Abstract
In 2013, the Organ Procurement and Transplantation Network in the United States approved a new national deceased donor kidney allocation policy that introduces the kidney donor profile index (KDPI), which gives scores of 0%–100% based on 10 donor factors. Kidneys with lower KDPI scores are associated with better post-transplant survival. Important features of the new policy include first allocating kidneys from donors with a KDPI≤20% to candidates in the top 20th percentile of estimated post-transplant survival, adding waiting time from dialysis initiation, conferring priority points for a calculated panel-reactive antibody (CPRA)>19%, broader sharing of kidneys for candidates with a CPRA≥99%, broader sharing of kidneys from donors with a KDPI>85%, eliminating the payback system, and allocating blood type A2 and A2B kidneys to blood type B candidates. We simulated the distribution of kidneys under the new policy compared with the current allocation policy. The simulation showed increases in projected median allograft years of life with the new policy (9.07 years) compared with the current policy (8.82 years). With the new policy, candidates with a CPRA>20%, with blood type B, and aged 18–49 years were more likely to undergo transplant, but transplants declined in candidates aged 50–64 years (4.1% decline) and ≥65 years (2.7% decline). These simulations demonstrate that the new deceased donor kidney allocation policy may improve overall post-transplant survival and access for highly sensitized candidates, with minimal effects on access to transplant by race/ethnicity and declines in kidney allocation for candidates aged ≥50 years.
The current deceased donor kidney allocation policy has not changed substantially in >20 years.1 During this time, the gap between supply and demand has widened. Waiting time has become the dominant factor in allocation, and less emphasis has been placed on biologic criteria such as the degree of immune system sensitization or HLA matching. For minority candidates, such as African Americans, who have difficulty gaining access to the waiting list, delay in listing contributes to racial disparities in access to transplant.2–4 The current allocation system favors candidates who have waited the longest, but does not improve outcomes after transplant and discourages use of kidneys with a potentially shorter duration of functioning. These shortcomings have created inequities. Waiting times for blood type B candidates are much longer than waiting times for blood type A candidates.5,6 Kidneys with the potential to function longer may be allocated to candidates with shorter life expectancy; conversely, candidates with a longer estimated life span may be allocated kidneys with limited duration of functioning. These types of transplants result in high retransplant rates and increase the demand for donor kidneys. A new allocation policy was approved by the Organ Procurement and Transplantation Network (OPTN) on June 24, 2013.
The new allocation policy risk-stratifies deceased donors using the kidney donor profile index (KDPI).7,8 The KDPI takes into account donor age, height, weight, ethnicity, history of hypertension and diabetes, cause of death, serum creatinine level, hepatitis C status, and donation after circulatory death status (Table 1). Lower KDPI kidneys are associated with better post-transplant survival. Similarly, transplant candidates on the waiting list are risk-stratified based on estimated post-transplant survival (EPTS), which takes into account candidate age, dialysis duration, prior solid organ transplant, and diabetes status. Generally, older age, longer dialysis duration, prior solid organ transplant, and presence of diabetes are associated with higher EPTS scores and shorter expected post-transplant survival. The new allocation policy prioritizes candidates in the top 20th EPTS percentile to receive kidneys from donors with a KDPI≤20% (so-called “longevity matching”). It also prioritizes candidates with a calculated panel-reactive antibody (CPRA)≥98% and provides broader sharing for candidates with a CPRA≥99% (Table 1). The new policy maintains the current restriction in which kidneys from blood type B and O donors are allocated strictly to candidates with identical blood types (except for the zero-HLA mismatch category; Table 1).
