Abstract
Podocyte injury has a critical role in the pathogenesis of HIV-associated nephropathy (HIVAN). The HIV-1 transactivator of transcription (Tat), combined with fibroblast growth factor-2 (FGF-2), can induce the dedifferentiation and proliferation of cultured human podocytes. Cellular internalization of Tat requires interactions with heparan sulfate proteoglycans and cholesterol-enriched lipid rafts (LRs). However, the specific distribution of Tat in human podocytes and its ability to associate with LRs have not been documented. Here, we found that Tat is preferentially recruited to LRs in podocytes isolated from children with HIVAN. Furthermore, we identified arginines in the basic domain (RKKRRQRRR) of Tat as essential for (1) targeting Tat to LRs, (2) Tat-mediated increases in the expression of Rho-A and matrix metalloproteinase-9 in LRs, and (3) Tat-mediated enhancement of FGF-2 signaling in human podocytes and HIV-transgenic mouse kidneys and the exacerbation of renal lesions in these mice. Tat carrying alanine substitutions in the basic domain (AKKAAQAAA) remained localized in the cytosol and did not associate with LRs or enhance FGF-2 signaling in cultured podocytes. These results show the specific association of Tat with LRs in podocytes isolated from children with HIVAN, confirm Tat as a regulator of FGF-2 signaling in LRs, and identify the key domain of Tat responsible for promoting these effects and aggravating renal injury in HIV-transgenic mice. Moreover, these results provide a molecular framework for developing novel therapies to improve the clinical outcome of children with HIVAN.
Keywords: podocyte, Cell Signaling, pediatric nephrology, HIV nephropathy, proteinuria, cytokines
HIV-associated nephropathy (HIVAN) is a progressive renal disease seen in HIV-infected patients from African ancestry. One feature characteristic of HIVAN is the collapse of glomerular capillaries associated with the dedifferentiation and proliferation of podocytes.1 These changes affect the glomerular filtration barrier2 and cause heavy proteinuria and renal failure. Previous studies suggest that podocytes can be productively infected in vivo3 and that cells derived from the parietal epithelium4,5 may also be affected. In addition, circulating viral proteins and cytokines released by HIV-infected cells can induce renal injury as well.1 In this regard, the HIV-1 transactivator of transcription (Tat) protein has received significant attention, because it is a transcription factor that enhances viral replication; it can also be released and taken up by noninfected cells. In this manner, Tat can affect the function of podocytes acting through at least two different mechanisms.6–8 A concentration of Tat within the range detected in sera of HIV-infected patients9,10 can cause glomerular permeability changes in vivo11 and induce the proliferation of cultured podocytes by stimulating the release of fibroblast growth factor-2 (FGF-2).12 Indeed, high plasma, kidney, and urinary levels of FGF-2 are detected in children with HIVAN,13,14 and FGF-2 is accumulated in the kidney of HIV-Tg26 mice with renal disease.15–17 Overall, these studies suggest that both Tat and FGF-2 may play a role in the pathogenesis of childhood HIVAN.
Extracellular Tat and FGF-2 specifically bind and interact with negatively charged cell-surface heparan sulfate proteoglycans (HSPGs).6,18–20 Tat entry into cells is facilitated by an endocytic pathway that originates in lipid rafts (LRs) and involves caveolar endocytosis21 or LR-dependent macropinocytosis.22 LRs are specialized membrane domains enriched in certain lipids, cholesterol, and proteins that serve as docking sites for signaling proteins, including G protein–coupled receptors.23–25 They are resistant to low-temperature solubilization by nonionic detergents, and this property allows for their separation by differential flotation after density-gradient centrifugation in low-density fractions that are called detergent-resistant membranes (DRMs).23 Actin binding proteins bind to polyphosphoinositides located in LRs, and these proteins link the actin cytoskeleton with signaling molecules that are enriched in the LRs.23 In fact, activation of G protein–coupled receptors by HIV-Tat in brain endothelial cells results in the stimulation of small guanosine 5′‑triphosphatase of the Rho-family, which in turn, activate Rho-kinase and the myosin binding subunit of the myosin light chain (MLC).26,27 Moreover, activation of the Rho-A pathway in podocytes can cause chronic renal failure in transgenic mice.28 Therefore, we carried out this study to determine whether Tat, alone or combined with FGF-2, can induce cytoskeletal changes in cultured podocytes acting through an LR-mediated Rho-signaling mechanism and to define the Tat binding motif regulating this process in podocytes harvested from children with HIVAN.
Results
Extracellular Tat Increases the Ability of FGF-2 to Induce Rho-A/Phospho–Extracellular Signal-Regulated Kinase Signaling in Cultured Primary Podocytes Harvested from Children with HIVAN
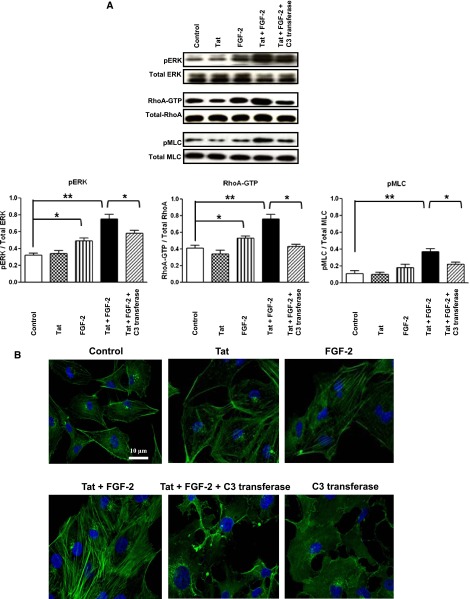
To determine how extracellular Tat and FGF-2 modulate Rho-A signaling in children with HIVAN, we cultured primary podocytes from the urine of these patients (Figure 1, Supplemental Figure 1). We found that Tat, alone or combined with FGF-2, induced the expression of Rho-A, phospho–extracellular signal-regulated kinase (pERK), and phospho-MLC2 (pMLC2) in these cells (Figure 2). These changes were partially inhibited by the exoenzyme C3-transferase from Clostridium botulinum, which inhibits Rho-proteins by ADP-ribosylation in the effector binding domain of the guanosine 5′‑triphosphatase (Figure 2), and did not involve other HIV-1 genes, because the cells were not infected. Similar results were reproduced in the cell lines generated from the primary podocytes (Figure 3A), validating their clinical relevance for the purpose of this study.
Figure 1.
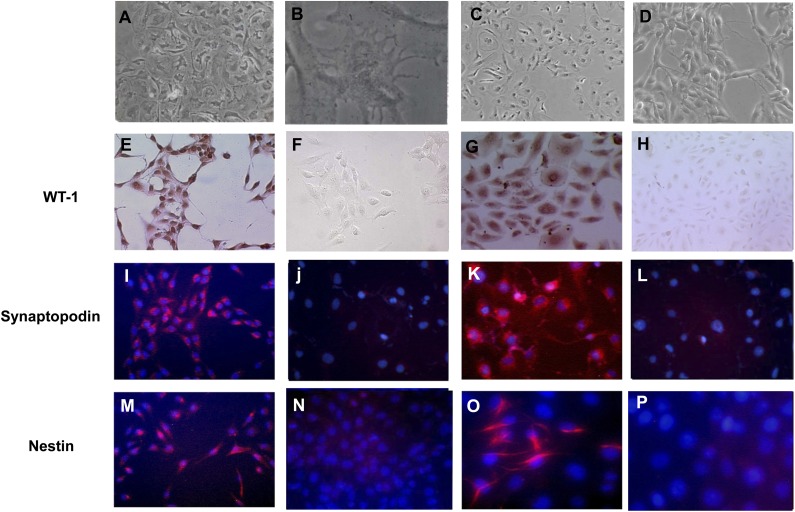
Podocytes cultured from the urine of children with HIVAN express WT-1, synaptopodin, and nestin. A–D show light microscopy images of (A and B) primary podocytes cultured from the urine of two children with HIVAN and (C and D) two cell lines derived from the primary podocytes. (E and G) Immunohistochemistry staining identified the nuclear localization of the WT-1 antigen in cultured podocytes (red). (F and H) No specific WT-1 staining was detected in cells incubated with nonimmune IgG. (I and K) By immunofluorescence microsocopy, synaptopodin was detected in red in the cytoplasm of cultured podocytes, whereas cell nuclei were counterstained in blue with diamino-2 phenylindol. (J and L) No specific synaptopodin staining was detected in podocytes incubated with irrelevant isotypic Igs. (M and O) Nestin was detected in red in the cytoplasm of podocytes, showing both a partial filamentous and homogenous pattern. Cell nuclei were counterstained in blue with diamino-2 phenylindol. (N and P) Nestin was not detected in podocytes incubated with irrelevant isotypic Igs. Original magnification, ×200 in A and E–P; ×400, B.
Figure 2.
