Abstract
Polycystin-1 (PC1) mutations result in proliferative renal cyst growth and progression to renal failure in autosomal dominant polycystic kidney disease (ADPKD). The transcription factor STAT3 (signal transducer and activator of transcription 3) was shown to be activated in cyst-lining cells in ADPKD and PKD mouse models and may drive renal cyst growth, but the mechanisms leading to persistent STAT3 activation are unknown. A proteolytic fragment of PC1 corresponding to the cytoplasmic tail, PC1-p30, is overexpressed in ADPKD. Here, we show that PC1-p30 interacts with the nonreceptor tyrosine kinase Src, resulting in Src-dependent activation of STAT3 by tyrosine phosphorylation. The PC1-p30–mediated activation of Src/STAT3 was independent of JAK family kinases and insensitive to the STAT3 inhibitor suppressor of cytokine signaling 3. Signaling by the EGF receptor (EGFR) or cAMP amplified the activation of Src/STAT3 by PC1-p30. Expression of PC1-p30 changed the cellular response to cAMP signaling. In the absence of PC1-p30, cAMP dampened EGFR- or IL-6–dependent activation of STAT3; in the presence of PC1-p30, cAMP amplified Src-dependent activation of STAT3. In the polycystic kidney (PCK) rat model, activation of STAT3 in renal cystic cells depended on vasopressin receptor 2 (V2R) signaling, which increased cAMP levels. Genetic inhibition of vasopressin expression or treatment with a pharmacologic V2R inhibitor strongly suppressed STAT3 activation and reduced renal cyst growth. These results suggest that PC1, via its cleaved cytoplasmic tail, integrates signaling inputs from EGFR and cAMP, resulting in Src-dependent activation of STAT3 and a proliferative response.
Autosomal dominant polycystic kidney disease (ADPKD) is a common, life-threatening genetic disease characterized by the progressive growth of renal cysts that eventually destroy the normal tissue architecture, leading to renal failure.1–3 There is currently no approved treatment to slow disease progression, and most patients eventually require dialysis or kidney transplantation. Renal cysts originate from tubule epithelial cells; cystic expansion is driven by proliferation and fluid secretion and is accompanied by fibrosis and excessive extracellular matrix deposition. Germline mutations in the genes encoding polycystin (PC) 1 or PC2, PKD1 and PKD2, respectively, are the root cause underlying ADPKD, with PKD1 mutations accounting for most cases. The normal functions of PC1 and PC2 are not well understood, but both proteins have been implicated in the regulation of numerous signaling molecules and pathways. In particular, PC1—a large multispanning membrane protein—has been shown to regulate heterotrimeric G proteins, mammalian target of rapamycin, STAT (signal transducer and activator of transcription 3) transcription factors, wnt signaling, and several other pathways. However, the exact molecular mechanisms that are involved, the purpose of their regulation, and the importance to renal cyst growth are still largely unclear.
We have recently shown that PC1 regulates the activity of the transcription factor STAT3 by a unique dual mechanism.4 PC1 can directly activate STAT3 by tyrosine phosphorylation due to its physical interaction with the tyrosine kinase Janus kinase 2 (JAK2).4 In addition, PC1 can also undergo proteolytic cleavage, which releases its cytoplasmic tail from the membrane.5,6 This soluble C-terminal fragment, termed PC1-p30, has lost the ability to directly activate STAT3 by JAK2-mediated phosphorylation but instead undergoes nuclear translocation, interacts with transcriptional coactivators, and can coactivate STAT3-dependent gene expression.4 We and others have shown that STAT3 is very strongly activated by tyrosine-phosphorylation in cyst-lining cells in ADPKD and several mouse models.4,7,8 Moreover, initial attempts to inhibit STAT3 in PKD mouse models have led to reduction of renal cyst growth.7,8 These results suggest that the STAT3 pathway could be exploited as a drug target for ADPKD therapy.9 STAT3 is known to be aberrantly activated in numerous forms of cancer, is a driving force of tumor cell proliferation, and is being pursued as a drug target for cancer therapy.9,10Although PC1 can regulate STAT3 activity, the actual mechanism leading to STAT3 activation in PKD is unknown. Several signaling pathways linked to the pathogenesis of ADPKD are known to signal via STAT3 in other cell types, including epidermal growth factor and EGF receptor (EGFR), TNF-α, Src, and hepatocyte growth factor/Met.9,11 In addition, suppressor of cytokine signaling 3 (SOCS3), an inhibitor of some STAT3 signaling pathways, is overexpressed in hepatocyte nuclear factor (HNF)-1β null mice that develop renal cysts.12 STAT3 is also activated in renal tubule cells during AKI and plays a critical role in the response to injury.13 Following AKI, SOCS3 levels are depressed, corresponding with increased STAT3 activity associated with the tubule repair process.14 Both the loss and overexpression of SOCS3 have been associated with renal disease.15,16 Renal injury can trigger rapid cyst growth in preconditioned mouse models,17 and there are numerous parallels between the response to renal injury and the pathogenesis of PKD, which has led to the hypothesis that PKD represents the aberrant, persistent activation of cellular pathways that are normally intended for tissue regeneration.18
We hypothesized that another potential link between renal cyst growth and STAT3 signaling may be aberrant cAMP signaling. Excessive cAMP levels may drive cyst fluid secretion and proliferation, and they appear to be largely due to aberrant vasopressin receptor 2 (V2R) activation.3 Cystic epithelial cells respond to increased cAMP by increased proliferation, whereas normal renal tubule cells do not.19,20 Inhibition of V2R signaling mitigates cyst growth in PKD animal models,21–24 and a recent phase 3 clinical trial showed beneficial effects of the V2R inhibitor tolvaptan in patients with ADPKD.25 Transformation of cancer cells by constitutively active G-α proteins involves Src-dependent STAT3 activation.26 Part of this mechanism may involve cAMP signaling because the cAMP-dependent kinase protein kinase A (PKA) has been shown to phosphorylate and activate Src.27
To understand the mechanisms underlying aberrant STAT3 activation in PKD, we have investigated the regulation of STAT3 by PC1. We and others have shown previously that PC1 is overexpressed in cyst-lining cells in ADPKD and mouse models and is proteolytically cleaved, which leads to accumulation of the nuclear-targeted cytoplasmic tail.4–6 Here, we show that STAT3 is strongly activated in PKD despite highly upregulated expression of SOCS3 indicating that the STAT3-activating mechanism is insensitive to inhibition by SOCS3. We show that direct STAT3 activation by membrane-anchored PC1/JAK2 is inhibited by SOCS3. Instead, the cleaved C-terminal PC1 tail is capable of interacting with Src, which leads to strong STAT3 activation in a mechanism that is insensitive to SOCS3. This pathway is amplified by both EGFR activation and by increased cAMP. These results suggest a model in which the cleaved PC1 tail integrates inputs from EGFR, Src, and cAMP signaling pathways to activate STAT3 while bypassing inhibition by SOCS3.