Table 1.
| Wait-Listed Candidates | |||
|---|---|---|---|
| KDPI≤0.20 | KDPI 0.21–0.34 | KDPI 0.35–0.85 | KDPI>0.85 |
| Local CPRA 100% | Local CPRA 100% | Local CPRA 100% | Local CPRA 100% |
| Regional CPRA 100% | Regional CPRA 100% | Regional CPRA 100% | Regional CPRA 100% |
| National CPRA 100% | National CPRA 100% | National CPRA 100% | National CPRA 100% |
| Local CPRA 99% | Local CPRA 99% | Local CPRA 99% | Local CPRA 99% |
| Regional CPRA 99% | Regional CPRA 99% | Regional CPRA 99% | Regional CPRA 99% |
| Local CPRA 98% | Local CPRA 98% | Local CPRA 98% | Local CPRA 98% |
| 0 HLA mm top 20 | 0 HLA mm | 0 HLA mm | 0 HLA mm |
| Prior living donors | Prior living donors | Prior living donors | Local, regional adult |
| Local pediatric | Local pediatric | Local | National adult |
| Local top 20 | Local adult | Regional | |
| 0 HLA mm bottom 80 | Regional pediatric | National | |
| Local bottom 80 | Regional adult | ||
| Regional pediatric | National pediatric | ||
| Regional top 20 | National adult | ||
| Regional bottom 80 | |||
| National pediatric | |||
| National top 20 | |||
| National bottom 80 | |||
0 HLA mm designates candidates with zero HLA mismatch at A, B, and DR loci; top 20 designates candidates in the top 20th percentile of survival; bottom 80 designates candidates not in the top 20th percentile of survival. Both the new and the current allocation policies give priority to candidates listed for simultaneous kidney and non-kidney organ transplants, including kidney-pancreas, kidney-liver, and kidney-heart transplants. This is not shown in the table above and is not included in the KPSAM modeling. Prior living donors represent a small number of candidates who are not included in the KPSAM modeling. SCr, serum creatinine; CVA, cerebrovascular accident.
KDPI is derived from the kidney donor risk index (KDRI) developed by Rao et al.7 The KDPI includes only the donor-specific elements of the KDRI, and is mapped to a reference population from the previous year, in order to yield percentiles. For KDRI, the reference population is all kidneys recovered for transplant between January 1, 2007, and December 31, 2009. The calculation is as follows: KDRI=exp(−0.0194×I[age<18 yr]×[age−18 yr]+0.0128×[age−40 yr]+0.0107×I[age>50 yr]+0.179×I[race=African American]+0.126×I [hypertensive]+0.130×I[diabetic]+0.220×[SCr−1 mg/dl] −0.209×I[SCr>1.5 mg/dl]×[SCr−1.5 mg/dl]+0.0881×I[cause of death=CVA]−0.0464×[{height−170 cm}/10]−0.0199×I[weight<80 kg]×[{weight–80 kg}/5]+0.133×I[donation after cardiac death] +0.240×I[hepatitis C]−0.0766, where I is equal to 1 if the condition is true and I is equal to 0 if the condition is false.
EPTS score=0.047×MAX (Age−25, 0)−0.015×Diabetes×MAX(Age–25,0)+0.398×Prior Organ Transplant−0.237×Diabetes×Prior Organ Transplant+0.315×log(Years on Dialysis+1)−0.099×Diabetes×log(Years on Dialysis+1)+0.130×(Years on Dialysis=0)−0.348×Diabetes×(Years on Dialysis=0)+1.262×Diabetes.
As in the current system, points will be used to rank candidates in each category listed in Table 1, with more points leading to higher priority for receiving a kidney offer. One point will be awarded for each year spent waiting once the eGFR measurement is <20 ml/min per 1.73 m2 (Table 2). However, under the new allocation policy, candidates receiving dialysis at the time of listing will also receive waiting time credit from the first day of maintenance dialysis. These waiting time points will be awarded based on fractional years, by dividing the number of days waiting by 365 (Table 2). A new priority point scale of 0–202 will be awarded based on the CPRA (Tables 2 and 3). This scale was based inversely on the probability of receiving an organ offer. Other points will be awarded as in the current allocation policy (Table 2).1 Other features of the current and new kidney allocation policies are compared in Table 4.
Table 2.