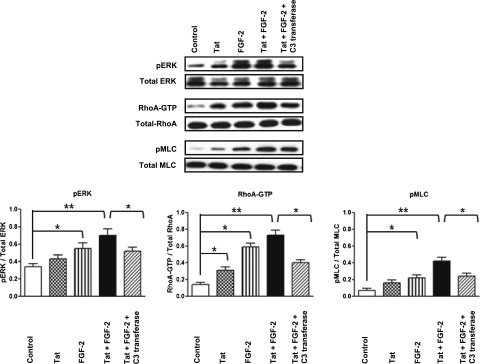
Extracellular Tat increases the ability of FGF-2 to induce Rho-A/pERK signaling in primary cultured podocytes isolated from the urine of children with HIVAN. Primary podocytes were starved overnight in serum-free media and treated with Tat (100 ng/ml) alone or combined with FGF-2 (50 ng/ml). The Rho-A inhibitor C3-transferase (20 ng/ml) was added 4 hours before stimulation. The cells were treated for 5 minutes as described above and then harvested to assess the phosphorylation of Rho-A, pERK, and pMLC as described in Concise Methods. The graph shows mean±SEM corresponding to three different experiments. Results were expressed in OD units expressed as a ratio of the total activity. Values significantly different from the corresponding control group are marked: *P<0.05; **P<0.01.
Figure 3.
Extracellular Tat increases the ability of FGF-2 to induce Rho-A/pERK signaling in a podocyte cell line generated from a child with HIVAN. (A) Cultured podocytes were starved overnight in serum-free media and treated with Tat (100 ng/ml) alone or combined with FGF-2 (50 ng/ml). The Rho-A inhibitor C3-transferase (20 ng/ml) was added 4 hours before stimulation. The cells were treated for 5 minutes as described above and then harvested to assess the phosphorylation of Rho-A, pERK, and pMLC as described in Concise Methods. The graph shows mean±SEM corresponding to three different experiments. Results were expressed in arbitrary OD units as a ratio of the total activity. Values significantly different from the corresponding control group are marked: *P<0.05; **P<0.01. (B) Cultured podocytes were treated as in A, and 20 minutes after treatment, F-actin fibers were visualized in cells by staining with 2 µg/ml Alexa Fluor 488–labeled phalloidin. Cell nuclei were stained with Hoechst 33342. Scale bar, 10 µm.
C3-Transferase Inhibits Tat+FGF-2–Induced Stress Fiber Formation and Rho-Activation in Cultured Podocytes
Follow-up experiments were done to determine how Tat+FGF-2 modulate the formation of stress fibers in the presence and absence of C3-transferase. As shown in Figure 3B, incubation of podocytes with Tat+FGF-2 resulted in a remarkable increase in stress fibers. These structures appeared as thick actin cables running along the length of the cell. In contrast, control cells or Tat+FGF-2–treated cells incubated with C3-transferase showed a more rounded shape, exhibited F-actin in the periphery of the cells, and formed fewer stress fibers on Tat+FGF-2 stimulation. The activation of pMLC2 was also inhibited by the C3-transferase (Figure 3A). These results suggest that Rho-A activation mediates the cytoskeletal changes induced by Tat+FGF-2 in cultured podocytes.
Tat Preferentially Associates with LRs in Cultured Human Podocytes
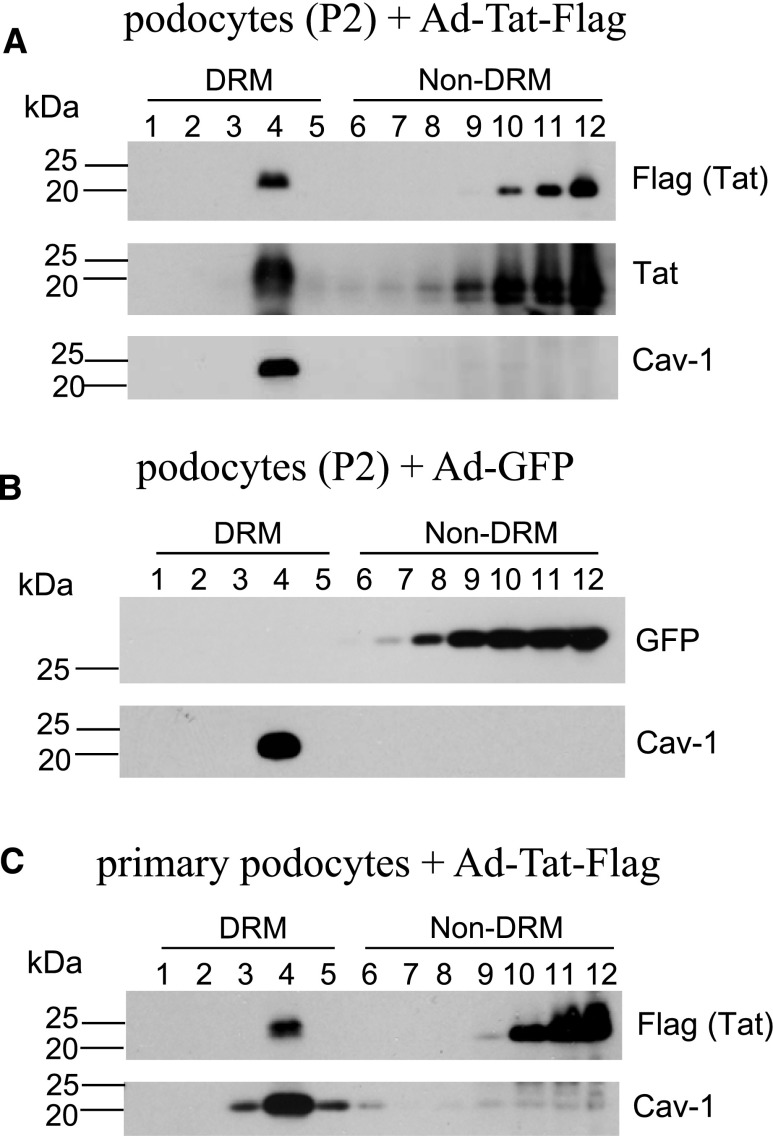
To investigate the intracellular distribution of Tat in cultured podocytes, we generated recombinant adenoviral vectors (rAds) carrying a cDNA fragment encoding the full-length Tat protein and the FLAG peptide sequence. As discussed in Concise Methods and Supplemental Figure 2, the cDNA fragment encoding Tat was derived from cultured renal tubular epithelial cells infected with HIV-1.29 This Tat variant, named Tat-HIVAN, was conserved in the N terminus but not the polymorphic C-terminal region downstream of the basic domain, and it was missing the RGD motif in the C-terminal region because of a frameshift deletion (Supplemental Figure 2). To validate the proper function of the Tat-HIVAN, we confirmed its ability to translocate to the nucleus in cultured podocytes and transactivate the HIV long terminal repeat in GHOST (GFP–human osteosarcoma) cells carrying a green fluorescent protein (GFP) reporter system30 (Supplemental Figures 3 and 4). Subsequently, using flotation sucrose gradients, we isolated LRs from cultured podocytes transduced with either rAd-Tat-FLAG or rAd-GFP control vectors. Using both FLAG and Tat antibodies, we found that a significant proportion of Tat was preferentially localized in fraction 4 of the DRMs, whereas additional Tat was detected in detergent-soluble fractions (non-DRMs) (Figure 4A). Caveolin-1, a protein known to associate with LRs, was almost exclusively detected in DRMs fraction 4, confirming that an efficient isolation of LRs was achieved (Figure 4). In contrast, GFP was not detected in DRMs (Figure 4B), but it was located in the bottom of the gradient, which is composed of non-DRMs that contain detergent-soluble cytoplasmic and nuclear components. Similar results were also found in cultured primary podocytes harvested from children with HIVAN (Figure 4C).
Figure 4.
HIV-1 Tat protein is associated with LRs in human podocytes isolated from the urine of children with HIVAN. LRs were isolated from a cultured podocyte cell line (P2) infected with adenovirus carrying (A) FLAG-tagged Tat (Ad-Tat-FLAG) or (B) GFP (Ad-GFP) or (C) primary podocytes infected with Ad-Tat-FLAG by sucrose gradient–based flotation assay. Twelve fractions of the gradient were run by SDS-PAGE to show the distribution of DRM and non-DRM fractions. FLAG-tagged Tat, GFP, and the LR marker Caveolin-1 (Cav-1) were analyzed by Western blotting.