Results
Regulation of STAT3 Activity and SOCS3 Expression in Renal Epithelial Cells and ADPKD
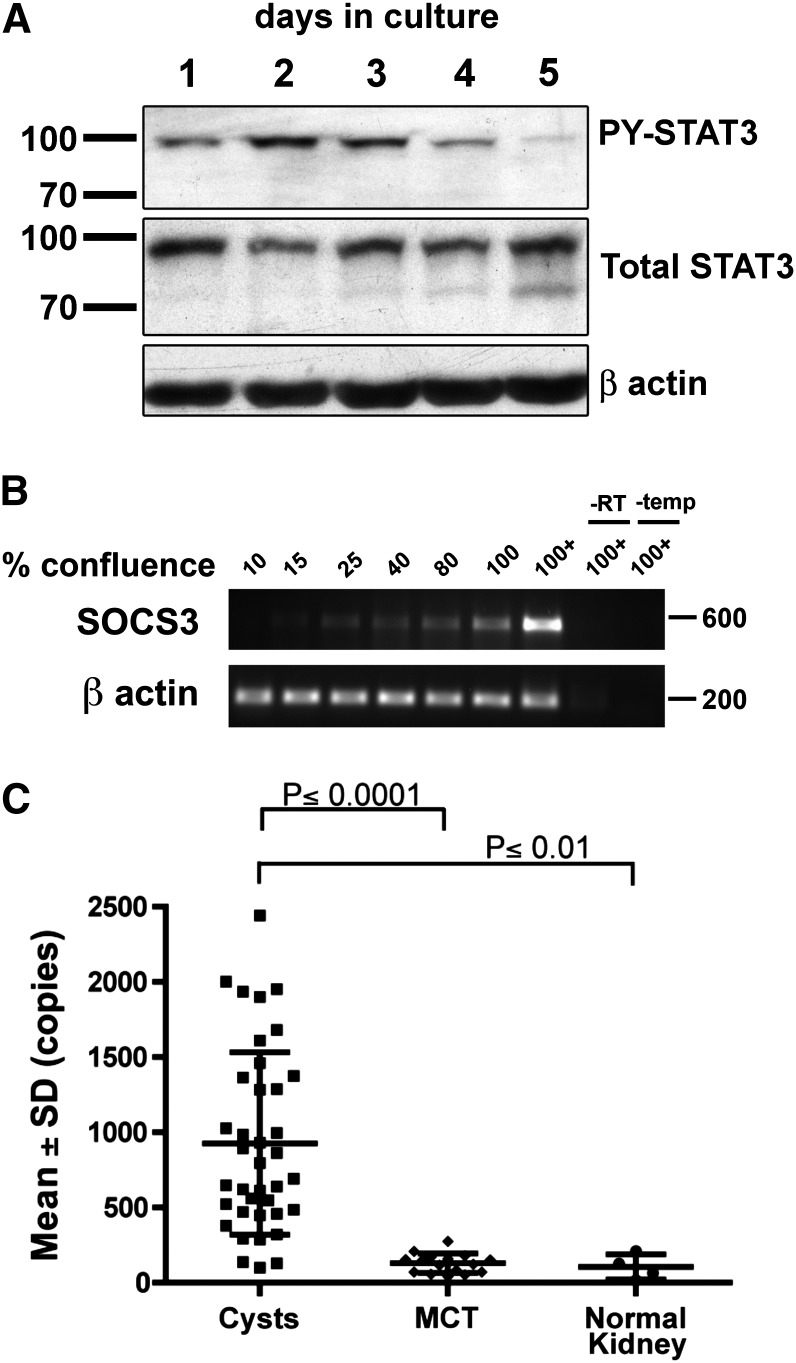
Aberrant STAT3 activation in PKD may be due to abnormal growth factor signaling or intrinsic STAT3 regulation. To test whether renal epithelial cells can regulate STAT3 activity intrinsically in the absence of changes in the growth factor environment, we assessed the level of STAT3 activation as Madin-Darby canine kidney (MDCK) cells transition from rapidly proliferating, subconfluent cultures to contact-inhibited, postconfluent cultures (Supplemental Figure 1). STAT3 activity peaks in rapidly dividing cells and is strongly diminished in postconfluent culture (Figure 1A). Concurrently, SOCS3 expression is strongly upregulated and becomes maximal in postconfluent cultures (Figure 1B). This result suggests that MDCK cells are capable of intrinsically downregulating STAT3 activity—possibly by upregulation of SOCS3—as they differentiate into a well organized, postmitotic monolayer.
Figure 1.
Regulation of STAT3 and SOCS3 in vitro and in ADPKD. (A and B) MDCK cells were cultured under minimal serum conditions (0.5% FBS) for the indicated periods resulting in increasing confluence, full confluence (100%), and further compacting postconfluence (100+). Refer to Supplemental Figure 1 for representative micrographs. (A) Immunoblotting shows increased activation of STAT3 (phosphorylation at Tyr705) in rapidly proliferating cells followed by downregulation in postconfluent cells. (B) This corresponds with an increase in mRNA levels of SOCS3 detected by RT-PCR. (C) Quantitative PCR analysis of microdissected human renal epithelial cells from cystis, minimally cystic tissue (MCT), and normal kidney tissues shows strong upregulation of SOCS3 expression in renal cysts of patients with ADPKD.
Previous gene expression results indicated that SOCS3 expression is increased in cyst-lining cells in ADPKD compared with normal kidneys.28 To confirm and quantify this, we analyzed SOCS3 expression in an expanded sample set of isolated human PKD1 cysts versus control tissues. SOCS3 expression is indeed greatly increased in PKD1 cysts (Figure 1C). These results suggest that the mechanism causing high-level STAT3 activation in PKD may be insensitive to inhibition by SOCS3.
JAK2-Dependent STAT3 Activation by Membrane-Anchored PC1 Is Inhibited by SOCS3
We tested whether the previously described PC1/JAK2-dependent activation of STAT34 is sensitive to SOCS3 inhibition. Overexpression of SOCS3 completely inhibits the activation of STAT3 by full-length PC1 (Figure 2A) or by a chimeric membrane–anchored construct containing the C-terminal cytoplasmic tail of PC1 (full length membrane-bound tail of PC1 [FLM-PC1]; Figure 2B, Supplemental Figure 2). We tested whether any of the four tyrosine residues present in the cytoplasmic tail of PC1 are required for SOCS3-sensitive STAT3 activation. Loss of all four tyrosine residues (FLM-4Y/F-PC1) does not eliminate the ability of PC1 to activate STAT3 (Figure 2B), and SOCS3 still inhibits this activity. Together, these results indicate that PC1/JAK2-dependent activation of STAT3 is sensitive to SOCS3 inhibition and that the inhibitory effect of SOCS3 probably occurs by direct interaction with JAK2 as opposed to interaction with phospho-tyrosines in PC1.
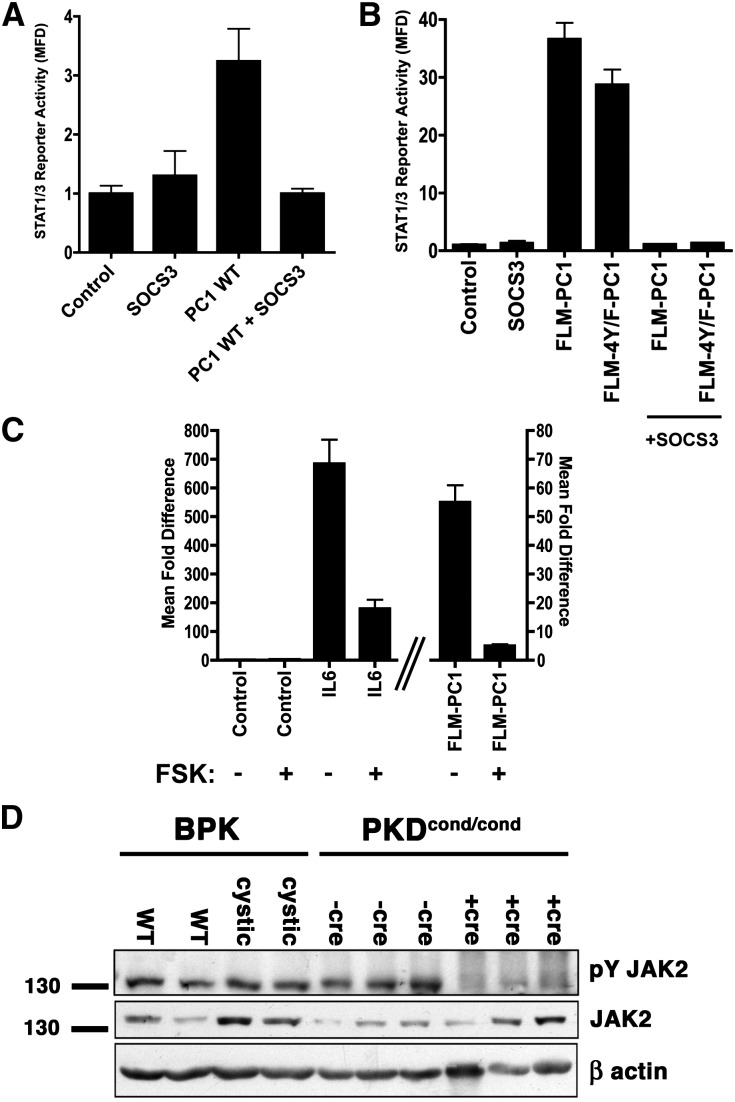
Figure 2.
STAT3 activation mediated by membrane-anchored PC1 is sensitive to SOCS3 inhibition. (A–C) Luciferase reporter assay using human embryonic kidney 293T cells transfected with the STAT1/3 luciferase reporter and control green fluorescent protein or wild-type (WT), full-length PC1 (A), chimeric membrane-anchored constructs containing the wild-type C-terminal cytoplasmic tail of PC1, or mutant in which all four tail tyrosines have been mutated to phenylalanine (B and C) in combination with SOCS3 (A and B) or the cAMP agonist forskolin (5 μM, 16 hours) (C). (D) Immunoblot of kidney lysates from age-matched cystic and control animals of the Bpk mouse model or the Pkd1cond/cond:Nestincre mouse model of PKD. Note that activated phospho-tyrosine JAK2 levels are not increased in cystic kidneys compared with normal control kidneys. Error bars are the mean±SEM.