Priority point system for new kidney allocation
| Factor | Points Awarded |
|---|---|
| For qualified time spent waiting | 1 per year (as 1/365 per day) |
| Degree of sensitization (CPRA) | 0–202 |
| Prior living organ donor | 4 |
| Pediatric candidate if donor KDPI<0.35 | 1 |
| Pediatric candidate (age 0–10 yr at time of match) when offered a zero antigen mismatch | 4 |
| Pediatric candidate (age 11–17 yr at time of match) when offered a zero antigen mismatch | 3 |
| Share a single HLA-DR mismatch with donor | 1 |
| Share a zero HLA-DR mismatch with donor | 2 |
These points will be used to rank candidates in each of the categories listed in Table 1, with more points leading to higher priority for receiving a kidney offer.
Table 3.
Priority points awarded based on CPRA>19%
| CPRA (%) | Points |
|---|---|
| 0–19 | 0 |
| 20–29 | 0.08 |
| 30–39 | 0.21 |
| 40–49 | 0.34 |
| 50–59 | 0.48 |
| 60–69 | 0.81 |
| 70–74 | 1.09 |
| 75–79 | 1.58 |
| 80–84 | 2.46 |
| 85–89 | 4.05 |
| 90–94 | 6.71 |
| 95 | 10.82 |
| 96 | 12.17 |
| 97 | 17.3 |
| 98 | 24.4 |
| 99 | 50.09 |
| 100 | 202.1 |
Table 4.
Comparison of allocation concepts for current and new allocation policy
| Features | Policy | |
|---|---|---|
| Current | New | |
| SCD allocation (defined as KDPI≤0.85 for new policy) | X | X |
| DCD allocation | X | |
| ECD allocation (defined as KDPI>0.85 for new policy) | X | X |
| Payback system | X | |
| Waiting time since listing | X | |
| Waiting time from dialysis initiation | X | |
| Waiting time points based on fractional years | X | |
| A2/A2B blood type donor to B candidates priority (local, regional, national) | X | |
| Highest scoring CPRA classification | X | |
| Pediatric candidates cannot receive non-0 mm ECD offers | X | |
| Longevity matching (top 20th percentile survivors first offered kidneys with KDPI<0.20) | X | |
| Share KDPI<0.35 kidneys pediatric priority (donor age<35 yr for current policy) | X | X |
| Priority points for CPRA>19% | X | |
| Priority points for CPRA>79% | X | |
| National priority sharing for CPRA 100%, regional priority sharing for CPRA 99%, local priority for CPRA 98% candidates | X | |
| Regional sharing for marginal kidneys (KDPI>0.85) | X | |
| Kidney pancreas/pancreas alone allocation policy: current (1) | X | |
| Kidney pancreas/pancreas alone allocation policy: future (1) | X | |
SCD, standard criteria donor; DCD, donation after circulatory death; ECD, expanded criteria donor.
In this study, we describe the final simulation models that were used to estimate how the current and new allocation systems allocate deceased donor kidneys. The results of these simulations were used to propose the new allocation system.
Results
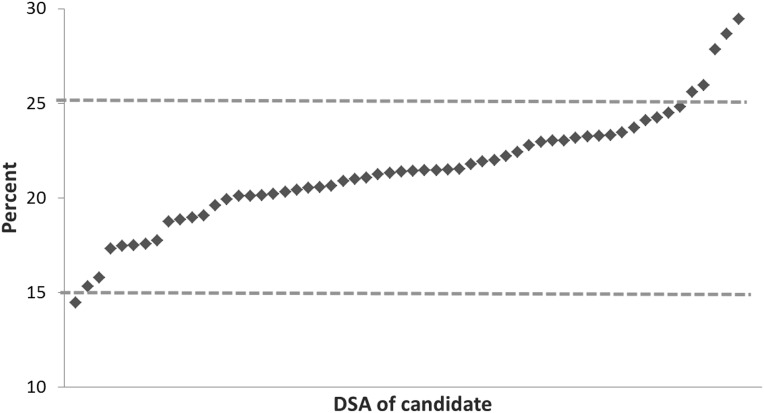
Candidates on the waiting list who are in the national top 20th percentile for EPTS constitute 15%–25% of all candidates on the waiting list for a donation service area (Figure 1). The percentage of kidney donors with KDPI in the top 20th percentile varied between 15% and 25% for most donation service areas (Figure 2). However, the candidate list is much larger than the number of donors available, with 122,669 candidates nationally and enough donors to allow <12,000 deceased donor kidney transplants annually (Table 5). The simulations resulted in 11,531 primary kidney and kidney-pancreas transplants (range, 11,463–11,586 across 10 iterations) under the current allocation policy and 11,599 (range, 11,538–11,681) under the new allocation policy. The 122,669 candidates represent the total number of kidney and kidney-pancreas candidates on the waiting list on the first day or added during the year, in simulations of current and new policy (Table 5).
