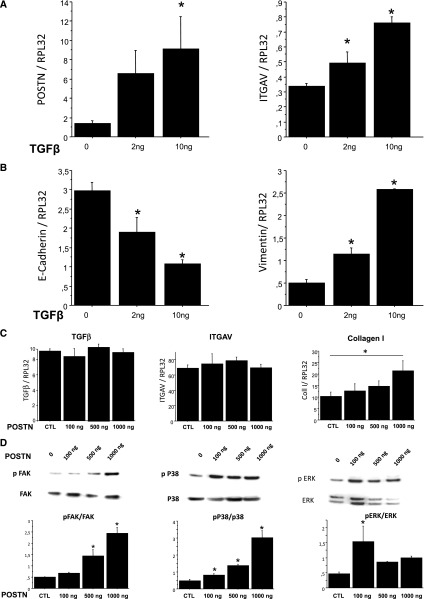
Because UUO first affects the collecting duct cells and because periostin synthesis and expression were found in these cells early after UUO (Figure 1), a human collecting duct cell line (H5 cells) was used in subsequent experiments to investigate the cellular mechanisms associated with the abnormal expression of periostin early in the disease. Exposure of H5 cells to TGF-β resulted in a dose-dependent increase of periostin expression (Figure 4A). This increase was accompanied with the induction of integrin receptor integrin alpha-V and paralleled their phenotype alterations, which are characterized by the decrease of E-cadherin and the simultaneous increase of vimentin expressions (Figure 4B).
Figure 4.
TGF-β induces Postn expression in cultured collecting duct cells. H5 cells were treated with either (A and B) 2 or 10 ng TGF-β or (C and D) 100, 500, or 1000 ng periostin for 24 hours. (A) Postn and ITGAV were increased in a dose-dependent manner. (B) At the same time, E-cadherin expression decreased, whereas vimentin expression increased, indicating a TGF-β–induced phenotype change to a fibroblast-like phenotype. (C) Evaluation of mRNA expressions of TGF-β, integrin alpha-V (ITGAV), and collagen I exposed to increasing doses of periostin. (D) Periostin-induced activation of FAK, p38, and ERK pathways assessed by Western blot. Three independent experiments in triplicate were performed in each condition. *P<0.05.
As shown in Figure 4C, periostin treatment increased collagen I expression in a dose-dependent manner but had no effect on TGF-β or integrin alpha-V expressions. However, periostin activated several major signaling pathways, such as focal adhesion kinase (FAK), p38, and extracellular signal-regulated kinase (ERK) (Figure 4D).
Periostin Silencing Prevented the Progression of L-NAME–Induced Renal Injury
Given that UUO is a particular model of renal disease, we measured periostin expression in other models of progressive renal disease. Thus, in transgenic mice overexpressing renin (a spontaneous model of renal fibrosis), periostin expression was increased at the early phases of progression (Supplemental Figure 1A). Furthermore, periostin was highly induced in two additional models characterized by severe proteinuria and fibrosis that ultimately lead to ESRD: a model of crescentic GN in mice (Supplemental Figure 1B) and a model combining hypertension and unilateral nephrectomy in rats (Supplemental Figure 1C).