Intracellular cAMP levels are commonly elevated in cysts in PKD.1 In other cell types, increased cAMP levels have been shown to lead to exchange protein activated by cAMP (EPAC)-dependent stimulation of SOCS3 expression,29,30 which would be expected to lead to inhibition of JAK2-dependent STAT3 activation. We found that treatment with the adenylate cyclase activator forskolin strongly inhibits STAT3 activation that is mediated by IL6 or by the membrane-anchored PC1 tail (Figure 2C), both of which signal via JAK.
Altogether, these results suggest that the high levels of cAMP and SOCS3 in renal cystic cells should be expected to suppress JAK-dependent activation of STAT3. However, because STAT3 is clearly strongly activated in PKD,4,7,8 this suggests that STAT3 activation in PKD is mediated by a JAK-independent pathway. To directly test this, we investigated the levels of activated JAK2 (P-Tyr1007/1008) in two PKD mouse models. As shown in Figure 2D, phospho-JAK2 levels are not increased in polycystic kidneys compared with controls but rather appear to be suppressed. This indicates that STAT3 activation in PKD does not occur via a JAK2-dependent, SOCS3-sensitive pathway but must be caused by a different mechanism.
The Cleaved, Soluble PC1 Tail Interacts with Src, Leading to Strong STAT3 Activation
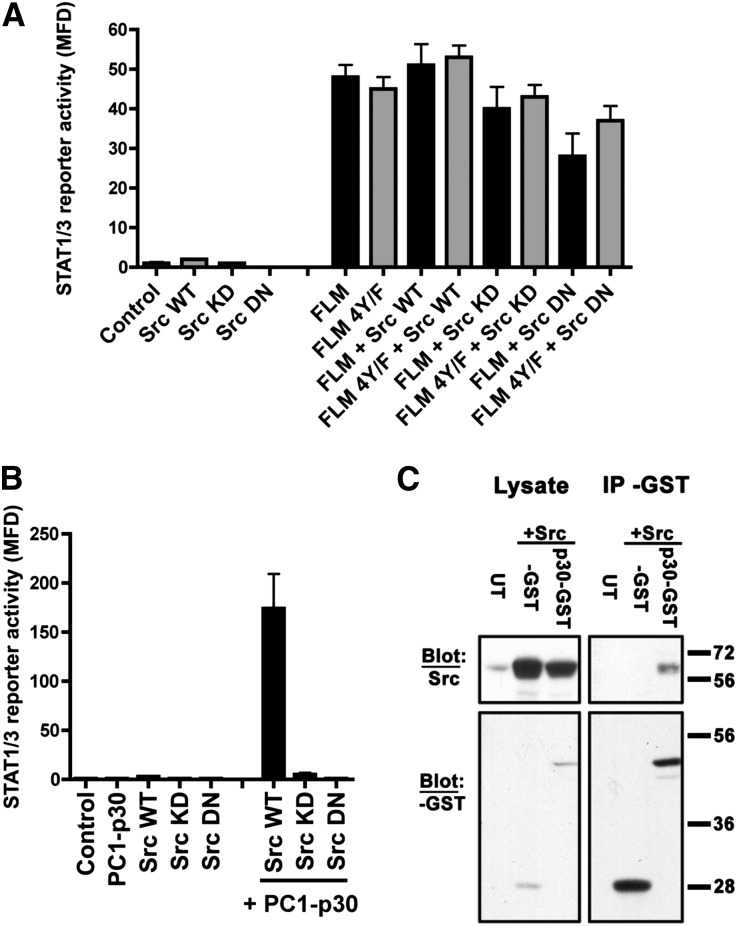
Because activation of STAT3 by membrane-anchored PC1 strictly depends on JAK2,4 it is unlikely to play a role in the observed STAT3 activation in PKD. The nonreceptor tyrosine kinase Src has previously been shown to be activated in PKD rodent models, and its inhibition leads to amelioration of renal cyst growth.31 Src was proposed to increase cyst cell proliferation by stimulating the B-Raf/mitogen-activated kinase kinase/extracellular signal-regulated kinase (ERK) pathway.31 However, Src is also known to be able to activate STAT3 by direct tyrosine-phosphorylation,32,33 and its role in PKD could therefore involve STAT3 activation. To test whether Src is involved in STAT3 activation by membrane-anchored PC1, we coexpressed wild-type Src, kinase-dead Src (K297M),34 or a dominant-negative mutant of Src (K297M Y529F)35 with FLM-PC1. None of these treatments affected the ability of FLM-PC1 to activate STAT3 (Figure 3A), indicating that this mechanism is Src-independent; this finding is consistent with its JAK2-dependency.
Figure 3.
The cleaved PC1 tail, PC1-p30, activates STAT3 in an Src-dependent manner. (A and B) Luciferase assays using human embryonic kidney 293T cells transfected with the STAT1/3 luciferase reporter and indicated genes. (A) The ability of membrane anchored PC1 to activate STAT3 is not altered by the expression of wild-type Src, a kinase-dead Src mutant (KD), or a dominant-negative Src mutant (DN). (B) In contrast, PC1-p30 strongly enhances Src’s ability to induce STAT3 transcription and requires Src kinase activity. (C) Coimmunoprecipitation analysis using GST-tagged PC1-p30 expressed in human embryonic kidney 293T cells. Immunoblotting for Src indicates that PC1-p30 and Src form a stable complex. Error bars are the mean±SEM. GST, glutathione S-transferase; IP, immunoprecipitation; MFD, mean fold difference; UT, untransfected; WT, wild-type.
We next tested whether the regulation of STAT3 activity by the cleaved, soluble PC1 tail (PC1-P30) may involve Src. Strikingly, coexpression of PC1-p30 with wild-type Src leads to extremely strong STAT3 activation compared with Src alone, and this effect depends on the kinase activity of Src (Figure 3B). We recently identified a C-terminal, approximately 15-kDa cleavage product of the PC1 tail (PC1-CTp15) that accumulates in ADPKD kidneys in addition to PC1-p30.4 Neither PC1-CTp15 nor the corresponding N-terminal half of PC1-p30 alone is sufficient to activate Src, indicating that the entire cytoplasmic fragment is required (Supplemental Figure 3).
Coimmunoprecipitation experiments indicate that PC1-p30 is able to physically interact with Src—directly or indirectly (Figure 3C) —which suggests that complex formation is involved in Src-dependent STAT3 activation. We tested a panel of PC1-p30 constructs containing ADPKD patient mutations. None of these mutations disrupts the ability of PC1-p30 to activate Src (Supplemental Figure 4), indicating that any PC1-p30 expressed in renal cysts in these patients would still be expected to stimulate Src and STAT3 activity.
Regulation of Src by the Cleaved PC1 Tail
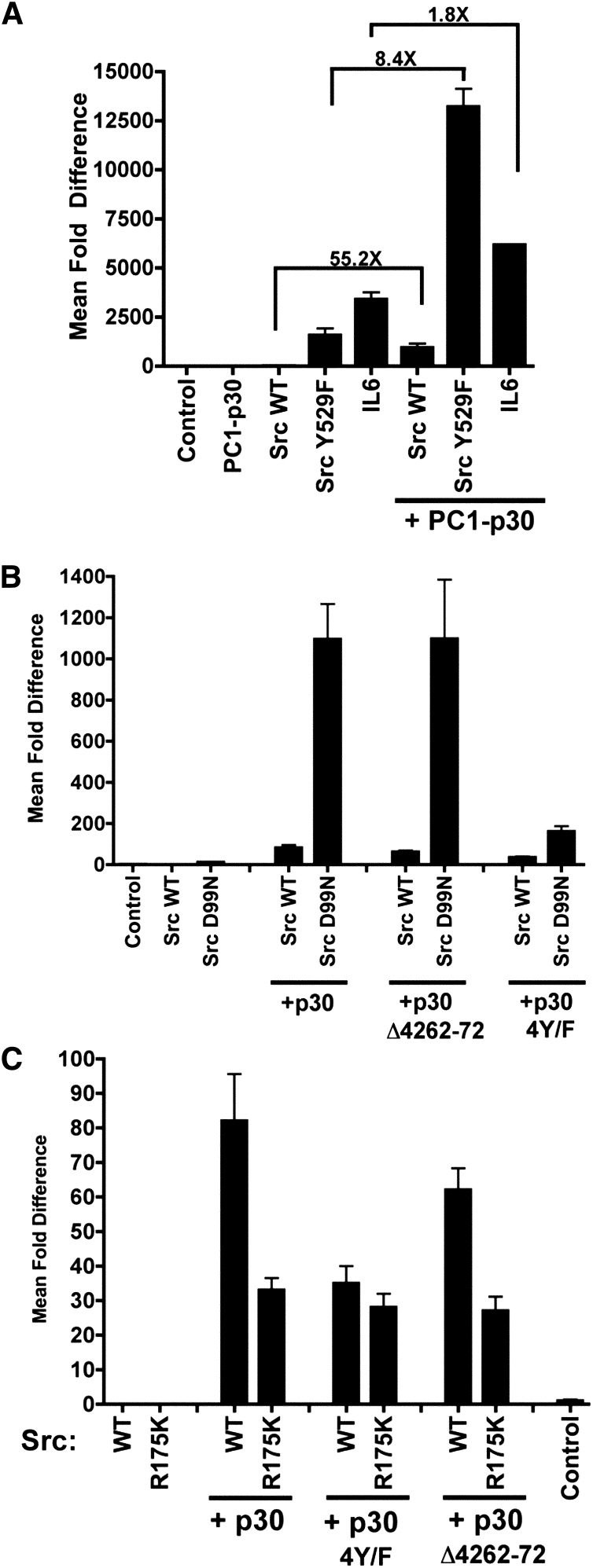
To explore how PC1-p30 may affect the activity of Src, we tested Src mutations that affect Src’s separate autoinhibitory mechanisms36 (Supplemental Figure 2): PC1-p30 is still capable of further enhancing the activity of Src (Y529F) about 8-fold (Figure 4A). Similarly, Src (D99N)37 can still be further activated by PC1-p30 (Figure 4B). These results indicate that the PC1-p30–dependent activation of Src is not exclusively due to relieving intramolecular autoinhibition mechanisms involving Tyr529 or the SH3 domain.