Arginines in the Tat Basic Domain Sequences Mediate LR Association in Podocytes
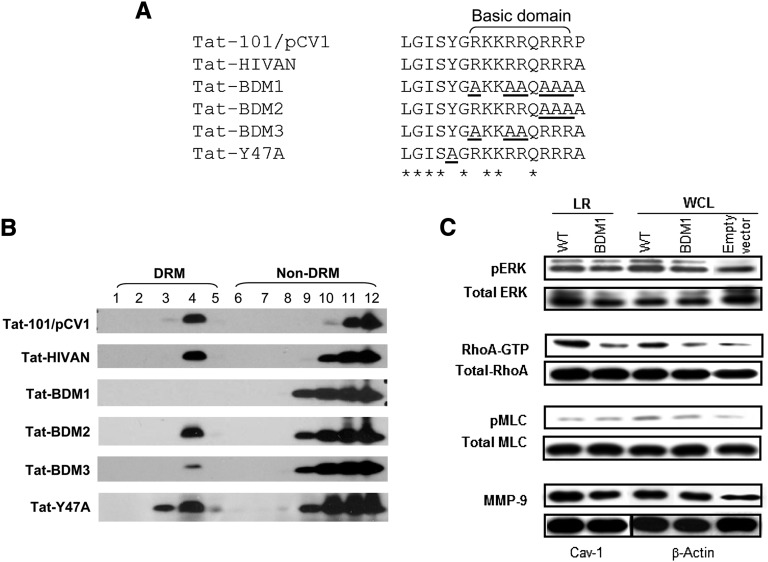
The basic domain of Tat, (RKKRRQRRR)49–57 (also called protein transduction domain), is known to mediate membrane crossing for protein transduction. In addition, arginines of the basic domain have been reported to specifically interact with negatively charged lipids in the plasma membrane.31 Therefore, we explored whether this domain is necessary and sufficient to mediate Tat association with LR in cultured podocytes. As shown in Figure 4A, we observed that two different wild-type (WT) –Tat variants, carrying or missing the RGD motifs, were localized in the LR domains and that the presence or absence of the RGD motif did not affect this localization. In contrast, we found that six alanine substitutions (AKKAAQAAA)49–57 carried by a mutant Tat, called Tat–basic domain mutant 1 (Tat-BDM1-HIVAN), almost completely abolished Tat association with LRs in cultured podocytes (Figure 5). Interestingly, partial substitutions of three arginines (55–57) did not affect the recruitment of Tat (Tat-BDM2-HIVAN) to the LRs (Figure 5B). Alternatively, another Tat mutant (Tat-BDM3-HIVAN) carrying partial substitutions of the other three arginines (49,52,53) showed a poor association with LRs (Figure 5B). Indeed, the OD ratio of DRM bands over non-DRM bands for Tat-BDM3-HIVAN was 5-fold lower than the ratio of Tat-BDM2-HIVAN or Tat-WT-HIVAN (data not shown). These data suggest that positions of arginines present in the Tat basic domain are important for its association with LRs.
Figure 5.
Arginines located in the basic domain are essential for targeting Tat to the LRs in cultured podocytes. (A) Several mutations were introduced in the basic domain of the WT Tat isolated from a child with HIVAN (Tat-HIVAN) to generate different Tat BDMs named for the purpose of this study: Tat-BDM1, Tat-BDM2, Tat-BDM3, and Tat-Y47A (mutation introduced in the putative cholesterol recognition consensus sequence). The Tat-101 control gene derived from the HIV-1 cDNA clone pCV1 (Supplemental Figure 2) carries the RDG motif, which is missing in Tat-HIVAN and all the other Tat BDMs. All these sequences were aligned using the Clustal Omega multiple sequence alignment program. Residues aligned identical are marked with an asterisk. Mutated residues are underlined. (B) Podocytes were transiently transfected with Tat-101/pCV1, Tat-HIVAN, Tat-HIVAN BDMs, or Tat-HIVAN-Y47A mutant. Cells were lysed with Triton X-100 and fractionated using a sucrose gradient flotation assay. FLAG-tagged Tat was detected by Western blot using the anti-FLAG antibody. (C) Western blot analyses showing changes in pERK, Rho-A, pMLC, and MMP-9 expression in isolated LRs and whole-cell lysates (WCLs) extracted from cultured podocytes transfected with WT Tat and Tat-mutated in BDM1 and the empty vector. GTP, guanosine triphosphate.
Because cholesterol is a major component of LRs, we also explored the potential role of the consensus cholesterol recognition/interaction amino acid consensus sequence (CRAC). The CRAC sequence is defined as (L/V–(X)1–5–Y–(X)1–5–R/K) and used by several proteins for binding cholesterol in the LRs, including Caveolin-1, HIV gp41, and human cytomegalovirus UL37 exon 1 protein.32,33 We found that arginines49,52,53 in the basic domain could form part of a potential CRAC motif (LGISYGRKKRR). Thus, because tyrosine in the CRAC motif is an essential residue for cholesterol binding,32,33 we generated a mutant Tat with Y47A (Tat-Y47A) and tested its association with LRs. We found that the Y47A mutation did not affect the association of Tat with the LRs (Figure 5B). These findings indicate that the CRAC sequence alone is not sufficient to warrant cholesterol binding and that other structural factors are needed for this process.32
Tat Basic Binding Domain Modulates Rho-A Signaling and the Expression of Matrix Metalloproteinase-9 in LRs
Rho-A signaling and matrix metalloproteinases (MMPs) modulate the migration of HIV-infected cells26 and facilitate the release of FGF-2 in patients with Kaposi's sarcoma.34,35 Children with HIVAN excrete high urinary levels of MMPs and FGF-2, which is in correlation with the progression of their renal disease.14,15 Therefore, we explored the effect of Tat-HIVAN on Rho-A signaling and MMP-9 expression. We found that Rho-A and MMP-9 were localized in the LRs of the podocytes and that the basic domain of Tat increased Rho-A phosphorylation and MMP-9 expression in LRs (Figure 5C).
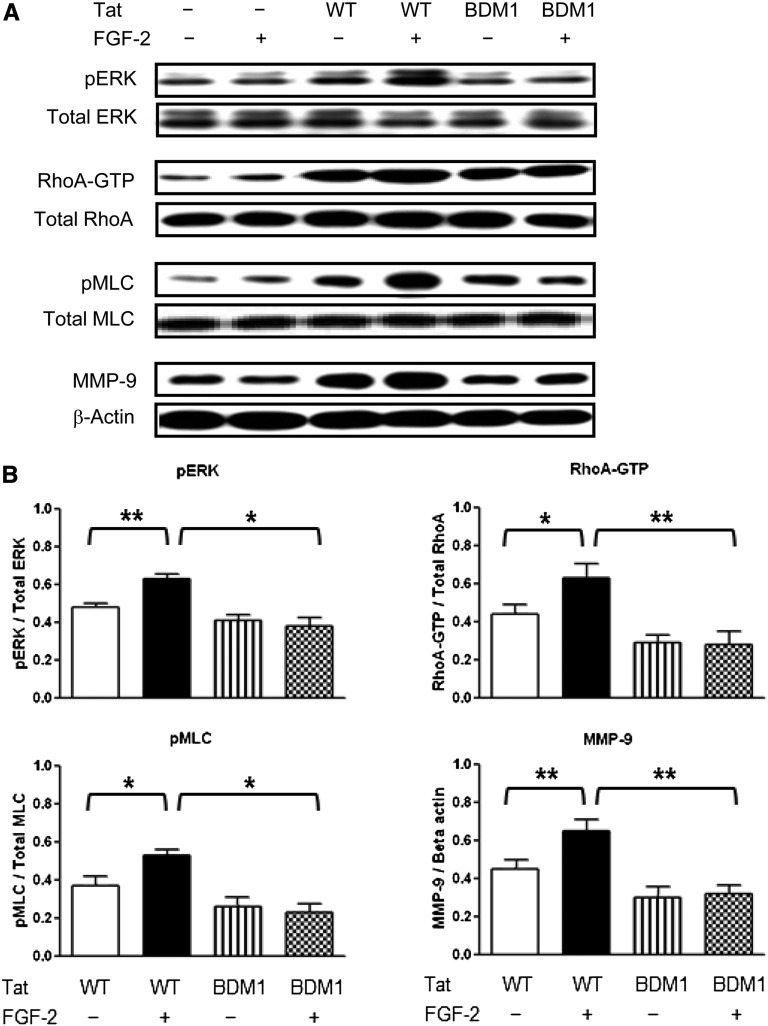
Tat Basic Domain Modulates FGF-2–Induced pERK, Rho-A, and pMLC2 in Cultured Podocytes
Because FGF-2 is accumulated in the kidney of children with HIVAN,13,14,16,17,36 we explored how the Tat basic domain modulated FGF-2 signaling in podocytes transfected with Tat-WT-HIVAN or Tat-BDM1-HIVAN. As shown in Figure 6, we found that Tat-WT-HIVAN, alone or combined with FGF-2, induced a more significant activation of pERK, Rho-A, and pMLC2 compared with Tat-BDM1-HIVAN. These changes are consistent with the results obtained in cultured primary podocytes using an extracellular Tat variant that carries the RGD motif (Figure 2). In contrast, Tat-BDM1-HIVAN was unable to increase the activity of FGF-2 (Figure 6). Overall, these findings confirm that the Tat basic domain plays a critical role modulating FGF-2 signaling in cultured podocytes and that the RGD motif is not essential for this process.
Figure 6.