Figure 1.
Percentages of candidates in the national top 20th percentile of survival, by DSA of candidate’s listing center. DSA, donation service area.
Figure 2.
Percentages of kidney donors with kidney donor profile index<0.20, by donor’s DSA. DSA, donation service area.
Table 5.
Results of averaged post-transplant and wait-list outcomes of 10 simulations of current and new policies for allocating deceased donor kidneysa
| Outcome | Simulated Current | Simulated New |
|---|---|---|
| Number of candidates on the waiting listb | 122,669 | 122,669 |
| Number of primary transplant recipients (min, max) | 11,531 (11,463–11,586) | 11,599 (11,538–11,681) |
| Median lifespan posttransplant (min, max) | 11.82 (11.75–11.85) | 12.65 (12.61–12.71) |
| Median allograft-years of life (min, max) | 8.82 (8.80–8.84) | 9.07 (9.05–9.08) |
| Median extra life-years for transplant versus waiting list candidates (min, max) | 5.01 (4.99–5.03) | 5.24 (5.22–5.27) |
| Number of deaths on the waiting list by age in years (min, max)c | ||
| <18 | 9 (7–11) | 8 (7–9) |
| 18–34 | 223 (218–226) | 221 (213–230) |
| 35–49 | 927 (921–932) | 926 (921–933) |
| 50–64 | 2353 (2342–2374) | 2367 (2357–2379) |
| ≥65 | 1330 (1318–1337) | 1338 (1326–1349) |
Values are given as the mean and median (minimum and maximum).
The total number of candidates on the waiting list on the first day or added during the year was 122,669 (in both simulations).
Includes candidates added to the waiting list during 2010.
The actual number of deaths on the waiting list in 2010 was 5444 (n=12, age<18 years; n=220, age 18–34 years; n=952, age 35–49 years; n=2762, age 50–64 years; and n=1498, age≥65 years).
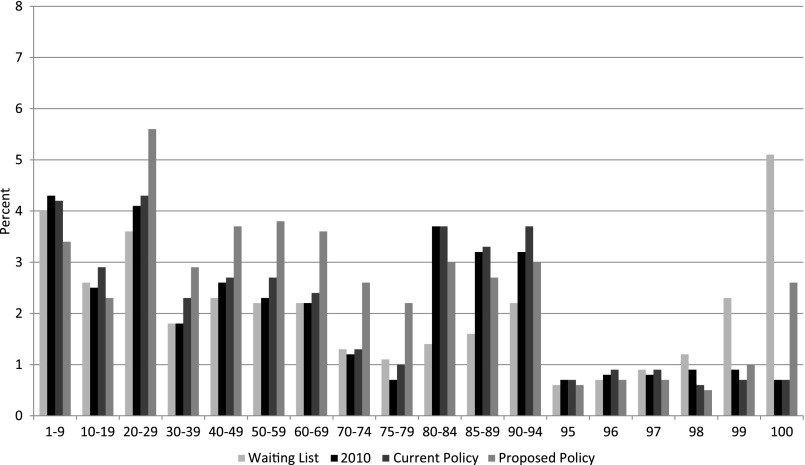
The characteristics of recipients and donors were compared for the following: (1) actual transplants in 2010, (2) 10 simulations using current allocation policy, and (3) 10 simulations using the new allocation policy (Table 6). The characteristics of actual transplant recipients were similar to the characteristics of recipients under simulations of the current allocation policy. The new allocation policy resulted in more transplants for candidates aged 18–49 years and fewer transplants for candidates aged ≥50 years. As expected, the new allocation policy also resulted in more transplants for candidates with blood type B and fewer transplants for candidates with blood type A compared with current allocation policy. Because of the change in prioritization points, the number of transplants in candidates with a CPRA>20% increased with the new allocation policy compared with the current policy (Figure 3). Median life spans and graft-year survival were longer for simulated transplant recipients under the new policy compared with recipients under the current policy (Table 5). The new policy results in transplants with more median extra life-years for recipients versus wait-list candidates compared with current policy, without increasing mortality on the waiting list (Table 5).