Figure 4.
Characterization of Src activation by PC1-p30. (A–C) Luciferase assays using human embryonic kidney 293T cells transfected with the STAT1/3 luciferase reporter and the indicated genes. (A) PC1-p30 can still further activate the conditionally active Src Y529F mutant, suggesting that regulation of phosphorylation at that residue is the sole mechanism of Src activation by PC1-p30. Lines indicate fold differences between the two conditions being compared. (B) PC1-p30 can also still further activate the conditionally active Src D99N mutant. Removal of a conserved PXXP motif in PC1-p30 (Δ4262–4272) does not alter Src/STAT3 activation. Loss of all four tyrosines in PC1-p30 (4Y/F) in combination with loss of PXXP-binding motif in Src significantly reduces Src/STAT3 activation. (C) Alone or in combination, mutation of the SH2 domain of Src (R175K) and the PC1-p30 phospho-tyrosine mutant (4Y/F) significantly reduce— but does not eliminate—STAT3 activation by Src. This level of activation is not altered by the loss of the PC1-p30 PXXP motif. Error bars are the mean±SEM. WT, wild-type.
To test whether recognition of phosphotyrosine residues by the SH2 domain of Src is involved, we used the Src (R175K)38 mutant (Supplemental Figure 5). The ability of PC1-p30 to activate Src is significantly inhibited by the R175K mutation but not completely eliminated (Figure 4C). To test whether phosphotyrosine residues in PC1-p30 may play a role, we mutated all four Tyr residues to Phe (PC1-p30–4Y/F). This prevented Src activation to a similar extent as mutating Src’s SH2 domain in the Src (R175K) mutant (Figure 4C). When the two mutants were combined, it did not lead to any further suppression of Src activation (Figure 4C). The PC1-p30–4Y/F mutant, was, however, much less capable of activating Src (D99N) (Figure 4B). Together, these results suggest that an interaction between phosphotyrosine residues in PC1-p30 and Src’s SH2 domain is not absolutely required but likely plays a role in positively modulating Src activity.
A conserved proline-rich (PXXP) motif in the cytoplasmic tail of PC1 can interact with the SH3 domain of nephrocystin-1.39 Removal of this PXXP motif (PC1-p30-Δ4262–72), however, did not affect the ability to activate wild-type Src, or the Src (D99N) or Src (R175K) mutants (Figure 4, B and C), suggesting that PC1’s PXXP motif is not critical for Src activation.
Altogether, these results indicate that the activation of Src by PC1-p30 is likely governed by a complex interaction that may depend on an adaptor protein and is subsequently regulated by effects involving the SH2 and SH3 domains of Src.
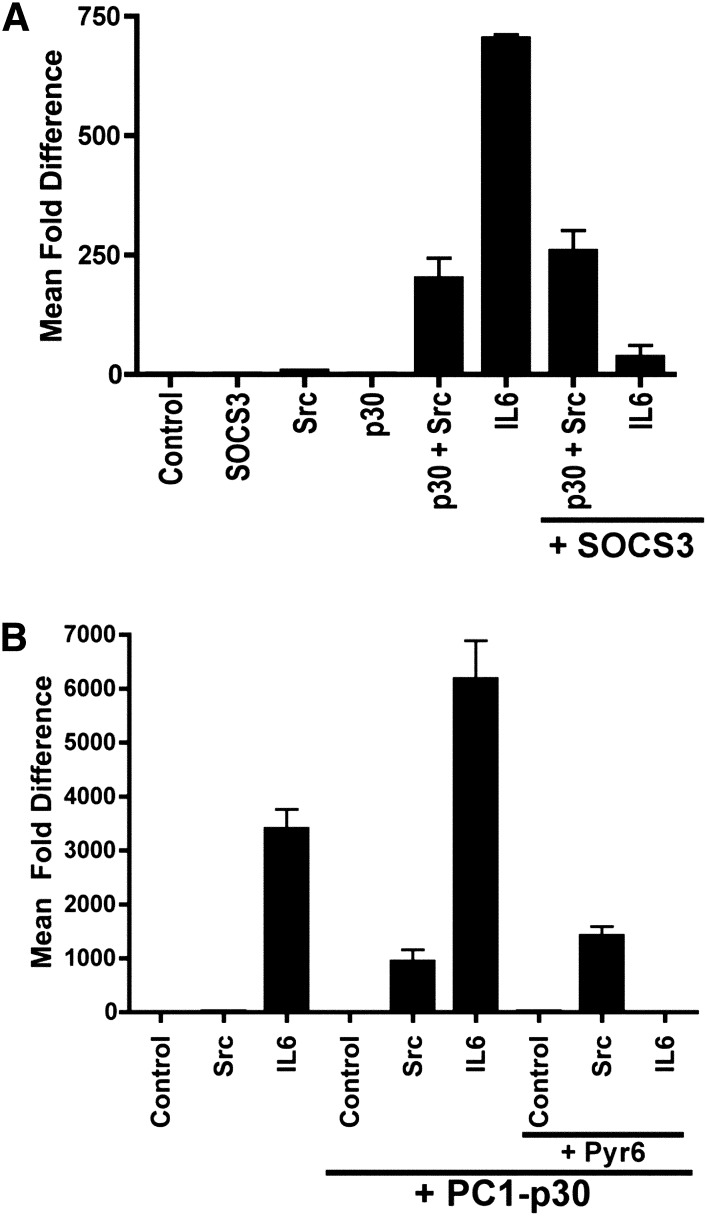
PC1-p30/Src-Dependent Activation of STAT3 Is Insensitive to SOCS3
STAT3 can participate both in a positive and negative feedback mechanism by transcriptionally upregulating its own expression40 and that of its inhibitor SOCS3,41 respectively. To test whether PC1-p30/Src-dependent activation of STAT3 can regulate both pathways, we used luciferase reporters driven by the native promotors of the STAT3 and SOCS3 genes, respectively. PC1-p30 indeed leads to Src-dependent enhancement of transcription from these native promotors (Supplemental Figure 6).
Next, we tested whether SOCS3 is capable of inhibiting the PC1-p30/Src-dependent activation of STAT3. As shown in Figure 5A, although SOCS3 strongly suppresses the IL-6–mediated activation of STAT3, it is not capable of suppressing the PC1-p30/Src-dependent activation of STAT3. Similarly, the pan-JAK inhibitor pyridone 6 completely inhibits the IL-6–dependent coactivation of STAT3 by PC1-p30 but fails to inhibit the Src/PC1-p30–dependent activation of STAT3 (Figure 5B). Altogether, these results indicate that PC1-p30 can act as an intrinsic Src activator that leads to transcriptional activity of STAT3 in a manner that is independent of JAK and cannot be suppressed by SOCS3. This mechanism would be consistent with the observation of concurrent high levels of STAT3 and SOCS3 expression and high levels of STAT3 activation in cysts in PKD.
Figure 5.
The PC1-p30/Src/STAT3 pathway is SOCS3- and JAK-independent. Luciferase reporter assays using human embryonic kidney 293T cells transfected with the STAT1/3 luciferase reporter. (A) Expression of SOCS3 inhibits STAT3 activation by IL-6 but has no effect on PC1-P30/Src-mediated STAT3 activation. (B) The pan-JAK inhibitor pyridone 6 (Pyr6) inhibits STAT3 activation by IL-6 but has no effect on PC1-P30/Src-mediated STAT3 activation. Error bars are the mean±SEM.