The basic domain of Tat is essential for enhancing the FGF-2–induced activation of pERK, Rho-A, and pMLC2 and increasing the expression of MMP-9 in cultured podocytes. (A) Western blot analyses showing representative results in podocytes transiently transfected Tat-WT (WT) or Tat-BDM1 (BDM1) for 44 hours. Transfected cells were subsequently treated with FGF-2 (50 ng/ml) for 5 minutes and processed for the signaling studies. (B) The graph shows mean±SEM corresponding to five samples per group. Results are expressed in arbitrary OD units as a ratio of the total activity or normalized for β-actin for the MMP-9 expression. Values significantly different from the corresponding control group are marked: *P<0.05; **P<0.01.
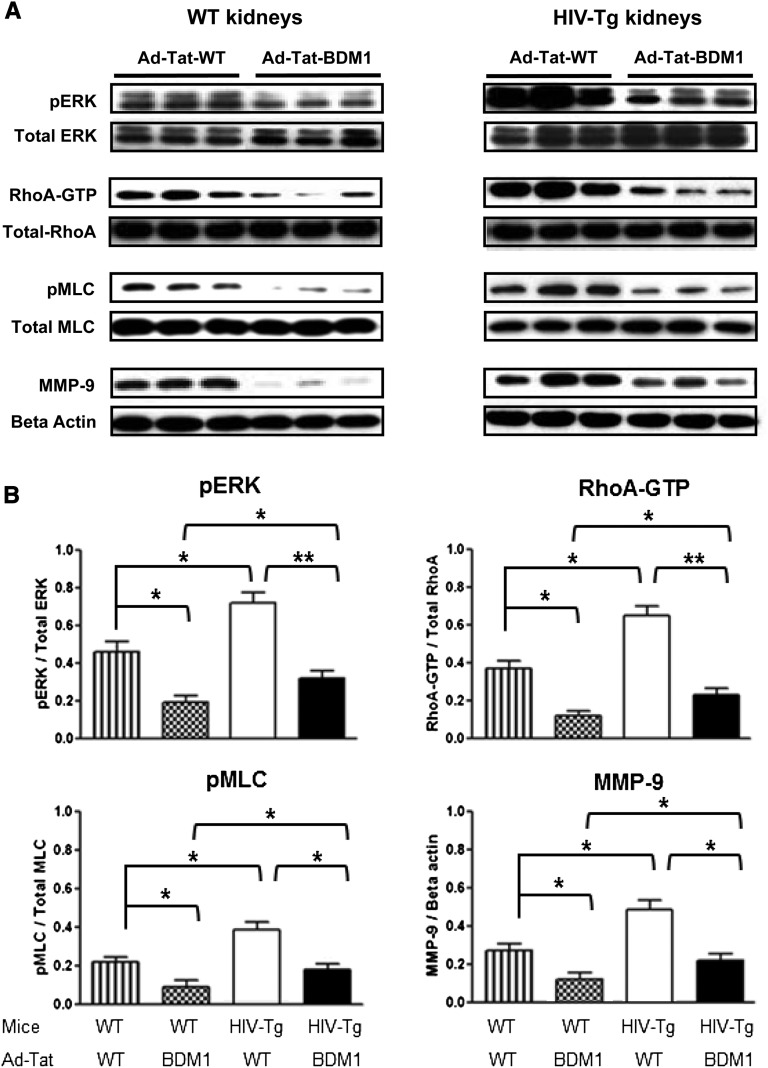
Tat Basic Domain Modulates the Expression of pERK, Rho-A, pMLC2, and MMP-9 in the Kidneys of Young HIV-Tg26 Mice
To determine the role of the Tat basic domain in vivo, we used an adenoviral gene transferring technique developed in our laboratory37 to express Tat in the kidney of young mice (Supplemental Figures 5 and 6). As shown in Figure 7, both WT and HIV-Tg26 mice infected with Ad-Tat-WT-HIVAN showed a significant upregulation of renal pERK, Rho-A, pMLC, and MMP-9 compared with mice infected with Ad-Tat-BDM1-HIVAN. These changes were more remarkable in HIV-Tg26 mice (Figure 7) and also associated with an upregulated expression of HIV-1 genes and the development of more severe renal lesions compared with HIV-Tg26 mice injected with rAd-Tat BDM1-HIVAN or rAd-LacZ vectors (Supplemental Figures 5 and 6).
Figure 7.
The basic domain of Tat is important for inducing the activation of pERK, Rho-A, and pMLC2 and increasing the expression of MMP-9 in the kidney of WT and HIV-Tg26 young mice. Newborn FVB/N WT and HIV-Tg26 mice (n=3 per group) were infected with adenoviral vectors carrying Tat-WT (Ad-Tat-WT) or Tat-BDM1 (Ad-Tat-BDM1). Mice were euthanized after 7 days, and their kidneys were processed for the signaling studies as described in Concise Methods. (A) The Western blots show representative results corresponding to three mice in each group. (B) The graphs summarize the results corresponding to all the kidney samples per group. Results were expressed in arbitrary OD units as a ratio of the total activity or normalized for β-actin for the MMP-9 expression. Values significantly different from the corresponding control group are marked: *P<0.05; **P<0.01.
Circulating FGF-2 Induces the Expression of pERK, Rho-A, pMLC, and MMP-9 in the Kidney of Adult HIV-Tg26 Mice
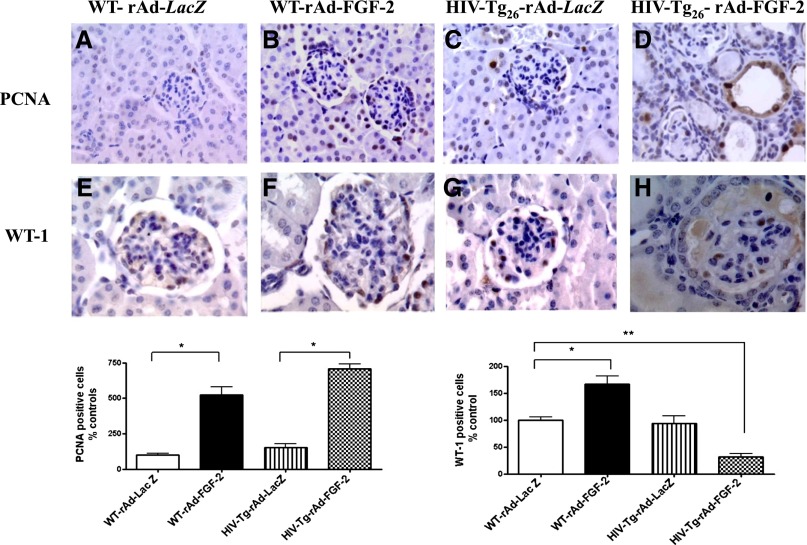
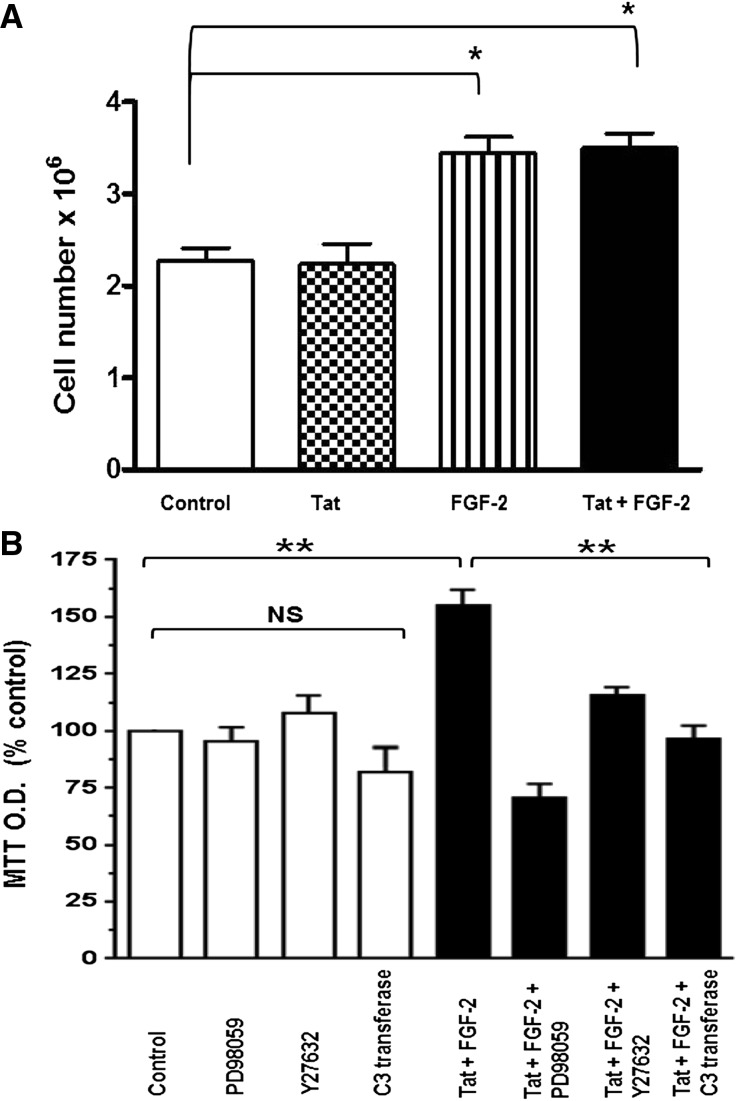
Because children with HIVAN show high plasma and urinary levels of FGF-2,13,14 we used Ad-FGF-2 vectors carrying a secreted form of FGF-2 to determine whether circulating FGF-2 can induce similar renal signaling changes in adult WT and HIV-Tg26 mice. In this experimental adult mouse model,38,39 only the hepatocytes are infected with the adenoviral vectors, and FGF-2 released into the circulation is trapped in the kidney bound to HSPGs.13,15,16,36,40 As shown in Figure 8, FGF-2 increased the renal expression of pERK, Rho-A, pMLC2, and MMP-9 in both WT and HIV-Tg26 mice. These changes, however, were more remarkable in HIV-Tg26 mice (Figure 8) and associated with the development of more severe renal lesions and albuminuria compared with all other groups (Figure 9, Supplemental Figure 7), Moreover, FGF-2, alone or combined with Tat, induced the proliferation and survival of cultured podocytes harvested from children with HIVAN acting through similar signaling pathways (Figure 10).