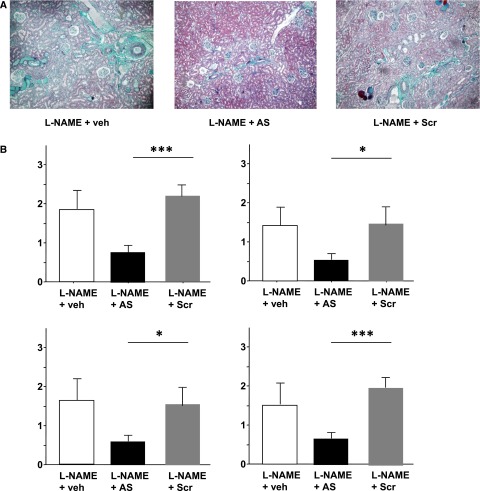
To test whether periostin could be considered as a therapeutic target, we performed additional studies in animals treated with specific AS ODN directed against periostin mRNA. Doses and sequences were validated in preliminary experiments. We used the l-NG-nitroarginine methyl ester (L-NAME)–induced model of CKD because of our previous experience with this model showing that it can be efficiently treated.3 L-NAME induced a 12-fold increase of periostin expression in the kidneys compared with controls (P<0.001), confirming our previous observations.15 Simultaneous administration of periostin AS ODN attenuated this overexpression (3.5-fold increase, P<0.01 versus L-NAME), whereas the scrambled (Scr) sequence did not have any effect on its expression (10.5-fold increase, P<0.01 versus AS). ODN administration did not alter hypertension (mean arterial pressure values were 190±16, 188±22, and 175±23 mmHg for the L-NAME, L-NAME+AS, and L-NAME+Scr groups, respectively). Animals treated with AS ODN showed decreased proteinuria compared with the Scr group (0.5±0.2 versus 1.2±0.3 g/mmol, P<0.05). In addition, several histologic parameters characteristic of the L-NAME model (glomerulosclerosis, perivascular fibrosis, vascular hypertrophy, and tubular dilation) were blunted in the kidneys of AS-treated rats in contrast to the Scr group (Figure 5).
Figure 5.
Treatment with specific periostin AS ODNs protects against the development of L-NAME–induced renal disease. (A) Representative renal sections stained by Masson’s Trichromic showing that rats treated with L-NAME and periostin AS display less fibrosis and tubular dilation compared with vehicle- or Scr-treated groups. (B) Quantifications of vascular hypertrophy, glomerulosclerosis, perivascular fibrosis, and tubular dilation confirm this protection. Data are mean±SEM. Six rats per treatment were used. Veh, vehicle. *P<0.05; ***P<0.001.
Discussion
To date, CKD is an incurable disease. It is well established that the local activation of the renin-angiotensin system is a key factor for inducing multiple mechanisms responsible for the progression of renal fibrosis.3,17,18 Timely blockade of angiotensin II action (angiotensin-converting enzyme inhibitors or angiotensin type 1 receptor antagonists) can promote reversal of renal lesions and ultimately prevent the progression to ESRD in animal models.3,19,20 However, renin-angiotensin system blockers do not exhibit the same efficiency in arresting or reversing chronic renal disease progression in humans, making the identification of novel targets for therapy urgent.21,22
The present study examined whether periostin could be such a target. Our results show that periostin is highly induced locally during renal disease and that its expression is associated with the development of renal lesions in wt animals. In contrast, mice lacking periostin expression are protected against structural and inflammatory alterations. This protection is probably caused by a dual mechanism: reduced inflammatory influx and decreased TGF-β signaling. A preservation of the renal function was also observed in a hypertensive model of CKD when periostin expression was inhibited by AS ODN administration. Thus, we provide evidence indicating that periostin mediates renal disease progression and suggest that the inhibition of its synthesis and/or action can lead to a therapeutic approach for CKD.
Angiotensin II can induce periostin expression in fibroblasts and vascular smooth muscle cells through the Ras/p38 mitogen-activated protein kinase/cAMP response element binding and ERK1/2/TGF-β1 pathways as well as through phosphatidylinositol 3-kinase signaling, respectively.23,24 Accordingly, periostin is induced in models of ischemic, hypertensive, and hypertrophic cardiomyopathies, and an angiotensin type 1 receptor antagonist was shown to decrease the cardiac expression of periostin.25–27 Periostin is transiently expressed during renal development, and the expression in the normal adult kidney is low.28 Our previous results found a strong association between periostin expression and classic indexes of renal function, including proteinuria, plasma creatinine, and renal blood flow.15 Importantly, these associations held true when arterial pressure was added as a covariate, which suggests that periostin is correlated to renal injury independently of the degree of hypertension. Immunohistochemistry revealed that the localization of periostin was predominantly perivascular, in areas where important deposits of ECM occur in this model. We also observed an intense extracellular staining for periostin in the injured fibrotic tubulointerstitial regions of chronic allograft nephropathy in human biopsies, which further shows the overexpression of periostin in the failing kidney.