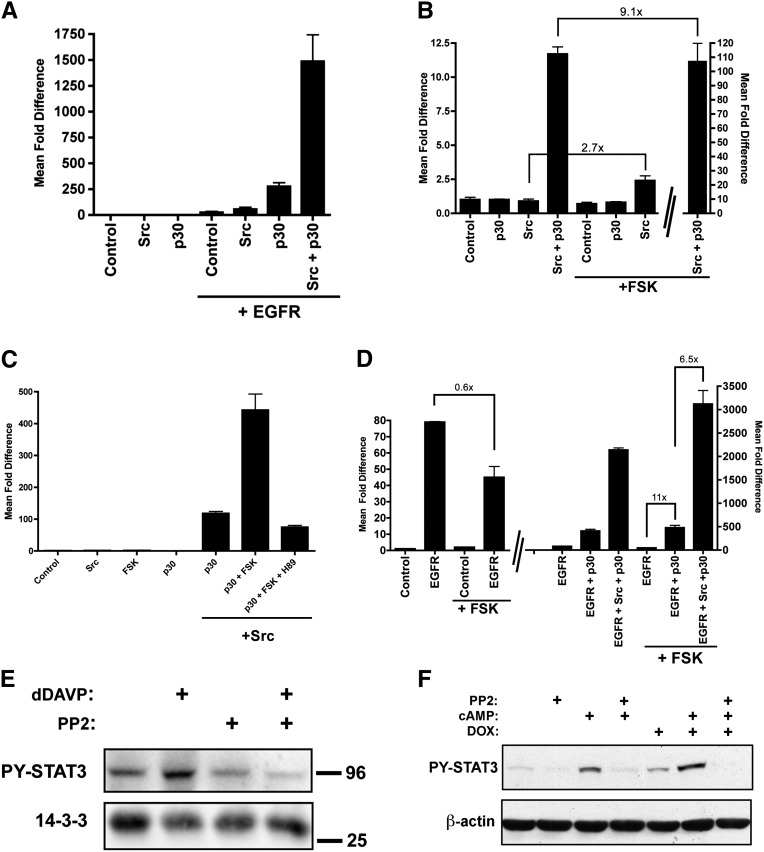
Increased cAMP and EGFR Signaling Both Amplify PC1-p30/Src-Dependent Activation of STAT3
Next, we investigated pathways that may be upstream regulators of Src/STAT3 in renal cysts. Both EGFR and cAMP signaling pathways are aberrantly activated in PKD1,42 and have been reported to affect Src/STAT3 in other cell types. EGFR activation leads to numerous downstream signaling events, including the ability of EGFR to phosphorylate STAT3 via its tyrosine kinase domain.43 EGFR activation can also lead to rapid recruitment of Src and Src-mediated STAT3 activation.44 cAMP may inhibit STAT3 activity by EPAC-dependent upregulation of SOCS3 expression.29 On the other hand, cAMP may increase STAT3 activity by PKA-dependent phosphorylation and activation of Src.27,45,46
As expected, EGFR expression leads to STAT3 transcriptional activity, which is further enhanced by Src expression (Figure 6A). Strikingly, expression of PC1-p30 alone or in combination with Src dramatically amplifies the EGFR-dependent activation of STAT3 (Figure 6A). Forskolin (FSK)—which increases intracellular cAMP levels—alone does not affect STAT3 activity in human embryonic kidney 293 cells in the absence of Src (Figure 6B). However, FSK dramatically increases the PC1-p30/Src-mediated activation of STAT3 (Figure 6B), which is inhibitable by the PKA inhibitor H89 (Figure 6C). These results suggest that the PKA-mediated phosphorylation of Src leads to full Src activation only in the presence of PC1-p30.
Figure 6.
PC1-p30 amplifies EGFR- and cAMP-mediated Src/STAT3 activation. (A–C) STAT1/3 luciferase reporter assays. (A) PC1-p30 significantly enhances EGFR-mediated activation of STAT3, and this is strongly further enhanced by Src. (B) The cAMP agonist FSK activates Src/STAT3 much more strongly in the presence than in the absence of PC1-p30. Note the difference in scale for the last bar. (C) The PKA inhibitor H89 suppresses PC1-p30/Src-mediated STAT3 activation. (D) Elevated cAMP (+FSK) inhibits EGFR-mediated STAT3 activation in the absence of PC1-p30 but increases EGFR-mediated STAT3 activation in the presence of PC1-p30. This effect is further amplified by Src. (E) Vasopressin responsive mpkCCD-cl11 cells were exposed to dDAVP (10−9 M, 5 minutes) with or without pretreatment with the Src-family kinase inhibitor PP2 (20 µM, 12 hours). Lysates were subjected to immunoblotting and probed for PY-STAT3 and pan-14–3-3 protein as a loading control. (F) Confluent MDCK cells stably expressing PC1-p30 under control of a DOX-inducible promoter were treated with DOX as indicated and serum starved for 16 hours. Afterward, cells were pretreated with PP2 for 3 hours, and then treated with a combination of 50 µM 8-Br-cAMP and 10 µM FSK for 1.5 hours. Error bars are the mean±SEM. Lines in B and D indicate the fold differences between the two conditions being compared; DOX, doxycycline.
The combination of EGFR and FSK leads to the strongest PC1-p30/Src-mediated activation of STAT3 (Figure 6D). Interestingly, the EGFR-dependent STAT3 activation is suppressed by FSK in the absence of PC1-p30 but is strongly enhanced in the presence of PC1-p30 (Figure 6D). This suggests that EGFR signaling is normally inhibited by cAMP, likely by EPAC-mediated upregulation of SOCS3,43,47 but that expression of PC1-p30 overcomes this inhibition by amplifying the EGFR/Src/STAT3 axis.
cAMP levels are thought to be elevated in renal cysts due to aberrant vasopressin V2 receptor (V2R) signaling.3 To test whether stimulation of the endogenous V2R in untransfected renal epithelial cells leads to Src-dependent STAT3 activation, we used the mpkCCDcl11 collecting duct cell line, which is responsive to the V2R agonist 1-deamino-8-d-arginine vasopressin (dDAVP).48 Treatment with dDAVP indeed leads to increased tyrosine phosphorylation of STAT3, and this response can be blocked by pretreatment with PP2, a selective inhibitor for Src-family kinases (Figure 6E). Next, we tested whether PC1-p30 expression leads to increased Src-dependent STAT3 activation in response to cAMP in a renal epithelial cell line. MDCK cells, stably expressing PC1-p30 under doxycycline control, were challenged by increasing intracellular cAMP. This leads to significantly increased STAT3 tyrosine phosphorylation, which is further enhanced in the presence of PC1-p30 (Figure 6F). Again, treatment with PP1 prevents this effect, indicating that STAT3 activation is Src-dependent.
Altogether, these results demonstrate that expression of PC1-p30 results in Src-dependent STAT3 activation and that this function is strongly amplified in conditions of elevated cAMP and/or EGFR signaling.