Table 6.
Characteristics of actual recipients in 2010 and of recipients in simulations of current policy and the new policy
| Characteristic | 2010 Actual | Simulated Current Policy | Simulated New Policy |
|---|---|---|---|
| Blood type | |||
| A | 34.9 (3551) | 35.2 (3801.4) | 30.1 (3298.7) |
| AB | 5.5 (556) | 5.1 (554.3) | 5.6 (609.5) |
| B | 13.3 (1357) | 12.7 (1368.8) | 17.7 (1945.0) |
| O | 46.1 (4686) | 47.0 (5070.3) | 46.6 (5117.7) |
| Age (yr) | |||
| <18 | 4.5 (455) | 4.9 (528.9) | 4.4 (487.0) |
| 18–34 | 9.6 (975) | 10.4 (1119.9) | 15.4 (1692.3) |
| 35–49 | 25.2 (2565) | 25.2 (2718.6) | 27.5 (3014.7) |
| 50–64 | 41.2 (4185) | 41.1 (4436.8) | 37.0 (4054.3) |
| ≥65 | 19.4 (1970) | 18.4 (1990.6) | 15.7 (1722.6) |
| Race/ethnicity | |||
| African American | 34.1 (3472) | 34.1 (3677.8) | 35.0 (3835.2) |
| Hispanic | 14.7 (1493) | 14.5 (1565.9) | 15.2 (1669.3) |
| White | 43.1 (4378) | 44.3 (4783.8) | 42.9 (4703.5) |
| Other/unknown | 7.9 (807) | 7.1 (767.3) | 7.0 (762.9) |
| Pre-emptive transplants | 8.3 (915) | 10.9 (1257.4) | 11.1 (1292.5) |
| Pre-emptive by race/ethnicity | |||
| African American | 3.6 (131) | 4.9 (186.8) | 5.5 (220.2) |
| Hispanic | 6.1 (96) | 6.9 (114.7) | 7.4 (128.8) |
| White | 12.8 (632) | 16.8 (885) | 16.8 (860.0) |
| Other/unknown | 6.7 (56) | 9.0 (70.9) | 10.7 (83.5) |
| Zero HLA-A, HLA-B, HLA-DR mismatches | 7.3 (742) | 6.4 (693.6) | 5.9 (643.5) |
| Local or shared kidneys | |||
| Shared | 20.4 (2070) | 14.8 (1596.4) | 16.9 (1851.7) |
| Local | 79.6 (8080) | 85.2 (9198.4) | 83.1 (9119.2) |
| Primary cause of disease | |||
| Diabetes, age<50 years | 4.9 (499) | 6.1 (660.6) | 5.8 (640.2) |
| Diabetes, age≥50 years | 20.9 (2127) | 25.7 (2776.2) | 23.4 (2565.3) |
| Hypertension | 27.2 (2769) | 21.1 (2274.9) | 21.7 (2376.1) |
| Glomerular | 23.9 (2435) | 20.8 (2241.4) | 22.7 (2485.7) |
| Polycystic | 8.3 (846) | 7.9 (848.0) | 7.3 (801.6) |
| Renovascular | 0.2 (21) | 0.2 (22.3) | 0.2 (22.1) |
| Other/unknown | 14.3 (1453) | 18.3 (1971.4) | 19.0 (2079.9) |
| Time on dialysis before listing (in yr) | |||
| 0 | 23.9 (2622) | 26.1 (3009.4) | 23.9 (2772.6) |
| >0–1 | 31.7 (3475) | 31.5 (3632.2) | 30.2 (3500.9) |
| >1–2 | 18.9 (2072) | 18.9 (2176.6) | 18.9 (2189.2) |
| >2–3 | 8.8 (962) | 8.7 (1006.0) | 9.3 (1082.2) |
| >3–4 | 5.4 (587) | 5.0 (572.6) | 5.6 (646.3) |
| >4–5 | 3.3 (360) | 3.0 (340.7) | 3.7 (426.4) |
| >5 | 8.1 (891) | 6.9 (793.6) | 8.5 (980.9) |
Data are listed as % (n). Values from simulations are averages.