The present study goes beyond these previous results, showing that periostin participates in the development of renal disease (at least in the ureteral obstruction model). Ureteral obstruction leads to an immediate increase of mechanical stress in the collecting duct cells, which is followed by macrophage infiltration and spreading of the tension increase and inflammation upstream to the tubular epithelial cells. Taking advantage of the transgenic animal model expressing β-galactosidase instead of periostin, we showed that, shortly after ureteral obstruction, periostin was synthesized by the collecting duct cells, the site of initial renal destruction in this model. It is possible that this early and local increase of periostin synthesis results from the increase of mechanical stress, which has been previously reported in pressure-overloaded cardiomyocytes.29
UUO is mainly characterized by a strong inflammatory response and an important invasion of macrophages in the tubular interstitium. Indeed, in our in vivo experiments, MCP-1 expression was increased several-fold and accompanied by significant macrophage infiltration in wt mice (Figure 3, A and B). The fact that the obstructed kidneys of Postn null mice exhibited a lesser increase of MCP-1 expression and decreased macrophage infiltration indicates an interaction between exaggerated periostin expression and the inflammatory response. Several recent studies support this hypothesis. Postn null mice were protected against the development of bleomycin-induced pulmonary fibrosis.30 This protection was caused by an impaired secretion of inflammatory cytokines, such as MCP-1, and the decreased subsequent recruitment of neutrophils and macrophages in lung fibroblasts. In addition, variations in the plasma concentrations of periostin were used as a readout for the efficiency of anti–IL-13 therapy in asthmatic patients.31 It is also proposed that periostin is a critical mediator for the amplification and persistence of allergic inflammation in an animal model of skin inflammation.32 In these studies, proinflammatory ILs stimulated periostin synthesis, which induced additional production of proinflammatory cytokines, accelerating and amplifying the inflammatory response. Inversely, genetic deletion of periostin expression caused impairment of the inflammatory response along with morphologic changes of the epidermis and prevented the development or progression of skin inflammation. Other investigators found that mechanical force activated the FAK and ERK pathways, which in turn, triggered the secretion of MCP-1 and lead to skin fibrosis33; this finding is in agreement with our in vitro results provided in Figure 4D, which show that periostin activated FAK and ERK pathways in collecting duct cells.
Several in vivo and in vitro studies investigated the interaction between TGF-β and periostin. Most studies agree that TGF-β is a major inducer of periostin secretion in a variety of tissues or cells, whereas inhibition of TGF-β is accompanied with decreased periostin expression.7,34,35 Other studies found that periostin can also induce TGF-β synthesis, interact with TGF-β cofactors, or activate TGF-β signaling.11,23,36 A recent study using a model of muscular dystrophy showed that mice lacking the periostin gene displayed major improvements in skeletal muscle structure and function, despite the persistence of increased levels of TGF-β.37 The proposed mechanism was that periostin is induced by TGF-β to promote the pathologic effects of TGF-β. Deletion of periostin does not act upstream in TGF-β synthesis but downstream by altering TGF-β signaling, which now enhances tissue regeneration and reduces levels of fibrosis. Indeed, Postn null mice embryo hearts exhibited reduced pSmad2,3 levels ,and loss of periostin reduced TGF-β responsiveness, resulting in an altered ECM.34 Our in vitro data show that TGF-β can strongly induce periostin synthesis and change the phenotype of collecting duct cells. Periostin does not induce TGF-β but activates several signaling pathways involved in tissue inflammation and fibrosis as well as cell migration. Our in vivo experiments show that periostin is synthesized early in collecting duct cells (before any significant inflammatory infiltration or structural or phenotype alteration). With the progression of disease, its expression expands into the renal epithelium and interstitium concurrently with inflammation, fibrosis, and structural destruction. Mice lacking periostin gene expression show inhibited TGF-β–pSmad3 signaling and are protected against inflammation, fibrosis, epithelial phenotype, and structural alterations. Taken together, our results are consistent with an inflammation-induced active fibrosis hypothesis, in which periostin is a central player. Thus, the early pressure increase in the collecting duct is the triggering signal of periostin synthesis. Periostin subsequently induces the secretion of proinflammatory cytokines that favor macrophage infiltration. TGF-β (produced by infiltrating cells) amplifies periostin synthesis and secretion, which leads to an additional increase of inflammation and ECM remodeling and promotes fibrosis. Thus, a vicious cycle is triggered, leading to the progression of renal disease and the ultimate destruction of the kidney.