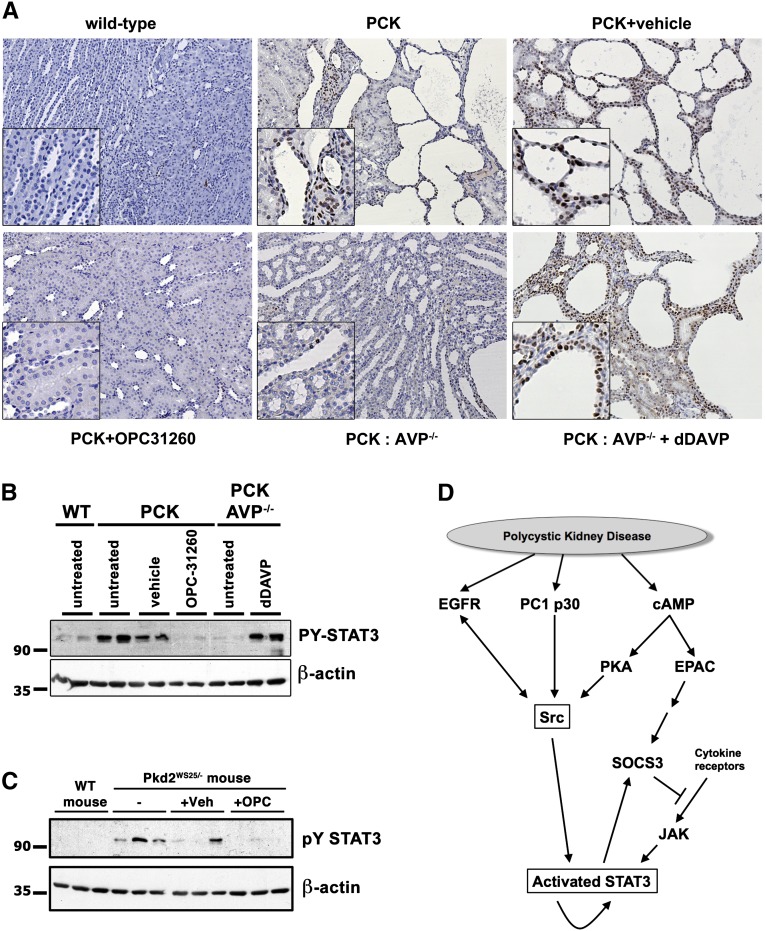
Pharmacologic and Genetic Inhibition of Vasopressin Signaling Prevents Renal STAT3 Activation In Vivo
If increased cAMP levels contribute to increased Src-dependent activation of STAT3 in renal cysts, we would expect that inhibiting cAMP signaling in a PKD rodent model should also result in inhibition of STAT3 activity. To test this prediction in vivo, we used the polycystic kidney (PCK) rat model, which has previously been shown to exhibit increased cAMP levels.21 STAT3 is strongly activated in renal cysts in this model (Figure 7). Treatment with the V2R antagonist OPC-31260 decreases renal cAMP and leads to inhibition of renal cyst growth in the PCK rat model.21,23 As shown in Figure 7, A and B, treatment of PCK rats with OPC-31260 eliminates STAT3 activation. To confirm that the effect of OPC-31260 on STAT3 activation is due to inhibition of the V2R, we used PCK rats that have been crossed with Brattleboro rats that lack arginine vasopressin (AVP), the ligand of the V2R. As shown previously, PCK AVP−/− rats exhibit lower renal cAMP and reduced renal cyst growth.24 As shown in Figure 7, A and B, PCK AVP−/− rats exhibit strongly reduced levels of activated STAT3 compared with PCK rats. Treatment of PCK AVP−/− rats with dDAVP again leads to strong activation of STAT3 in cyst-lining cells and resumption of cyst growth (Figure 7). Similar to the PCK model, mice with an engineered unstable Pkd2 gene, the orthologous gene to human ADPKD, exhibit aberrantly activated STAT3, and treatment with OPC-31260 eliminates STAT3 activation (Figure 7C). Altogether, these results suggest that increased STAT3 activation is mediated, at least in part, by increased levels of cAMP in renal tubule epithelial cells in vivo and is likely an important factor that drives renal cyst growth.
Figure 7.
Pharmacologic or genetic inhibition of vasopressin/V2R signaling in vivo inhibits STAT3 activation and renal cyst growth. (A and B) Immunohistochemistry (A) or immunoblot (B) of wild-type (WT) and PCK rat kidney sections, and kidney lysates, respectively. Animals were treated with vehicle alone, the V2R-inhibitor OPC31260, or dDAVP as indicated. Wild-type rats, PCK rats, or PCK rats crossed with Brattleboro rats (AVP−/−) are compared. (C) Pkd2WS25/- or control mouse were similarly treated with or without OPC31260 or vehicle. (D) Model depicting pathways activated in PKD disease in which PC1-p30 acts as an activator of Src. This results in driving EGFR and cAMP signaling toward Src-dependent STAT3 activation in a manner that is independent of SOCS3.
Discussion
Previous findings by us4 and others7,8 have demonstrated that STAT3 is strongly activated in renal cyst-lining cells in human ADPKD and several PKD mouse models, and suggested that STAT3 is a driving force for cyst growth.9,11 However, the mechanism of STAT3 activation in renal cysts remained unknown. The observation that increased expression of SOCS3, an inhibitor of STAT3 activation, is associated with renal cyst growth in HNF-1β null mice12 and human ADPKD (Figure 1D) appeared to contradict a role of STAT3 in cyst growth. Moreover, our finding that wild-type, membrane-anchored PC1 can activate STAT3 via JAK24 might lead to the conclusion that the loss of functional PC1 in patients with ADPKD should lead to diminished STAT3 activity as opposed to the observed increased STAT3 activity. The results presented here help to resolve these apparent contradictions and suggest a model (Figure 7C) for the integration of multiple signaling pathways that converge on the regulation of STAT3 activity in renal cysts.
We demonstrate that the cleaved, cytoplasmic tail of PC1 (PC1-p30) can interact with Src and that this leads to strong Src-dependent, and SOCS3-insensitive, activation of STAT3. This pathway is amplified by increased cAMP levels and by EGFR signaling, both of which are associated with renal cyst growth. Our results suggest that Src is the major activator of STAT3 in renal cysts which is consistent with, and supported by, the following.
Aberrant activation of Src has been reported in renal cysts in the Bpk mouse and PCK rat models of PKD, and treatment with the Src inhibitor SKI-606 strongly suppressed renal cyst growth in these models.31 Effects on STAT3 were not investigated in that study; however, given that STAT3 is activated in both models (Talbot et al.4 and Figure 7), it is highly likely that Src inhibition would have been found to lead to consequent STAT3 inhibition.
We demonstrate that the PC1-p30/Src-dependent activation of STAT3 is insensitive to inhibition by SOCS3. Therefore, the high expression levels of SOCS3 observed in human ADPKD here (Figure 1) and in renal cysts in HNF-1β null mice12 would not be able to dampen STAT3 activated by this pathway. Interestingly, the high level of SOCS3 in renal cysts may be expected to suppress the response to cytokines that signal via receptors that are affected by SOCS3. Although this prediction remains to be tested, such a mechanism could serve to desensitize cystic epithelial cells to certain stimuli. Indeed, we show that JAK2 is not activated in cystic kidneys (Figure 2D). In the PKD1cond/cond mouse model, JAK2 activity appears to be even suppressed, which is consistent with inhibition by SOCS3. Altogether, these results indicate that JAK-dependent and SOCS3-inhibitable pathways are unlikely to be major contributors to STAT3 activation in renal cysts.
Paradoxically, both reducing/eliminating the expression of PC1 (by gene knockout or hypomorphic alleles) and overexpression of PC1 lead to renal cyst growth in transgenic mice.49,50 ADPKD has traditionally been regarded as resulting from the loss of functional PC1 due to loss of heterozygosity. However, loss of PC1 in inducible Pkd1 knockout mice leads to rapid renal cyst growth only if the gene is ablated during the first 2 weeks of life, while the kidneys are still growing. In contrast, loss of PC1 in mice with mature kidneys has no effect for several months.51 Kidneys/cysts of patients with ADPKD have consistently been found to overexpress PC1.4,5,52,53 Moreover, lack of luminal fluid flow leads to cleavage and nuclear accumulation of the cytoplasmic tail of PC1.6 The cleaved PC1 tail is abundant in cyst-lining cells in human ADPKD4,5 and a PKD mouse model.6 Increased abundance of the cleaved C-terminal tail of PC1 can be detected in the nuclei of renal epithelial cells from two mouse models with reduced PC2 protein levels (i.e., Pkd2+/– and Pkd2WS25/–).6 PC2 appears to regulate the tail cleavage of PC1, but the exact mechanism remains unknown.54 Overexpression of the cleaved PC1 tail in zebrafish embryos leads to massive pronephric cyst growth.5 Altogether, these findings suggest that the cleaved PC1 tail promotes renal cyst growth.
Our findings reported here suggest that the cleaved PC1 tail can promote renal cyst growth by Src-dependent activation of STAT3. Importantly, the presence of PC1-p30 sensitizes cells to cAMP and EGFR signaling. In the absence of PC1-p30, cAMP suppresses STAT3 activation caused by EGFR signaling (Figure 6C), IL-6 signaling (Figure 2C), or signaling by membrane-anchored PC1 (Figure 2C). Because all of these mechanisms are sensitive to suppression by SOCS3, and because cAMP is known to lead to EPAC-mediated upregulation of SOCS3 expression,29,30 we suggest that increased levels of cAMP in normal renal epithelial cells dampen STAT3 signaling in this way. EPAC signaling in response to cAMP agonists has previously been demonstrated in renal epithelial cells.55 In contrast, in the presence of PC1-p30, increased levels of cAMP lead to STAT3 activation mediated by Src (Figure 6B). Similarly, in the presence of PC1-p30, EGFR/Src-mediated STAT3 activation is amplified (Figure 6A). A prediction from these results is that cystic epithelial cells that express PC1-p30 should differ from normal tubule epithelial cells (expressing membrane-anchored PC1 but lacking PC1-p30) in their response to cAMP. Indeed, it has long been recognized that cAMP stimulates proliferation in ADPKD cyst cells but has either no effect or an antiproliferative effect on normal renal tubule epithelial cells.19,20 Similarly, ouabain—a hormone that activates EGFR, Src, and mitogen-activated kinase kinase–ERK pathways—stimulates proliferation in ADPKD cyst cells but not in normal renal tubule cells.56 Overall, our results suggest that accumulation of the cleaved PC1 tail in renal epithelial cells in ADPKD changes their response to cAMP stimuli. Although normal cells would respond by dampening STAT3 activation via EPAC/SOCS3, ADPKD cells respond to cAMP by activating STAT3 via PKA/PC1-p30/Src, resulting in a proliferative response.