Figure 3.
Percentages of wait-list candidates, actual recipients in 2010, and recipients in simulations of the current kidney allocation policy, and the new policy by CPRA.
Discussion
The new allocation policy uses KDPI and EPTS to rank order kidneys and candidates, respectively. We found that the number of candidates in the top 20th percentile for survival constitutes 15%–25% of all candidates on the waiting list, depending on the donation service area. Similarly, the percentage of kidney donors with a KDPI<20% varied between 15% and 25% for most donation service areas. Because candidates far outnumber donors, not all candidates in the top 20th percentile for survival will undergo transplant within 1 year. The new allocation policy results in increases in average projected median lifespan after transplantation and increases in time with a functional allograft. The distribution of transplants did not change substantially by candidate race or HLA mismatches. The number of kidneys that were shared beyond the local area increased only modestly due to broader sharing for candidates with a CPRA≥99% and regional sharing of kidneys from donors with a KDPI>85%. The new allocation policy results in more transplants for highly sensitized candidates, particularly those with a CPRA≥99%, and increases the number of transplants for candidates with blood type B.
The new allocation policy is projected to result in improved patient and allograft survival (Table 5). The simulations predict an average 7.0% increase in median patient life-years per transplant and an average 2.8% increase in median allograft years of life under the new allocation policy compared with the current policy (Table 5). Assuming 11,000 transplants, this could lead to a gain of 9130 life-years of patient survival and 2750 years of allograft survival. This increase is likely due to a greater number of younger candidates (aged 18–49 years) undergoing transplant than older candidates (aged≥50 years), and to better matching of patient and graft expected longevity. Adolescent and young adult age is a risk factor for poor adherence to immunosuppressive medications,9 and this increases the theoretical risk of overall reduced allograft years of life under the new policy. However, the simulations also predict improved graft survival.
Despite transplants in some higher-risk candidates, the simulations show that the net benefit of the new policy is to improve patient and allograft survival (Table 5). Giving priority to high-CPRA candidates could result in worse outcomes, because high CPRA is a risk factor for rejections and poor outcomes. However, finding immunologically compatible donors will remain challenging for candidates with a CPRA>80% (Figure 3).
The new allocation policy maintains many features of the current policy, including the local, regional, and national categories, and organ offers would be made first to all local candidates before regional candidates in the top 20th percentile of survival. Likewise, organ offers would be made to all regional candidates before national candidates in the top 20th percentile of survival. Pediatric candidates in general maintain the same priority over adult candidates as in current policy, but they are prioritized to receive local offers from donors with a KDPI<35% instead of from donors aged<35 years. The candidates with offers of zero HLA-A, HLA-B, and HLA-DR mismatched organs are prioritized over candidates with one or more HLA mismatches at the A, B, and DR loci. This priority for zero HLA mismatched organs could continue to incentivize candidates to list even before eGFR falls to <20 ml/min per 1.73 m2, despite the waiting time being back-dated to dialysis initiation for candidates who are listed after starting dialysis. The new policy does not change current policy allowing candidates to list even before eGFR falls to <20 ml/min per 1.73 m2, and candidates not on dialysis can start accruing waiting time as soon as eGFR falls to <20 ml/min per 1.73 m2. Currently, only three donation service areas, namely One Legacy in California, Iowa Donor Network, and Gift of Life Michigan, have an approved variance to policy allowing calculation of waiting time from the start of dialysis, even if this occurred before listing.