To our knowledge, this study is the first attempt to block overexpression of periostin in vivo by daily treatment with specific AS ODN. We did not observe any adverse effects in control animals treated with AS or Scr ODN, at least for the period of the protocol. Scr ODN did not modify the course of the disease, whereas specific AS ODN induced a significant protection. The inhibition of the L-NAME–induced increase of periostin expression was not complete, and this finding can explain why treatment with periostin AS certainly preserved (but did not normalize) renal function and structure. In agreement with our results, it was shown that the use of a periostin neutralizing antibody protected mice from ovarian tumor growth and metastasis, a disease in which aberrant expression of periostin is also involved.38 It would be interesting to develop a similar strategy and test its efficiency in models of CKD.
In conclusion, our study shows that periostin is involved in the inflammatory and fibrotic mechanisms associated with the progression of renal disease. Furthermore, the observed protection in Postn null mice and rats treated with AS ODN provides hope that inhibition of periostin could represent a novel, promising target for the treatment of chronic renal disease.
Concise Methods
Animals
The strain of Postn null mice was created in the laboratory of S.J.C. These mice are characterized by the lack of the periostin gene and the replacement of the translation start site and the first exon with an lacZ reporter gene.
Tubulointerstitial nephropathy was induced by applying UUO in Postn null and C57BL/6 wt littermate female mice aged 4–6 months. After induction of general anesthesia (intraperitoneal injection of pentobarbital of 50 mg/kg), Postn null mice and wt littermate controls were subjected to a left flank incision. UUO was performed by complete ligation of the left ureter at the ureteropelvic junction using double silk sutures. Contralateral kidneys were used as controls. Mice were euthanized on days 2, 5, and 15. Nine mice per strain and time point were used.
In the AS experiments, male Sprague–Dawley rats, weighing 250 g, were maintained on a normal salt diet and had free access to chow and tap water. NO synthesis was inhibited by L-NAME (15 mg⋅kg−1 day−1 orally). We have previously found that this dose produced a gradual elevation of BP accompanied by the progression of renal disease. Animals were divided in three groups: L-NAME+saline, L-NAME+AS periostin, and L-NAME+Scr periostin (n=6 per group). Control groups consisted of animals receiving saline or AS or Scr periostin (n=4 per group). Mean arterial pressure was measured as previously described.15 All groups were euthanized 3 weeks after treatment. Details about the ODN sequences and administration are provided below.
Animals were euthanized under general anesthesia (pentobarbital=150 mg/kg). Kidneys were removed and divided into four equal parts (for RNA, protein, cryosection, and paraffin sections). All procedures were in accordance with the European Union Guidelines for the Care and Use of Laboratory Animals and approved by the local ethics committee.
Immunohistological Analyses
Staining for periostin was performed on 5-μm frozen sections. Sections were incubated with monoclonal rat anti-mouse periostin antibody (1/1000; R&D Systems).
Staining of proliferation and macrophages was performed on 5-μm sections of paraffin-embedded kidneys incubated with Ki 67 (polyclonal rabbit, 1/1000; Abcam) and F4/80 (monoclonal rat anti-mouse, 1/200; AbD Serotec), respectively.
Secondary antibodies were from the N-Histofine Kit (Nichirei Biochemicals, Japan). AEC (Dako) was used for a substrate, and slides were counterstained with hematoxylin QS (Vector, Burlingame, CA) and mounted with Permanent Aqueous Mounting Media (Innovex).
Staining for p-Smad3 was performed on 3-μm frozen sections. Sections were incubated with polyclonal rabbit anti-mouse p-Smad3 antibody (1/500; Abcam). The secondary antibody was Alexa anti-rabbit (1/1000; Abcam), and Evans blue was used to stain tissue.