Increased vasopressin/V2R signaling has been identified as a major source of cAMP stimulation leading to renal cyst growth,22,24 and treatment with the V2R inhibitor tolvaptan leads to disease amelioration in patients with ADPKD.25 Our results show that STAT3 activation strictly corresponds to V2R signaling in the PCK rat model (Figure 7, A–C). This suggests that V2R/cAMP signaling may be largely responsible for STAT3 activation in renal cysts. In turn, the results suggest that STAT3 activation is a major outcome of V2R/cAMP signaling that is likely to drive cells to a proliferative outcome.
The model emerging from our results places Src and PC1-p30 at the convergence point of cAMP and EGFR signaling (Figure 7D). EGFR overexpression, mislocalization, and activation have been reported in human ADPKD and rodent models.57 Treatment with EGFR tyrosine kinase inhibitors inhibits renal cyst growth in several PKD rodent models,58,59 but not all.60 The EGFR and Src interact with each other, Src can phosphorylate multiple tyrosine residues of the EGFR, and both kinases are thought to be able to mutually activate each other.61 STAT3 activation in response to EGFR signaling requires Src.44,61 In addition to Src/STAT3 signaling, EGFR activation leads to the activation of several signaling pathways, most notably the mitogen-activated protein kinase (MAPK)/ERK pathway. MAPK/ERK activation has been reported in human ADPKD and almost all rodent PKD models. MAPK/ERK activation has also been reported to be responsible for the proliferative effect of cAMP signaling in renal cysts.19 Consequently, MAPK/ERK signaling has been thought to be a major driving force of proliferation and cyst growth in PKD. However, pharmacologic inhibition of MAPK/ERK signaling failed to affect renal cyst growth in a Pkd1 mouse model,62 raising doubts about the sole importance of this pathway in PKD. Our results suggest that activation of the Src/STAT3 axis—possibly in conjunction with MAPK/ERK signaling—is an important driver of renal cyst growth.
Our studies using several mutant forms of Src and PC1-p30 (Figures 3 and 4) suggest that the regulation of Src activity by PC1-p30 is governed by a complex interaction. Tyr4237 in the PC1 tail has been shown to be a substrate of Src in vitro.63 Mutation of all four tyrosines in PC1-p30 suppresses the ability to fully activate Src (Figure 4). This may suggest that Y-phosphorylation of PC1-p30 by Src plays a role in Src activation, perhaps by stabilizing the interaction between these two proteins. Activation of Src by PC1-p30 appears to only partially involve several known mechanisms of Src regulation: the relieving of intramolecular autoinhibition mechanisms involving Tyr529 and the SH3 domain of Src, and the recognition of phosphotyrosine residues by the SH2 domain of Src (Figure 4). Overall, our results are consistent with a model of multiple binding interactions in which both the SH2 and SH3 domains of Src bind the phosphotyrosine residues and PXXP motifs of PC1-p30, respectively, allowing it to adopt a maximally open conformation, thus being fully active. The complexity of the interaction also suggests that it may be facilitated by an adaptor protein. It is possible that the interaction of the PC1 tail with Src may also serve to recruit Src to the vicinity of other targets. In this regard, NPHP1—a protein mutated in the renal cystic disease nephronophthisis—binds via its SH3 domain with the PXXP motif in the PC1 tail.39 Because NPHP1 has been shown to be phosphorylated by Src,64 it is possible that Src acts on the complex of PC1 and NPHP1. In addition, EGFR interacts with the C-terminal part of the cytoplasmic tail of PC2, the same domain that interacts with the PC1 tail.65 Furthermore, PC1-mediated Src activity may be regulated by receptor protein tyrosine phosphatases because receptor-type tyrosine-protein phosphatase-γ has been shown to interact with the PC1 tail and to dephosphorylate the Src phosphorylated residue, Y4237.66
What is the physiologic role of the PC1-p30/Src/STAT3 pathway identified here? Numerous similarities exist in the activation of signaling pathways and biologic responses between PKD kidneys and kidneys that respond to insults such as ischemia reperfusion injury and obstruction. This has led to the hypothesis that the pathogenesis of PKD involves the aberrant activation of innate renal response mechanisms to insults that normally serve the purposes of tissue regeneration and defense against pathogens.18 Indeed, Src is activated in tubule epithelial cells in response to ischemia reperfusion injury and appears to participate in tubular regeneration.67 PC1 expression is increased after renal ischemia reperfusion injury,68 and the cleaved PC1 tail accumulates in tubule epithelial cells in response to ureteral obstruction.6 Therefore, we speculate that the PC1-p30/Src/STAT3 pathway is normally activated in response to renal insults and integrates signaling inputs (such as EGF and cAMP) to provide a proliferative outcome to facilitate tissue regeneration. Constitutive activation of this pathway in ADPKD would lead to aberrant proliferation of renal epithelial cells and cyst growth. Because Src/STAT3 lie at the converging point of numerous signaling pathways implicated in PKD, their inhibition appears to be a promising therapeutic avenue to pursue.
Concise Methods
SOCS3 mRNA Expression Analysis
Semi-quantitative RT-PCR was done using total RNA from MDCK cells. Microarray and quantitative PCR analyses from human tissues were performed as previously described.28 For the complete methods, see the Supplemental Information.
Luciferase Transcriptional Reporter Assays
Luciferase reporter assays were carried out in triplicate in human embryonic kidney 293T cells as previously described.4 All experiments were repeated at least three times, and representative experiments are shown.
Plasmids
cDNAs of membrane-anchored and soluble PC1 constructs in pCDNA4/TO have been described previously,4,5,69 and additional mutated constructs were made by site-directed mutagenesis. The following plasmids were kindly provided by the following: full-length PC1 (G. Germino, National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, MD); STAT1/3 luciferase reporter4 (T. Hamilton, Cleveland Clinic, Cleveland, OH); luciferase reporters containing native STAT3 and SOCS3 promoter regions70 (G. Stark, Cleveland Clinic); EGFR (D. Wheeler, University of Wisconsin, Madison, WI); SOCS3 71 Addgene plasmid 11486 (R. Kahn, Harvard Medical School, Boston, MA); and wild-type c-Src (Addgene plasmid 13663;, J. Brugge, Harvard Medical School). Kinase dead (K297M), dominant negative (K297M Y529F), constitutively active (Y529F), SH2-binding (R175K), and SH3-binding (D99N) mutants of Src were all made on the same pCMV5 backbone using site-directed mutagenesis.
Rodent Models
Animal studies were approved by the Institutional Animal Care and Use Committees and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The bpk and Pkd1cond/cond:NestinCre mouse models have been described previously.4,69,72–74 PCK rats and crossed PCK/Brattleboro rats have been described previously.24 OPC31260 was administered by adding to chow at a concentration of 0.1% between 3 and 10 weeks of age. dDAVP was administered via osmotic minipumps (Alzet, CA) at a dose of 10 ng/hr per 100 g body weight between 3 and 10 weeks of age.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by grants DK62338 (to T.W.), DK44863 and DK090728 (to V.T.) from the National Institutes of Health, and MOP77806 from Canadian Institutes of Health Research (to Y.P.). M.M.R. was supported by an intramural scholarship from the University of Cologne (Koeln-Fortune).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013091026/-/DCSupplemental.