Figure 8.
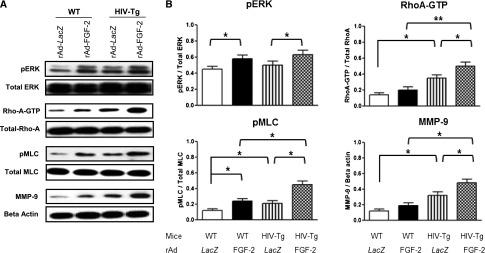
Circulating FGF-2 induces the expression of pERK, Rho-A, pMLC2, and MMP-9 in the kidneys of WT and HIV-Tg26 adult mice. Adult FVB/N WT and HIV-Tg26 mice (n=3 per group) were infected with adenoviral vectors carrying the Lac-Z gene (rAd-Lac-Z) or a secreted form of human FGF-2 (rAd-FGF-2). Mice were euthanized after 28 days, and their kidneys were processed for the signaling studies as described in Concise Methods. (A) The Western blots show representative results corresponding to one experiment. (B) The graphs summarize the results corresponding to six kidney samples per group. Results were expressed in arbitrary OD units as a ratio of the total activity or normalized for β-actin for the MMP-9 expression. Values significantly different from the corresponding control group are marked: *P<0.05; **P<0.01.
Figure 9.
Circulating FGF-2 precipitated the development of HIVAN in adult HIV-Tg26 mice. WT and HIV-Tg26 male adult mice without preexisting renal disease were infected with adenoviral viral vectors carrying a secreted form of human recombinant FGF-2 or the Lac-Z gene (n=5 per group) as described in Concise Methods. Renal sections were harvested 28 days after the adenoviral infection. (B and D) Both WT and HIV-Tg26 mice infected with rAd-FGF-2 showed a significant recruitment of glomerular and tubular epithelial cells expressing proliferating cell nuclear antigen (PCNA), a marker of DNA synthesis, replication, and intrinsic repair activity when compared to mice (A and C) injected with rAd-LacZ vectors. (H) HIV-Tg26 mice infected with rAd-FGF-2 showed a decreased number of WT-1+ cells compared with all other groups (E–G) (*P<0.01; ANOVA). (D and H) These changes were associated with the presence of FSGS lesions, tubular casts, and microcysts. The bar graphs show the immunohistochemistry staining scores corresponding to each group. Results are expressed as percent changes in PCNA or WT-1+ cells relative to the control group (WT mice infected with rAd-Lac-Z). Values significantly different from the corresponding control group are marked: *P<0.05; **P<0.01. Original magnification, ×200 in A–D; ×400 in E–H.
Figure 10.
FGF-2, alone or combined with Tat, increased the proliferation and survival of cultured podocytes. (A) Proliferation assay. Podocytes were seeded at a density of 5×104 cells/well and cultured in DMEM media supplemented with 1% FBS and penicillin/streptomycin for 4 days. HIV-Tat, FGF-2, or both combined were added daily at a concentration of 50 ng/ml each. On day 4, all cells were trypsinized and counted as described in Concise Methods. Values significantly different from controls are marked: *P<0.05. (B) The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) survival assay. Podocytes were seeded at a density of 6×104 cells per well and starved overnight on DMEM serum-free media containing antibiotics. Subsequently, all cells were treated with FGF-2+Tat at a concentration of 10 ng/ml each (black bars) in the presence or absence of the corresponding inhibitors. The kinase inhibitor PD98059 (5 µM), the Rho-associated protein kinase inhibitor Y27632 (10 µM), and the Rho-A inhibitor C3-transferase (20 ng/ml) were added 2 hours before the FGF-2+Tat treatment. On day 3, 10 µl MTT solution (5 mg/ml) was added to each well, incubated for 3 hours at 37°C, and then, treated with 100 µl MTT solvent (4 mM HCl and 0.1% Triton X-100 in isopropronal) as described in Concise Methods. Results were recorded in MTT absorbance units and expressed as a percent of control values (open bars) considering six independent readings. Values significantly different from controls are marked: **P<0.01.
Discussion
This study shows that Tat is preferentially recruited to LRs in podocytes harvested from children with HIVAN and induces cytoskeletal changes through the stimulation of the Rho-A/pMLC pathways. In addition, we found that alanine substitution of the six arginines in the Tat basic binding domain prevented the association of Tat with LRs, impaired its ability to enhance FGF-2 signaling or MMP-9 expression in cultured podocytes, and failed to induce severe renal disease in HIV-Tg26 mice. Taken together, these findings provide compelling evidence to support the notion that the Tat basic domain is essential for the recruitment of Tat to LRs and the regulation of FGF-2 signaling in podocytes isolated from children with HIVAN.
The Tat protein is a powerful transcriptional factor encoded by two exons. The first exon encodes the activation domain, which interacts with cyclin T1, and the basic domain (amino acids 49–57), which is required for the nuclear localization of Tat,41,42 HIV-1 transcription,7 and many other functions.7,41,42 The second exon encodes the RGD motif (C-terminal amino acids 73–86), which enhances the angiogenic activity of Tat acting through integrin receptors.43,44 However, the Tat protein derived from a child with HIVAN used in this study had an incomplete RGD sequence, and our results show that this sequence is not essential for the association of Tat with LRs or the regulation of FGF-2 signaling. Indeed, we found that Tat variants carrying the RGD motif were also recruited to LRs and induced FGF-2 signaling. Thus, our findings should be relevant for children infected with viruses carrying different Tat variants.
In contrast to the RGD motif, the Tat basic binding domain contains a cluster of basic residues (RKKRRQRRR) that are known to carry proteins and DNA molecules across the cell membranes and affect the activity of extracellular Tat peptides in human podocytes.11,12 LRs were also reported to play a critical role modulating Tat activity in other cell types26,45; however, these studies did not explore the specific localization of Tat in LRs. Moreover, to date, the interactions between Tat and LRs in podocytes are unclear, and the residues responsible for the association of Tat with LRs remain undefined. Here, we used purified LRs to show that three arginine residues in the basic domain are essential for the stable association of Tat with LRs and the regulation of FGF-2 signaling in cultured podocytes harvested from children with HIVAN. It is possible that Tat may be recruited to LRs microdomains by binding to anionic lipids that are enriched in the LRs.31 However, the basic residues are not equally important in membrane insertion or binding to polyphosphoinositides, because basic residues 49–51 but not arginines55–57 are critical for this process.31 Similarly, we found that three alanine substitutions in arginines49,52,53 but not arginines55–57 diminished Tat association with LRs by over 80%. These results indicate that lack of positive charges in the Tat-BDM1-HIVAN per se cannot fully explain its defective association with LRs. In that regard, there may be conformation-specific interactions between the basic domain and the LRs proteins to allow this stable association to occur.
Interesting findings of this study are the localization of MMP-9 within LRs in cultured podocytes and the ability of Tat-WT-HIVAN to induce the expression of MMP-9 in this location. MMP-9 belongs to a family of zinc binding endopeptidases that degrade extracellular matrix proteins, including HSPG and collagen.46 Previous studies have shown that MMP-9 was associated with LRs in several cancer cell lines, where they modulate angiogenesis and cell migration.47–49 In children with HIVAN, the urinary levels of MMP-9 are increased in correlation with the progression of their renal disease.14,16 Therefore, because MMP-9 facilitates the release of FGF-2,34 our findings may provide an alternative mechanism by which Tat can regulate the activity of FGF-2 in podocytes.