Evaluation of β-Galactosidase Activity
β-Galactosidase staining was performed according to a previously described protocol.39 Tissues were fixed with 4% paraformaldehyde at 4°C for 1 hour, transferred to PBS containing 18% sucrose overnight (4°C), and then, stored at −80°C; 5-μm sections were cut, washed in PBS for 10 minutes, incubated in blocking buffer (1 mM MgCl2, 0.01% Na-deoxycholate, 0.02% IGEPAL-CA630, and 5 mM EGTA in PBS) for 20 minutes at room temperature, and incubated in the X-gal mixture [1 mM MgCl2, 0.01% Na-deoxycholate, 0.02% IGEPAL-CA630, 5 mM EGTA, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6.3H2O, and 1 µg X-gal; Invivogen]. Care was taken to perform X-gal staining at neutral pH, and the incubation was carried out for 16 hours at 37°C. After a 5-minute PBS wash, the sections were counterstained with eosin (Sigma-Aldrich, St. Louis, MO) and mounted.
Evaluation of Renal Histology
Formalin-fixed, paraffin-embedded tissues were cut into 4-μm sections and stained with Masson’s Trichrome. The slides were independently and blindly examined for the level of tubular dilation and fibrosis using a zero to four injury scale. The mean value was used to compare the animals’ groups.
Interstitial fibrosis was assessed on similar tissue sections stained with Sirius Red. Five cortical fields (excluding interlobular arteries) were selected randomly from each kidney, and the red-stained area per total area, reflecting interstitial fibrosis, was quantified using computer-based morphometric analysis software (Axionplan, Axiophot2; Zeiss, Germany). Scoring was performed in a blinded manner on coded slides. Data are expressed as the mean value of the percentage of the positive area examined.
Analysis of mRNA Expressions
We extracted RNA from the renal cortex using TRIzol solution (Life Technologies BRL, Gaithersburg, MD). RNA quality was checked by measuring the ratio of ODs at 260 and 280 nm, and residual genomic DNA was removed by DNase I treatment for 30 minutes at 37°C (Fermentas). We used reverse transcription with Revert Aid H minus First Strand DNA Synthesis Kit (Fermentas) to convert 1 μg RNA into cDNA. Transcriptomic analyses were performed with the RT2Profiler PCR Array (Superarray; Bioscience Corp, Tebu Bio, Le Perray en Yvelines, France). cDNA was amplified by PCR using a LightCycler 480 (Roche Diagnostic) using SYBR Green (Fast Start DNA Master SYBRGreen I; Roche Applied Science/Roche Diagnostic), specific primers for selected mRNA, and the 60S ribosomal protein L32 (RPL32) housekeeping gene under the following conditions: 95°C for 5 minutes, 45 cycles at 95°C for 15 seconds and 60°C for 15 seconds, and then, 72°C for 15 seconds. Specific primers were designed by Universal Probe Library system (Roche Applied Science), and sequences are shown in Tables 1 and 2. To normalize the quantitative RT-PCR results, we used Roche LightCycler 2.0 software (Roche Diagnostics). We expressed results as 2−∆Cp, where Cp is the cycle threshold number. We analyzed dissociation curves after each run for every amplicon to assess the specificity of quantification when using SYBR Green.
Table 1.
Primers (mouse) used in the in vivo experiments
| Studied Gene | Sequence of Oligonucleotides |
|---|---|
| RPL 32 | |
| Sense | 5′-GCTGCCATCTGTTTTACGG-3′ |
| AS | 5′-TGACTGGTGCCTGATGAACT-3′ |
| Periostin | |
| Sense | 5′-TCCAGCAGATATTCCAGTTG-3′ |
| AS | 5′-TTTCGCCTTCTTTAATCAGC-3′ |
| Collagen III | |
| Sense | 5′-TCCCCTGGAATCTGTGAATC-3′ |
| AS | 5′-TGAGTCGAATTGGGGAGAAT-3′ |
| Vimentin | |
| Sense | 5′-CCAACCTTTTCTTCCCTGAA-3′ |
| AS | 5′-TTGAGTGGGTGTCAACCAGA-3′ |
| MCP-1 | |
| Sense | 5′-CATCCACGTGTTGGCTCA-3′ |
| AS | 5′-GATCATCTTGCTGGTGAATGAGT-3′ |
Table 2.