Despite these simulations, the potential effect of the new policy remains to be determined. For example, the simulations project a slight increase in the number of transplants, from 11,531 to 11,599 (Table 5). It is unclear whether this increase will be borne out in reality, or whether it results from the organ acceptance criteria in the kidney-pancreas simulated allocation model (KPSAM) being based on current organ acceptance behavior. The new allocation policy would also remove the kidney allocation variances (deviations in organ allocation policy approved by the OPTN) that are currently in place. The KPSAM does not take these variances into account when it simulates the current allocation policy. Once the policy is enacted, the OPTN Kidney Committee will evaluate its intended and unintended consequences.
Our study has several limitations. The KPSAM cannot account for changes in organ acceptance behaviors.10 Therefore, if the new policy results in dramatic changes in organ acceptance behavior, the estimates of number of transplants from the simulations will differ from reality. However, given the limited supply of kidney allografts and the large number of candidates on the waiting list, the overall number of transplants is unlikely to decrease. The KPSAM simulates transplants, discards, and removals and deaths on the waiting list for a 1-year period.10 Therefore, it is not possible to determine whether the new allocation policy could lead to changes in numbers of transplants in subsequent years of policy implementation. The KPSAM was not designed to predict outcomes at the level of a center or a donation service area because it assumes similar organ acceptance behavior across the country. The majority of the data shown in this study were used by the OPTN Kidney Transplantation Committee to submit the policy for public comment. Based on feedback from the public and other OPTN committees and regions, the new allocation policy underwent minor changes that were further incorporated. These include the following: (1) zero HLA mismatched candidates will receive priority in the categories of candidates with a CPRA≥98%; (2) pediatric candidates will be excluded from EPTS, thereby allowing more adults (approximately 700, data not shown) to be in the top 20th percentile of survival; (3) pediatric candidates will be offered kidneys with a KDPI>85% only for zero HLA mismatched kidneys; (4) EPTS scores will be updated daily or whenever EPTS factors change; and (5) each transplant center will be required to establish center-specific criteria in order to receive offers for blood type A2 and A2/B kidneys for candidates with blood type B. These changes were not accounted for by the simulations, but would affect only a small number of candidates and kidneys, thereby not invalidating the findings of this study.
Development of the new kidney allocation policy represents a 9-year effort by the OPTN committee. The simulations predict that the new policy could potentially improve kidney transplant outcomes. After implementation of the new policy, expected at the end of 2014, OPTN and the Scientific Registry of Transplant Recipients (SRTR) will evaluate the policy’s intended and unintended consequences on an ongoing basis.
Concise Methods
Study Population
SRTR data were used. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of OPTN, and has been described elsewhere.11 The Health Resources and Services Administration of the US Department of Health and Human Services provides oversight of the activities of the OPTN and SRTR contractors. All kidney transplant candidates on the kidney, kidney-pancreas, and pancreas waiting lists from January 1, 2010, to December 31, 2010, and any kidney or pancreas donors whose organs were offered for transplant during this period were included. The EPTS was calculated for each candidate at listing or using the candidate’s data on January 1, 2010, whichever was later. EPTS thresholds for determining whether a candidate is in the top 20th percentile of survival were based on the national EPTS distribution from candidates for each blood type on the waiting list between January 1, 2007, and December 31, 2009. The KDPI for each kidney allograft was calculated using the distribution of kidney donor risk index of donors between January 1, 2007, and December 31, 2009, as the reference donors. We assumed that 20% of blood type A or AB white, African American, or Hispanic donors had a blood type A2 or A2B, respectively. We also assumed that 70% of the candidates with blood type B had low anti-A antibody titers and thus could accept kidneys from donors with an A2 or A2B blood type.