Also, Rho is a small guanosine triphosphate binding protein that plays a central role regulating the dynamic organization of contractile actin–myosin filaments and the formation of stress fibers in mammalian cells.50 When bound to guanosine triphosphate, Rho-proteins activate the Rho-kinase and other downstream effector proteins, including the phosphorylation of the myosin binding subunit of MLC.50 All these factors are important to maintain the cytoskeletal structure of podocytes and the integrity of the glomerular basement membrane.2 In line with this notion, our data suggest that Tat, combined with FGF-2, can induce the crosslinking of F-actin and the formation of stress fibers in human podocytes through activation of Rho-A and pMLC signaling pathways. In addition, we found that Tat increased the FGF-2–induced phosphorylation of pERK, a pathway leading to cell proliferation. Podocytes are terminally differentiated cells and have limited in vivo ability to undergo nuclear division in response to FGF-2.51 Thus, differentiated podocytes that are forced to re-enter the cell cycle under the influence of Tat and FGF-2 may be unable to divide and could detach or undergo apoptosis. Considering that FGF-2 is accumulated in the circulation, glomeruli, and urine of children with HIVAN,13,14,16,17,36 it is tempting to speculate that the podocytes carrying the genetic risk variants that predispose to HIVAN52,53 may be more sensitive to this pathogenic mechanism. In support of this notion, we found that both Tat and FGF-2 can precipitate the development of HIVAN in HIV-Tg26 mice. However, HIVAN is a complex renal disease, and other viral proteins like Nef, which inhibits Rho-A activation in conditionally immortalized cultured murine podocytes,54 also play a key role in this disease. Nonetheless, children may be more sensitive to the renal accumulation of FGF-2, because their kidneys are growing, and they have high expressions of HSPG, MMPs, FGF receptors, and binding proteins and higher plasma and tissue levels of FGF-2 relative to all other HIV-1 proteins.13,14,16,17,36 They also develop more severe immunosuppression and higher viral loads, leading to additional secretion of Tat and inflammatory cytokines that release more FGF-2.6,34 Moreover, our data did not rule out the possibility that Tat, acting through the release of systemic cytokines that upregulate the expression of HIV-1 genes through NF-κB activation, may play an additional role in HIVAN as well. Both Tat and FGF-2 are cleared from the circulation and stored bound to HSPG,6,18 and they can reach high tissue concentrations in organs with a high blood flow and HSPG content.15,17,40
In conclusion, we found that Tat is preferentially recruited to LRs in cultured podocytes from children with HIVAN. This event is mediated by the Tat basic domain, because mutations of six arginines in this region abolished Tat association with LRs and impaired its ability to induce Rho-A activation, MMP-9 expression, and FGF-2 signaling both in vitro and in vivo. These findings may provide a molecular framework to define the role of LRs in the pathogenesis of childhood HIVAN and/or identify new therapeutic targets to improve the outcome of children with other HIV renal diseases.
Concise Methods
Collection of Human Samples
The collection of human samples was carried out in accordance with the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Children’s National Medical Center, and a waiver of Documentation of Informed Consent and Health Insurance Portability and Accountability Act Authorization was obtained to allow for anonymous data and specimen collection after a verbal agreement was obtained from the patients or their parents.
Construction of Tat Expression Vectors and Adenoviruses
The cDNA fragment encoding Tat was derived from renal epithelial cells harvested from the urine of a child with HIVAN and infected with PBMCs isolated from the same child as previously described.29 Infected renal epithelial cells were then lysed using TRIzol (Invitrogen) to isolate total mRNA. The Tat gene was amplified from the subsequently synthesized cDNA with the high-fidelity DNA polymerase Pfu (Invitrogen) using PCR primers flanking the open reading frame of the full-length Tat.55 A cDNA fragment encoding the full-length Tat protein was cloned into the pCXN2-FLAG vector and used to generate E1-deleted recombinant adenoviruses as previously described.56 Both Tat-FLAG and GFP adenoviruses were purified, desalted, and titrated as described before.57 To generate adenoviruses that express secreted Tat protein, the signal peptide for secretion was amplified by PCR from the sp-FGF4:FGF-11–154–pMEXneo vector58 using forward 5′-ATACTCGAGATGGCGGGGCCCGGGACGGC-3′ and reverse 5′- GCGAAGCTTGGGCGCCAGCAAGGCCAGCAG-3′ primers. The PCR product of this 78-bp signal peptide was then ligated with the PCR product of the full-length Tat-FLAG (WT or BDM1) from the pCXN2 vector using forward 5′-ACTAAGCTTGACTACAAGGACGACGATGA-3′ and reverse 5′-TACGGATCCCTAACTAGCTAATCGAATCG -3′ primers. The resulting recombinant Tat-WT-FLAG or Tat-BDM1-FLAG gene with the FGF4 signal peptide sequence was cloned into the pVQAd CMV K-NpA shuttle plasmid (provided by ViraQuest, Inc., North Liberty, IA) to generate Tat adenoviruses (rAd-Tat-WT* and rAd-Tat-BDM1*) as described previously.59
Podocyte Cultures
Primary podocytes were isolated from clean-catch urine as previously described.29,60 Proliferating podocytes were obtained consistently from the urine of two children with HIVAN. Primary colonies that showed typical podocyte morphology (Figure 1, A–E), were further characterized by immunohistochemistry or immunofluorescence with the podocyte markers Wilms' tumor 1 (WT-1), synaptopodin, and nestin (Figure 1, F–P). Cells were fixed in 4% paraformaldehyde permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) and stained using well established methods with specific antibodies against WT-1 (Dako), synaptopodin (Maine Biotechnology, Inc.), and nestin (Chemicon Int., Inc.). Subsequently, selected colonies were transduced with adenoviral vectors carrying DNA sequences encoding the SV-40 large T antigen (provided by Janice Chou, National Institutes of Health, Bethesda, MD)61 and human telomerase (rAd-TERT; Applied Biologic Materials, Inc.). Colonies of transformed podocytes were characterized again by RT-PCR using specific primers for the podocyte markers WT-1, synaptopodin, podocalyxin, nestin, podocin, and nephrin as described in previous studies60,62 and shown in Supplemental Figure 1 and Supplemental Table 1. Podocyte colonies expressing all these markers, with the exception of podocin (which was not detected in any of these colonies), were selected and tested again by immunohistochemistry with WT-1, synaptopodin, and nestin antibodies (Figure 1, G, K, and O). Two podocyte colonies, named for the purpose of this study as P-2 and P-3 (Supplemental Figure 1), were expanded and cultured in DMEM supplemented with 10% FBS and 1% antibiotic–antimycotic (Invitrogen), which contains 100 units/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml Fungizone (amphotericin B). Western blots were done to confirm the expression of WT-1, synaptopodin, and nestin using antibodies from Dako (6F-H2), Santa Cruz Biotechnology (P-19), and Chemicon (MAb5326), respectively. Control sections were stained omitting the first antibody and using nonimmune IgG. All podocytes clones were screened and tested for the presence of HIV-1 genes by PCR as previously described.29
Mutagenesis
Site-specific mutations were introduced to the Tat gene using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) following the manufacturer’s manuals. To generate mutations in the basic domain RKKRRQRRR, forward primer 5′-GGCAGGAAGAAGCGGAGACAGGCAGCAGCAGCTCCTCAAGACAGTCAGAC-3′ and reverse primer 5′-GTCTGACTGTCTTGAGGAGCTGCTGCTGCCTGTCTCCGCTTCTTCCTGCC-3′ were first used to mutate arginines55–57 and generate a mutant Tat (RKKRRQAAA)49–57. Forward primer 5′-TTAGGCATCTCCTATGGCGCGAAGAAGGCGGCACAGGCAGCAGCAGCTCC-3′ and reverse primer 5′-GGAGCTGCTGCTGCCTGTGCCGCCTTCTTCGCGCCATAGGAGATGCCTAA-3′ were then used to produce mutant Tat with (AKKAAQAAA)49–57. Forward 5′-CTTAGGCATCTCCTATGGCGCGAAGAAGGCGGCACAGCGACGAAGAGCTCCT-3′ and reverse 5′-AGGAGCTCTTCGTCGCTGTGCCGCCTTCTTCGCGCCATAGGAGATGCCTAAG-3′ were used to generate mutant Tat with (AKKAAQRRR)49–57. Forward primer 5′-AGGCTTAGGCATCTCCGCTGGCAGGAAGAAGCGG-3′ and reverse primer 5′-CCGCTTCTTCCTGCCAGCGGAGATGCCTAAGCCT-3′ were used to introduce the Y47A mutation in the potential CRAC sequence, LGISYGRKKRR.