Primers (human) used in the in vitro experiments
| Studied Gene | Sequence of Oligonucleotides |
|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | |
| Sense | 5′-AGCCACATCGCTCAGACAC-3′ |
| AS | 5′-GCCCAATACGACCAAATCC-3′ |
| Periostin | |
| Sense | 5′-GAACCAAAAATTAAAGTGATTGAAGG-3′ |
| AS | 5′-TGACTTTTGTTAGTGTGGGTCCT-3′ |
| E-cadherin | |
| Sense | 5′-CCCGGGACAACGTTTATTAC-3′ |
| AS | 5′-GCTGGCTCAAGTCAAAGTCC-3′ |
| Vimentin | |
| Sense | 5′-GACCAGCTAACCAACGACAAA-3′ |
| AS | 5′-GAAGCATCTCCTCCTGCAAT-3′ |
| TGF-β | |
| Sense | 5′-GCAGCACGTGGAGCTGTA-3′ |
| AS | 5′-CAGCCGGTTGCTGAGGTA-3′ |
| Collagen I | |
| Sense | 5′-TCTGGAGAGGCTGGTACTGC-3′ |
| AS | 5′-GAGCACCAAGAAGACCCTGA-3′ |
Cell Culture
Human collecting duct cells (H5 cell line donated by R. Piedagnel) were cultured at 37°C in a 5% carbon dioxide atmosphere in DMEM mixed 1:1 (vol:vol) with F12 medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% FBS. Cells were grown to approximately 70%–80% confluence and subjected to serum deprivation for 24 hours before experimental manipulation.
Human recombinant proteins TGF-β and Periostin were purchased from R&D Systems. Cells were stimulated for 24 hours before protein or RNA extraction (using EZ-10 Spin Column Total RNA Minipreps Super Kit) according to the manufacturer’s instructions.
Western Blot Analysis
Proteins were extracted from renal cortex or cells using RIPA buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich); 20 μg protein diluted in SDS sample buffer was heated at 70°C for 10 minutes, separated on a 4%–15% Tris⋅HCL gel, and then, transferred to a poly(vinylidene difluoride) membrane (Thermo Scientific). After blocking in 1× PBS and 5% milk (Thermoscientific) for 2 hours at room temperature, the membrane was incubated with the primary antibodies (Periostin; R&D Systems; 1/500, E-cadherin; Santa Cruz Biotechnology; 1/1000, TGF-β; Cell Signaling Technology; 1/1000, pFAK, FAK and pERK, ERK; Cell Signaling Technology; 1/1000, pP38 and P38; Santa Cruz Biotechnology) overnight at 4°C, washed, and incubated with secondary antibodies conjugated to horseradish peroxidase (SouthernBiotech or Vector) for 2 hours at room temperature. Protein bands were visualized using enhanced Supersignal West picochemiluminescent reagents according to the manufacturer’s instructions (Thermoscientific) and quantified using densitometry (Chemicapt; Fisher Bioblock Scientific).
Administration of AS against Periostin
To block periostin expression, we used a cocktail of two specific AS ODNs designed on IDT DNA (Integrated DNA Technologies) (Table 3) modified with phosphorothioate to prevent their in vivo hydrolysis by nucleases (Sigma-Aldrich, St. Quentin Fallavier, France). The absence of crossreactivity with related sequences in GenBank was checked. The AS or Scr control ODNs were diluted in 0.9% saline sodium chloride and administrated by intraperitoneal injections every 24 hours (100 pmol/ODN per injection), with a preinjection 48 hours before the administration of L-NAME. In addition, two groups of control rats received the AS or Scr ODNs.
Table 3.