Modeling Approach
This study conducted simulations using the KPSAM, which is a program that has been used routinely by the OPTN committees to assess policy proposals.10 The KPSAM simulates the arrival of donated organs and new candidates on the waiting list over a 1-year period, checks compatibility of organs with candidates on the waiting list at the time an organ becomes available, creates ordered lists of compatible candidates (candidates with more points have priority for receiving the organ over candidates with fewer points in each ordered list), simulates candidate acceptance or refusal of organ offers using a logistic regression model based on organ acceptance behavior in 2010, calculates the number of transplants and number of organs discarded, and uses linear approximations to Cox proportional hazard models12 to project outcomes such as median allograft and patient survival for each transplant. Allograft failure was defined as the need for dialysis or retransplant. The KPSAM repeated this process 10 times each for the current and new allocation policies (Tables 1–4), each time randomly permuting the order of donor arrivals and generating new random numbers to determine organ offer acceptance. Because the same donors and candidates are used in each of the simulations, and they are the actual donors and candidates from calendar year 2010 and not independent samples, statistical tests of comparisons are not possible. Instead, the average and the minimum to maximum range of results for the 10 iterations are described for the current and the new allocation policies. Of note, this range reflects variability of the simulation modeling, not variability in actual organ allocations.
Disclosures
None.
Acknowledgments
The authors thank SRTR colleagues Delaney Berrini for manuscript preparation, and Nan Booth, MPH, ELS, for manuscript editing.
This work was conducted under the auspices of the Minneapolis Medical Research Foundation, contractor for the SRTR, as a deliverable under US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation Contract HHSH250201000018C. As a US Government–sponsored work, there are no restrictions on its use. The views expressed herein are those of the authors and not necessarily those of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Simulating the New Kidney Allocation Policy in the United States: Modest Gains and Many Unknowns,” on pages 1617–1619.
References
- 1.Smith JM, Biggins SW, Haselby DG, Kim WR, Wedd J, Lamb K, Thompson B, Segev DL, Gustafson S, Kandaswamy R, Stock PG, Matas AJ, Samana CJ, Sleeman EF, Stewart D, Harper A, Edwards E, Snyder JJ, Kasiske BL, Israni AK: Kidney, pancreas and liver allocation and distribution in the United States. Am J Transplant 12: 3191–3212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasiske BL, London W, Ellison MD: Race and socioeconomic factors influencing early placement on the kidney transplant waiting list. J Am Soc Nephrol 9: 2142–2147, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT: Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol 13: 1358–1364, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Alexander GC, Sehgal AR: Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA 280: 1148–1152, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Matas AJ, Smith JM, Skeans MA, Lamb KE, Gustafson SK, Samana CJ, Stewart DE, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2011 annual data report: kidney. Am J Transplant 13(s1): 11–46, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Hurst FP, Sajjad I, Elster EA, Falta EM, Patel P, Abbott KC, Agodoa LY, Jindal RM: Transplantation of A2 kidneys into B and O recipients leads to reduction in waiting time: USRDS experience. Transplantation 89: 1396–1402, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Organ Procurement and Transplantation Network (OPTN): Kidney Donor Profile Index Calculator. Available at: http://optn.transplant.hrsa.gov/resources/allocationcalculators.asp?index=81 2013. Accessed December 19, 2013
- 9.Shaw RJ, Palmer L, Blasey C, Sarwal M: A typology of non-adherence in pediatric renal transplant recipients. Pediatr Transplant 7: 489–493, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Scientific Registry of Transplant Recipients: Kidney-Pancreas Simulation Allocation Model (KPSAM) User Guide, Version 5. Available at: http://www.srtr.org/sam/KPSAM.pdf 2012. Accessed December 19, 2013
- 11.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL: Scientific Registry of Transplant Recipients: Collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 27: 50–56, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RA, McCullough KP, Schaubel DE, Kalbfleisch JD, Murray S, Stegall MD, Leichtman AB: Calculating life years from transplant (LYFT): Methods for kidney and kidney-pancreas candidates. Am J Transplant 8: 997–1011, 2008 [DOI] [PubMed] [Google Scholar]