Extracellular Treatments, Adenovirus Infections, and Transient Transfection
Podocytes were exposed for 5 minutes to the control buffer, 100 ng/ml HIV-1 Tat protein (catalog number 2222; National Institutes of Health AIDS Reagent Program),63 or 50 ng/ml recombinant human FGF-2 (R&D Systems) alone or combined with HIV-Tat and harvested for the signaling or immunofluorescence studies. Both Tat and FGF-2 preparations were screened for endotoxin contamination (<0.1 ng/μg protein). As indicated, C3-transferase toxin (20 ng/ml) was added 4 hours before stimulation to block Rho-A activity. Alternatively, podocytes were infected with 2–4×109 particles/ml rAd-Tat or rAd-GFP vectors (Quantum Biotechnologies, Inc.) for 24–30 hours and then harvested for LRs isolation. For transfection, podocytes were transiently transfected with pCXN2-Tat-FLAG, pcDNA3.1-TAT-1–101-FLAG64 (Addgene), which was originally derived from the HIV-1 cDNA clone pCV1,65 or the corresponding Tat mutant or control vectors using Lipofectamine 2000 (Invitrogen) and harvested 36–48 hours later for LR isolation as described before.33 Cell proliferation and survival assays were done as previously described.36
LR Isolation
LR fractions were isolated using the flotation assay as described previously.33 In brief, podocytes were lysed on ice, homogenized, and sonicated using a Polytron tip sonicator. The cell lysate was then mixed with an equal volume of 80% sucrose dissolved in the isolation buffer and overlaid with discontinuous layers of 35% and 5% sucrose solutions at a volume ratio of 1:2:1 in a 4-ml ultracentrifuge tube (Beckman). The tubes were loaded on a Beckman SW60i rotor, and gradients were separated by centrifugation at 187,813×g (39,000 rpm) for 16 hours at 4°C. After centrifugation, 12 fractions (about 330 μl each) were sequentially collected from top to bottom, and 25 μl of each fraction were resolved by SDS-PAGE and analyzed by Western blot.
Western Blot Analyses
Cells were lysed using RIPA lysis buffer containing protease inhibitor and phosphatase inhibitor cocktail 2 (Sigma-Aldrich) and processed for Western blots as described before.66 The following primary antibodies were used: phospo-p44/42 mitogen-activated protein kinase (Thr202/Tyr204), p44/42 mitogen-activated protein kinase (ERK1/2), phospho-MLC2 (Thr18/Ser 19), and total MLC2 from Cell Signaling Technology, anti–MMP-9 (Calbiochem), β-actin (AC-15; Sigma-Aldrich), α-tubulin (DM1A; Abcam), Caveolin 1 (BD Biosciences), FLAG (M2; Sigma-Aldrich), GFP (Santa Cruz Biotechnology), and Tat Rabbit pAb (catalog number 705; National Institutes of Health AIDS Reagent Program).67 Protein bands were detected using Supersignal West Pico Chemiluminescent Substrate (Thermo Scientific) or an ECL Western Blot Detection Kit (GE Healthcare) following the manufacturer’s instructions.
Glutathione S-Transferase Pull-Down Assays
Rho-A activation was monitored by glutathione S-transferase (GST) pull-down using GST-Rhotekin recombinant protein bound to glutathione slurry resin using the protocol described before.66 Total and phosphorylated Rho-A were assessed by Western blotting using Rho-A (67B9) rabbit mAb (Cell Signaling Technology). For total Rho-A, we used an equal amount of protein corresponding to the cell lysates obtained before they were mixed with the GST beads.
Immunofluorescence Staining
Podocytes were cultured on cell growth-promoting coverslips (Fisher) or coverslips coated with type I collagen for 24 hours. Cells were treated as indicated and then fixed and permeablized as described before.66 After blocking with 1% BSA, cells were stained with 2 µg/ml Phalloidin–Alexa Fluor 488 (Invitrogen), and nuclei were stained with Hoechst 33342 (1:2000; Invitrogen) for 20 minutes at room temperature. After mounting, the confocal imaging was performed using an Olympus FV1000 confocal microscope, and a magnification of 60× was used for imaging.
Studies in WT and HIV-Tg26 Mice
These experiments were approved by the Children’s Research Institute Animal Care and Use Committee. WT and HIV-Tg26 FVB/N mice were housed in a pathogen-free environment on a 12:12-hour light/dark cycle in the animal facility at Children’s National Medical Center. All mice had free access to water and standard food and were treated in accordance with the National Institutes of Health guidelines for care and use of research animals. Both the generation of the HIV-Tg26 mouse colony and the adenoviral gene-transferring technique to express foreign genes in newborn mouse kidneys have been described in detail.37,68,69 Briefly, newborn FVB/N WT and HIV-Tg26 mice (n=3–8 per group) were injected through the retro-orbital plexus with adenoviruses carrying the Escherichia coli LacZ gene (rAd-LacZ), Tat-WT-FLAG, or Tat-BDM1-FLAG (1×108 pfu/pup). Tat mRNA expression was assessed by RT-PCR using the following primers: forward 5′-ATGGAGCCAGTAGATCCTAGAC-3′ and reverse 5′- CTAATCGAATCGATCTGTCTCTGC-3′. In other experiments, WT or HIV-Tg26 adult male mice without preexisting renal disease were divided in two groups (n=3–5 mice per group) and injected through the retro-orbital vein plexus with adenoviral vectors carrying LacZ or a 700-bp cDNA sequence encoding a secreted form of human FGF-2 (rAd-FGF-2; 5×108 pfu/mouse) as previously described.38,69,70 Mice were euthanized 7 or 28 days after the adenoviral infection, and their kidneys were processed for signaling or renal histologic studies as described above. Renal injury was assessed in sections stained with period acid–Schiff by counting the percentage of glomeruli exhibiting segmental/global sclerosis and the percentage of tubular casts and microcysts. In addition, we counted the number of cells that stained positive for proliferating cell nuclear antigen and WT-1 and assessed the magnitude of albuminuria as previously described.38
Statistical Analyses
If not specified otherwise, the data are expressed as means±SEMs. Multiple sets of data were compared by ANOVA with Newman–Keuls post hoc comparisons. The significant differences between the means of two groups were analyzed by unpaired t tests. Statistical analyses were performed using GraphPad Prism software (version 5.00; GraphPad Software, San Diego, CA). Values of P<0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The following reagents were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: Ghost(3)X4/R5 from Drs. Vineet N. Kewal Ramani and Dan R. Littman, HIV-1 Tat from Dr. John Brady, and HIV-1 BH10 Tat antiserum from Dr. Bryan Cullen. We thank Children's Research Institute Intellectual and Developmental Disabilities Research Center light microscopy and image analysis core at Children’s National Medical Center for help with the immunofluorescence studies. We thank the group of Dr. Valente for contributing the pcDNA3.1-TAT-1-101-FLAG plasmid to Addgene as well as Lian Xu, Dr. Jyoti Jaiswal, and Dr. Luana Scheffer for technical advice and helpful scientific discussions.
This study was supported, in part, by National Institutes of Health Grants R01-HL55605, R01-HL102497, and R01-DK049419.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013070710/-/DCSupplemental.