Design of AS and Scr ODNs
| Studied Gene | Sequence of Oligonucleotides |
|---|---|
| Periostin AS 1 | G*A*GAGGAACCATCTTCAGCCCTGAGCTC*C*G |
| Periostin AS 2 | T*C*T*CCCTCACACCCTATT*T*C*A |
| Periostin Scr 1 | C*T*C*TCCGGAGAGCCACCGAGATCTGAG*T*C*A |
| Periostin Scr 2 | T*C*C*TACTCCACCTCTCAC*A*T*T |
Statistical Analyses
Statistical analyses were performed using ANOVA followed by protected least significance difference Fisher’s test (Statview software). Results with P<0.05 were considered statistically significant. All values are means±SEM.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Zela Keuylian for carefully reading the manuscript.
Financial support for this work was provided by the Institut National de la Santé et de la Recherche Médicale and UPMC Univ Paris 06. M.M.-A. and A.A. were the recipients of fellowships from the Ecole Doctorale 394, Sorbonne Universités, UPMC Univ Paris 06, and the CROUS.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013060664/-/DCSupplemental.
References
- 1.Rüster C, Wolf G: Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 22: 1189–1199, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA: Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med 2: 418–423, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C: Regression of renal vascular and glomerular fibrosis: Role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol 14: 1132–1144, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R: BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9: 964–968, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ: Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev 103: 183–188, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Norris RA, Kern CB, Wessels A, Moralez EI, Markwald RR, Mjaatvedt CH: Identification and detection of the periostin gene in cardiac development. Anat Rec A Discov Mol Cell Evol Biol 281: 1227–1233, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A: Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14: 1239–1249, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y: BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev Biol 315: 383–396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR: Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101: 695–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD: Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR: Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol 302: 256–266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T: Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 295: F1463–F1471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD: Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol 179: 1756–1767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG: Periostin: Novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant 27: 2702–2711, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrot D, Dussaule JC, Mael-Ainin M, Xu-Dubois YC, Rondeau E, Chatziantoniou C, Placier S: Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy. PLoS One 7: e31974, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiu K, Manabe I, Nagai R: Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM: Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC: Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway. Hypertension 36: 330–336, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Boffa JJ, Tharaux PL, Dussaule JC, Chatziantoniou C: Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 37: 490–496, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Adamczak M, Gross ML, Amann K, Ritz E: Reversal of glomerular lesions involves coordinated restructuring of glomerular microvasculature. J Am Soc Nephrol 15: 3063–3072, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R: Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatziantoniou C, Dussaule JC: Is kidney injury a reversible process? Curr Opin Nephrol Hypertens 17: 76–81, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL: Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc Res 91: 80–89, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, Conway SJ, McNamara CA, Sarembock IJ: Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis 188: 292–300, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohjolainen V, Rysä J, Näpänkangas J, Kööbi P, Eräranta A, Ilves M, Serpi R, Pörsti I, Ruskoaho H: Left ventricular periostin gene expression is associated with fibrogenesis in experimental renal insufficiency. Nephrol Dial Transplant 27: 115–122, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG: Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest 120: 3520–3529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iekushi K, Taniyama Y, Azuma J, Katsuragi N, Dosaka N, Sanada F, Koibuchi N, Nagao K, Ogihara T, Morishita R: Novel mechanisms of valsartan on the treatment of acute myocardial infarction through inhibition of the antiadhesion molecule periostin. Hypertension 49: 1409–1414, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sorocos K, Kostoulias X, Cullen-McEwen L, Hart AH, Bertram JF, Caruana G: Expression patterns and roles of periostin during kidney and ureter development. J Urol 186: 1537–1544, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H: P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J 27: 3104–3115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, Toda S, Sagara H, Aizawa H, Hoshino T, Conway SJ, Hayashi S, Izuhara K: Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 46: 677–686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, Mosesova S, Eisner MD, Bohen SP, Matthews JG: Lebrikizumab treatment in adults with asthma. N Engl J Med 365: 1088–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izuhara K: Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 122: 2590–2600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC: Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18: 148–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ: Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res 102: 752–760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC: Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13: 204–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV: Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A 107: 14170–14175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD: Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-β pathway. Proc Natl Acad Sci U S A 109: 10978–10983, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC, Slamon DJ: Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther 10: 1500–1508, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.