References
- 1.Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher AR, Germino GG, Somlo S: Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC: Polycystic kidney disease in 2011: Connecting the dots toward a polycystic kidney disease therapy. Nat Rev Nephrol 8: 66–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T: Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci U S A 108: 7985–7990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T: Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell 10: 57–69, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ: Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114: 1433–1443, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J: Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJM: Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Weimbs T, Olsan EE, Talbot JJ: Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAK-STAT 2: e23650, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debnath B, Xu S, Neamati N: Small molecule inhibitors of signal transducer and activator of transcription 3 (Stat3) protein. J Med Chem 55: 6645–6668, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Weimbs T, Talbot JJ: STAT3 signaling in polycystic kidney disease. Drug Discov Today Dis Mech 2013,. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, Gong Y, Patel V, Karner CM, Fischer E, Hiesberger T, Carroll TJ, Pontoglio M, Igarashi P: Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci U S A 104: 20386–20391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH: IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogata K, Shimamura Y, Hamada K, Hisa M, Bun M, Okada N, Inoue K, Taniguchi Y, Ishihara M, Kagawa T, Horino T, Fujimoto S, Terada Y: Upregulation of HNF-1β during experimental acute kidney injury plays a crucial role in renal tubule regeneration. Am J Physiol Renal Physiol 303: F689–F699, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Mancusi S, La Manna A, Bellini G, Scianguetta S, Roberti D, Casale M, Rossi F, Della Ragione F, Perrotta S: HNF-1β mutation affects PKD2 and SOCS3 expression causing renal cysts and diabetes in MODY5 kindred. J Nephrol 26: 207–212, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Ortiz-Muñoz G, Lopez-Parra V, Lopez-Franco O, Fernandez-Vizarra P, Mallavia B, Flores C, Sanz A, Blanco J, Mezzano S, Ortiz A, Egido J, Gomez-Guerrero C: Suppressors of cytokine signaling abrogate diabetic nephropathy. J Am Soc Nephrol 21: 763–772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weimbs T: Third-hit signaling in renal cyst formation. J Am Soc Nephrol 22: 793–795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weimbs T: Polycystic kidney disease and renal injury repair: Common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293: F1423–F1432, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ: cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hanaoka K, Guggino WB: cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11: 1179–1187, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Gattone VH, 2nd, Wang X, Harris PC, Torres VE: Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH, 2nd: Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10: 363–364, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Gattone V, 2nd, Harris PC, Torres VE: Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16: 846–851, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE: Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19: 102–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 26.Ram PT, Horvath CM, Iyengar R: Stat3-mediated transformation of NIH-3T3 cells by the constitutively active Q205L Galphao protein. Science 287: 142–144, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Schmitt JM, Stork PJ: PKA phosphorylation of Src mediates cAMP’s inhibition of cell growth via Rap1. Mol Cell 9: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y: Systems biology of autosomal dominant polycystic kidney disease (ADPKD): Computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18: 2328–2343, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM: Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol 26: 6333–6346, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarwood SJ, Borland G, Sands WA, Palmer TM: Identification of CCAAT/enhancer-binding proteins as exchange protein activated by cAMP-activated transcription factors that mediate the induction of the SOCS-3 gene. J Biol Chem 283: 6843–6853, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED: Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol 19: 1331–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrmann A, Vogt M, Mönnigmann M, Clahsen T, Sommer U, Haan S, Poli V, Heinrich PC, Müller-Newen G: Nucleocytoplasmic shuttling of persistently activated STAT3. J Cell Sci 120: 3249–3261, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Cao X, Tay A, Guy GR, Tan YH: Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol 16: 1595–1603, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT: Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature 385: 650–653, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Bagrodia S, Chackalaparampil I, Kmiecik TE, Shalloway D: Altered tyrosine 527 phosphorylation and mitotic activation of p60c-src. Nature 349: 172–175, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Ingley E: Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta 1784: 56–65, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Weng Z, Rickles RJ, Feng S, Richard S, Shaw AS, Schreiber SL, Brugge JS: Structure-function analysis of SH3 domains: SH3 binding specificity altered by single amino acid substitutions. Mol Cell Biol 15: 5627–5634, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian M, Martin GS: Reduced phosphotyrosine binding by the v-Src SH2 domain is compatible with wild-type transformation. Oncogene 12: 727–734, 1996 [PubMed] [Google Scholar]
- 39.Wodarczyk C, Distefano G, Rowe I, Gaetani M, Bricoli B, Muorah M, Spitaleri A, Mannella V, Ricchiuto P, Pema M, Castelli M, Casanova AE, Mollica L, Banzi M, Boca M, Antignac C, Saunier S, Musco G, Boletta A: Nephrocystin-1 forms a complex with polycystin-1 via a polyproline motif/SH3 domain interaction and regulates the apoptotic response in mammals. PLoS ONE 5: e12719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Stark GR: Roles of unphosphorylated STATs in signaling. Cell Res 18: 443–451, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Badgwell DB, Bevers JJ, 3rd, Schlessinger K, Murray PJ, Levy DE, Watowich SS: IL-6 signaling via the STAT3/SOCS3 pathway: Functional analysis of the conserved STAT3 N-domain. Mol Cell Biochem 288: 179–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweeney WE, Jr, Avner ED: Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD). Cell Tissue Res 326: 671–685, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Xia L, Wang L, Chung AS, Ivanov SS, Ling MY, Dragoi AM, Platt A, Gilmer TM, Fu XY, Chin YE: Identification of both positive and negative domains within the epidermal growth factor receptor COOH-terminal region for signal transducer and activator of transcription (STAT) activation. J Biol Chem 277: 30716–30723, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE: ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem 274: 17209–17218, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Obara Y, Labudda K, Dillon TJ, Stork PJ: PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J Cell Sci 117: 6085–6094, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G: cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Gotoh N: Feedback inhibitors of the epidermal growth factor receptor signaling pathways. Int J Biochem Cell Biol 41: 511–515, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA: Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci U S A 107: 3882–3887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris PC: What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 21: 1073–1076, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, Leonhard WN, van de Wal A, Ward CJ, Verbeek S, Deruiter MC, Breuning MH, de Heer E, Peters DJ: Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet 13: 3069–3077, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG: A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward CJ, Turley H, Ong AC, Comley M, Biddolph S, Chetty R, Ratcliffe PJ, Gattner K, Harris PC: Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc Natl Acad Sci U S A 93: 1524–1528, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanoix J, D’Agati V, Szabolcs M, Trudel M: Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene 13: 1153–1160, 1996 [PubMed] [Google Scholar]
- 54.Bertuccio CA, Caplan MJ: Polycystin-1C terminus cleavage and its relation with polycystin-2, two proteins involved in polycystic kidney disease. Medicina (B Aires) 73: 155–162, 2013 [PubMed] [Google Scholar]
- 55.Wang Y, Klein JD, Blount MA, Martin CF, Kent KJ, Pech V, Wall SM, Sands JM: Epac regulates UT-A1 to increase urea transport in inner medullary collecting ducts. J Am Soc Nephrol 20: 2018–2024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen AN, Wallace DP, Blanco G: Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol 18: 46–57, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Wilson PD: Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta 1812: 1239–1248, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Sweeney WE, Jr, Hamahira K, Sweeney J, Garcia-Gatrell M, Frost P, Avner ED: Combination treatment of PKD utilizing dual inhibition of EGF-receptor activity and ligand bioavailability. Kidney Int 64: 1310–1319, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Torres VE, Sweeney WE, Jr, Wang X, Qian Q, Harris PC, Frost P, Avner ED: EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int 64: 1573–1579, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Torres VE, Sweeney WE, Jr, Wang X, Qian Q, Harris PC, Frost P, Avner ED: Epidermal growth factor receptor tyrosine kinase inhibition is not protective in PCK rats. Kidney Int 66: 1766–1773, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW: Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp Cell Res 284: 31–53, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li HP, Geng L, Burrow CR, Wilson PD: Identification of phosphorylation sites in the PKD1-encoded protein C-terminal domain. Biochem Biophys Res Commun 259: 356–363, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Liebau MC, Höpker K, Müller RU, Schmedding I, Zank S, Schairer B, Fabretti F, Höhne M, Bartram MP, Dafinger C, Hackl M, Burst V, Habbig S, Zentgraf H, Blaukat A, Walz G, Benzing T, Schermer B: Nephrocystin-4 regulates Pyk2-induced tyrosine phosphorylation of nephrocystin-1 to control targeting to monocilia. J Biol Chem 286: 14237–14245, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L: PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boucher CA, Ward HH, Case RL, Thurston KS, Li X, Needham A, Romero E, Hyink D, Qamar S, Roitbak T, Powell S, Ward C, Wilson PD, Wandinger-Ness A, Sandford RN: Receptor protein tyrosine phosphatases are novel components of a polycystin complex. Biochim Biophys Acta 1812: 1225–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R: Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol 163: 277–286, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prasad S, McDaid JP, Tam FW, Haylor JL, Ong AC: Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am J Pathol 175: 1493–1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, Chance MR, Chen X, Du Y, Wang Y, An L, Wang Q, Lu T, Zhang X, Wang Z, Stark GR: Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci U S A 107: 21499–21504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ueki K, Kondo T, Kahn CR: Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 24: 5434–5446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shillingford JM, Piontek KB, Germino GG, Weimbs T: Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T: Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci U S A 108: 18067–18072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shillingford JM, Leamon CP, Vlahov IR, Weimbs T: Folate-conjugated rapamycin slows progression of polycystic kidney disease. J Am Soc Nephrol 23: 1674–1681, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.