References
- 1.Ray PE, Hu CA: Advances in our understanding of the pathogenesis of HIV-1 associated nephropathy in children. Future Virol 6: 883–894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh J, Reiser J, Mundel P: Dynamic (re)organization of the podocyte actin cytoskeleton in the nephrotic syndrome. Pediatr Nephrol 19: 130–137, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D’Agati VD, Winston JA, Klotman ME, Klotman PE: Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 11: 2079–2087, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barillari G, Ensoli B: Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Clin Microbiol Rev 15: 310–326, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkhout B, Silverman RH, Jeang KT: Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 59: 273–282, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Vivès E, Brodin P, Lebleu B: A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 272: 16010–16017, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH: Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375: 497–500, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT: Selective CXCR4 antagonism by Tat: Implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci U S A 97: 11466–11471, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doublier S, Zennaro C, Spatola T, Lupia E, Bottelli A, Deregibus MC, Carraro M, Conaldi PG, Camussi G: HIV-1 Tat reduces nephrin in human podocytes: A potential mechanism for enhanced glomerular permeability in HIV-associated nephropathy. AIDS 21: 423–432, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Conaldi PG, Bottelli A, Baj A, Serra C, Fiore L, Federico G, Bussolati B, Camussi G: Human immunodeficiency virus-1 tat induces hyperproliferation and dysregulation of renal glomerular epithelial cells. Am J Pathol 161: 53–61, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray PE, Liu XH, Xu L, Rakusan T: Basic fibroblast growth factor in HIV-associated hemolytic uremic syndrome. Pediatr Nephrol 13: 586–593, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Soler-García AA, Rakhmanina NY, Mattison PC, Ray PE: A urinary biomarker profile for children with HIV-associated renal diseases. Kidney Int 76: 207–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray PE, Bruggeman LA, Weeks BS, Kopp JB, Bryant JL, Owens JW, Notkins AL, Klotman PE: bFGF and its low affinity receptors in the pathogenesis of HIV-associated nephropathy in transgenic mice. Kidney Int 46: 759–772, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Liu XH, Aigner A, Wellstein A, Ray PE: Up-regulation of a fibroblast growth factor binding protein in children with renal diseases. Kidney Int 59: 1717–1728, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ray PE, Xu L, Rakusan T, Liu XH: A 20-year history of childhood HIV-associated nephropathy. Pediatr Nephrol 19: 1075–1092, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F, Noonan D: HIV-tat protein is a heparin-binding angiogenic growth factor. Oncogene 12: 289–297, 1996 [PubMed] [Google Scholar]
- 19.Chu CL, Buczek-Thomas JA, Nugent MA: Heparan sulphate proteoglycans modulate fibroblast growth factor-2 binding through a lipid raft-mediated mechanism. Biochem J 379: 331–341, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urbinati C, Nicoli S, Giacca M, David G, Fiorentini S, Caruso A, Alfano M, Cassetta L, Presta M, Rusnati M: HIV-1 Tat and heparan sulfate proteoglycan interaction: A novel mechanism of lymphocyte adhesion and migration across the endothelium. Blood 114: 3335–3342, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Fittipaldi A, Ferrari A, Zoppé M, Arcangeli C, Pellegrini V, Beltram F, Giacca M: Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J Biol Chem 278: 34141–34149, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Wadia JS, Stan RV, Dowdy SF: Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med 10: 310–315, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Chini B, Parenti M: G-protein coupled receptors in lipid rafts and caveolae: How, when and why do they go there? J Mol Endocrinol 32: 325–338, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Pike LJ: The challenge of lipid rafts. J Lipid Res 50[Suppl]: S323–S328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons K, Ikonen E: Functional rafts in cell membranes. Nature 387: 569–572, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Zhong Y, Hennig B, Toborek M: Intact lipid rafts regulate HIV-1 Tat protein-induced activation of the Rho signaling and upregulation of P-glycoprotein in brain endothelial cells. J Cereb Blood Flow Metab 30: 522–533, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Y, Smart EJ, Weksler B, Couraud PO, Hennig B, Toborek M: Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci 28: 7788–7796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T: Activation of Rho-A in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray PE, Liu XH, Henry D, Dye L, 3rd, Xu L, Orenstein JM, Schuztbank TE: Infection of human primary renal epithelial cells with HIV-1 from children with HIV-associated nephropathy. Kidney Int 53: 1217–1229, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Mörner A, Björndal A, Albert J, Kewalramani VN, Littman DR, Inoue R, Thorstensson R, Fenyö EM, Björling E: Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol 73: 2343–2349, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayne F, Debaisieux S, Yezid H, Lin YL, Mettling C, Konate K, Chazal N, Arold ST, Pugnière M, Sanchez F, Bonhoure A, Briant L, Loret E, Roy C, Beaumelle B: Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J 29: 1348–1362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epand RM: Cholesterol and the interaction of proteins with membrane domains. Prog Lipid Res 45: 279–294, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Williamson CD, Zhang A, Colberg-Poley AM: The human cytomegalovirus protein UL37 exon 1 associates with internal lipid rafts. J Virol 85: 2100–2111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang HK, Brady JN, Gallo RC: Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature 371: 674–680, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Barillari G, Gendelman R, Gallo RC, Ensoli B: The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci U S A 90: 7941–7945, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray PE, Tassi E, Liu XH, Wellstein A: Role of fibroblast growth factor-binding protein in the pathogenesis of HIV-associated hemolytic uremic syndrome. Am J Physiol Regul Integr Comp Physiol 290: R105–R113, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Jerebtsova M, Liu XH, Ye X, Ray PE: Adenovirus-mediated gene transfer to glomerular cells in newborn mice. Pediatr Nephrol 20: 1395–1400, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Mattison PC, Soler-García AA, Das JR, Jerebtsova M, Perazzo S, Tang P, Ray PE: Role of circulating fibroblast growth factor-2 in lipopolysaccharide-induced acute kidney injury in mice. Pediatr Nephrol 27: 469–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerebtsova M, Wong E, Przygodzki R, Tang P, Ray PE: A novel role of fibroblast growth factor-2 and pentosan polysulfate in the pathogenesis of intestinal bleeding in mice. Am J Physiol Heart Circ Physiol 292: H743–H750, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Whalen GF, Shing Y, Folkman J: The fate of intravenously administered bFGF and the effect of heparin. Growth Factors 1: 157–164, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Hauber J, Malim MH, Cullen BR: Mutational analysis of the conserved basic domain of human immunodeficiency virus tat protein. J Virol 63: 1181–1187, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruben S, Perkins A, Purcell R, Joung K, Sia R, Burghoff R, Haseltine WA, Rosen CA: Structural and functional characterization of human immunodeficiency virus tat protein. J Virol 63: 1–8, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boykins RA, Mahieux R, Shankavaram UT, Gho YS, Lee SF, Hewlett IK, Wahl LM, Kleinman HK, Brady JN, Yamada KM, Dhawan S: Cutting edge: A short polypeptide domain of HIV-1-Tat protein mediates pathogenesis. J Immunol 163: 15–20, 1999 [PubMed] [Google Scholar]
- 44.Mitola S, Soldi R, Zanon I, Barra L, Gutierrez MI, Berkhout B, Giacca M, Bussolino F: Identification of specific molecular structures of human immunodeficiency virus type 1 Tat relevant for its biological effects on vascular endothelial cells. J Virol 74: 344–353, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong Y, Zhang B, Eum SY, Toborek M: HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci 32: 143–150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page-McCaw A, Ewald AJ, Werb Z: Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8: 221–233, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chetty C, Lakka SS, Bhoopathi P, Gondi CS, Veeravalli KK, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS: Urokinase plasminogen activator receptor and/or matrix metalloproteinase-9 inhibition induces apoptosis signaling through lipid rafts in glioblastoma xenograft cells. Mol Cancer Ther 9: 2605–2617, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raghu H, Sodadasu PK, Malla RR, Gondi CS, Estes N, Rao JS: Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer 10: 647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Chen X, Zhou J, Zhang L, Zhao Q, Chen G, Xu J, Qian F, Chen Z: CD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patients. Cancer Biol Ther 5: 808–814, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Etienne-Manneville S, Hall A: Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Sasaki T, Hatta H, Osawa G: Cytokines and podocyte injury: The mechanism of fibroblast growth factor 2-induced podocyte injury. Nephrol Dial Transplant 14[Suppl 1]: 33–34, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollak MR, Genovese G, Friedman DJ: APOL1 and kidney disease. Curr Opin Nephrol Hypertens 21: 179–182, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE: HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem 283: 8173–8182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emiliani S, Fischle W, Ott M, Van Lint C, Amella CA, Verdin E: Mutations in the tat gene are responsible for human immunodeficiency virus type 1 postintegration latency in the U1 cell line. J Virol 72: 1666–1670, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozarsky K, Grossman M, Wilson JM: Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia. Somat Cell Mol Genet 19: 449–458, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Jerebtsova M, Ye X, Ray PE: A simple technique to establish a long-term adenovirus mediated gene transfer to the heart of newborn mice. Cardiovasc Hematol Disord Drug Targets 9: 136–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forough R, Xi Z, MacPhee M, Friedman S, Engleka KA, Sayers T, Wiltrout RH, Maciag T: Differential transforming abilities of non-secreted and secreted forms of human fibroblast growth factor-1. J Biol Chem 268: 2960–2968, 1993 [PubMed] [Google Scholar]
- 59.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL: A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 7: 1034–1038, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV: Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 285: F40–F48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Doren K, Gluzman Y: Efficient transformation of human fibroblasts by adenovirus-simian virus 40 recombinants. Mol Cell Biol 4: 1653–1656, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakairi T, Abe Y, Kajiyama H, Bartlett LD, Howard LV, Jat PS, Kopp JB: Conditionally immortalized human podocyte cell lines established from urine. Am J Physiol Renal Physiol 298: F557–F567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bohan CA, Kashanchi F, Ensoli B, Buonaguro L, Boris-Lawrie KA, Brady JN: Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr 2: 391–407, 1992 [PMC free article] [PubMed] [Google Scholar]
- 64.Mousseau G, Clementz MA, Bakeman WN, Nagarsheth N, Cameron M, Shi J, Baran P, Fromentin R, Chomont N, Valente ST: An analog of the natural steroidal alkaloid cortistatin A potently suppresses Tat-dependent HIV transcription. Cell Host Microbe 12: 97–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arya SK, Guo C, Josephs SF, Wong-Staal F: Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science 229: 69–73, 1985 [DOI] [PubMed] [Google Scholar]
- 66.Gavard J, Patel V, Gutkind JS: Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14: 25–36, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Hauber J, Perkins A, Heimer EP, Cullen BR: Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A 84: 6364–6368, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE: Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci U S A 89: 1577–1581, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Jerebtsova M, Liu XH, Tang P, Ray PE: Novel cystogenic role of basic fibroblast growth factor in developing rodent kidneys. Am J Physiol Renal Physiol 291: F289–F296, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Gupta AR, Dejneka NS, D’Amato RJ, Yang Z, Syed N, Maguire AM, Bennett J: Strain-dependent anterior segment neovascularization following intravitreal gene transfer of basic fibroblast growth factor (bFGF). J Gene Med 3: 252–259, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.