Abstract
Mutations in polycystin 1 and polycystin 2 are responsible for autosomal dominant polycystic kidney disease, the most common heritable human disease. Polycystins function as calcium ion channels, but their impact on cell physiology is not fully known. Recent findings suggest that polycystins could function in the maintenance of extracellular matrix integrity. In zebrafish, polycystin 2 knockdown induces kidney cysts, hydrocephalus, left/right asymmetry defects, and strong dorsal axis curvature. Here, we show that increased notochord sheath collagen deposition in polycystin 2–deficient embryos is directly linked to axis defects. Increased collagen II protein accumulation did not associate with increased col2a1 mRNA or a decrease in matrix metalloproteinase activity but, instead, it associated with increased expression of the endoplasmic reticulum/Golgi transport coat protein complex II Sec proteins. sec24D knockdown prevented dorsal axis curvature and kidney cystogenesis in polycystin 2 morphants. Nontoxic doses of brefeldin A also prevented the dorsal axis curvature formation in polycystin 2 morphants and curly up polycystin 2 mutants. Brefeldin A treatment after the onset of polycystin deficiency phenotypes reversed the curved axis phenotype but not kidney cyst progression. Our results suggest that polycystin 2 deficiency causes increased collagen II synthesis with upregulation of secretory pathway coat protein complex II components. Restoration of normal rates of secretory protein synthesis and secretion may be a new target in the treatment of autosomal dominant polycystic kidney disease.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common heritable human disease, with an incidence of 1 in 800 births, and it is caused by mutations in polycystic kidney disease 1 (PKD1) and PKD2 encoding polycystin 1 and polycystin 2.1 ADPKD is characterized by the presence of epithelial cysts in both kidneys as well as cysts in the liver and pancreas. In addition to cystic pathology, ADPKD is also linked to abdominal hernia, and it is one of the best defined heritable disorders associated with intracranial aneurysm2 (conditions associated with both altered extracellular matrix (ECM) composition and integrity). Other manifestations of ADPKD include gastrointestinal cysts and cardiac valve defects.3 Aortic aneurysm and vascular fragility are also observed in mouse models of ADPKD.4,5 These features of ADPKD suggest that altered tissue structure or ECM integrity could play an important role in this disease; however, a mechanism linking ECM abnormalities and the cellular functions of polycystins has been elusive.
Polycystin 1 and polycystin 2 are multipass transmembrane proteins that function together as a receptor–ion channel complex,1 leading to the widely held view that primary cellular defects in polycystin-deficient cells are caused by a disruption of normal Ca2+ signaling. The polycystin 1/2 complex in apical cilia participates in mechanosensory Ca2+ signaling in response to fluid flow,6 suggesting that loss of either polycystins or cilia structure would lead to acute loss of flow sensation and cyst formation. However, the relatively slow and sporadic development of kidney cysts in polycystin 1 and cilia protein conditional knockout models has suggested that other factors may contribute to cyst formation. Polycystin 1 and polycystin 2 are also localized to the endoplasmic reticulum (ER) membrane, where they function to regulate ER calcium stores, calcium release, and intracellular calcium homeostasis.7–9 The ER and Golgi apparatus are also the main vesicular organelles associated with secretory protein synthesis and vesicle trafficking; altered ER calcium handling in ADPKD and the involvement of multiple calcium binding proteins in vesicular trafficking suggest that ADPKD could affect secretion. Indeed, early studies of ADPKD patient cells revealed defects in ECM proteoglycan synthesis in ADPKD cells,10–13 whereas more recently, Golgi function and basolateral exocytosis have been shown to be altered in PKD1 mutant epithelial cells,14 with altered intracellular vesicle trafficking linked to mislocalization of sec6, sec8, and E-cadherin proteins. Although the data are suggestive of a link between altered secretory protein production and disease pathology, it remains to be shown that modulating the secretory pathway can impact tissue morphogenesis defects associated with ADPKD.
We and others have modeled ADPKD in the zebrafish by knockdown or mutation of orthologous pkd genes.15–17 Polycystin loss of function results in left–right asymmetry, kidney cysts, hydrocephalus, and a strong dorsal axis curvature. Dorsal axis curvature is a fully penetrant phenotype associated with overaccumulation of collagen type II protein in the notochord sheath.18 Here, we show that accumulation of collagen type II in the notochord sheath is a consequence of an increased protein production and secretion linked to upregulation of the cytoplasmic coat protein II (COPII) components sec23B, sec24C, and sec24D. Inhibition of the secretory pathway by knockdown of sec24D or chemical inhibition with brefeldin A prevents the dorsal axis curvature and cyst formation observed in polycystin 2–deficient embryos. Our data suggest a causative role for increased ECM synthesis and secretion in the initiation of pkd2 deficiency phenotypes and raise the possibility that vesicle trafficking may be a target to ameliorate disease pathogenesis in human ADPKD patients.
Results
Altered Collagen Type II Accumulation Mediates Axis Defects in pkd2-Deficient Embryos
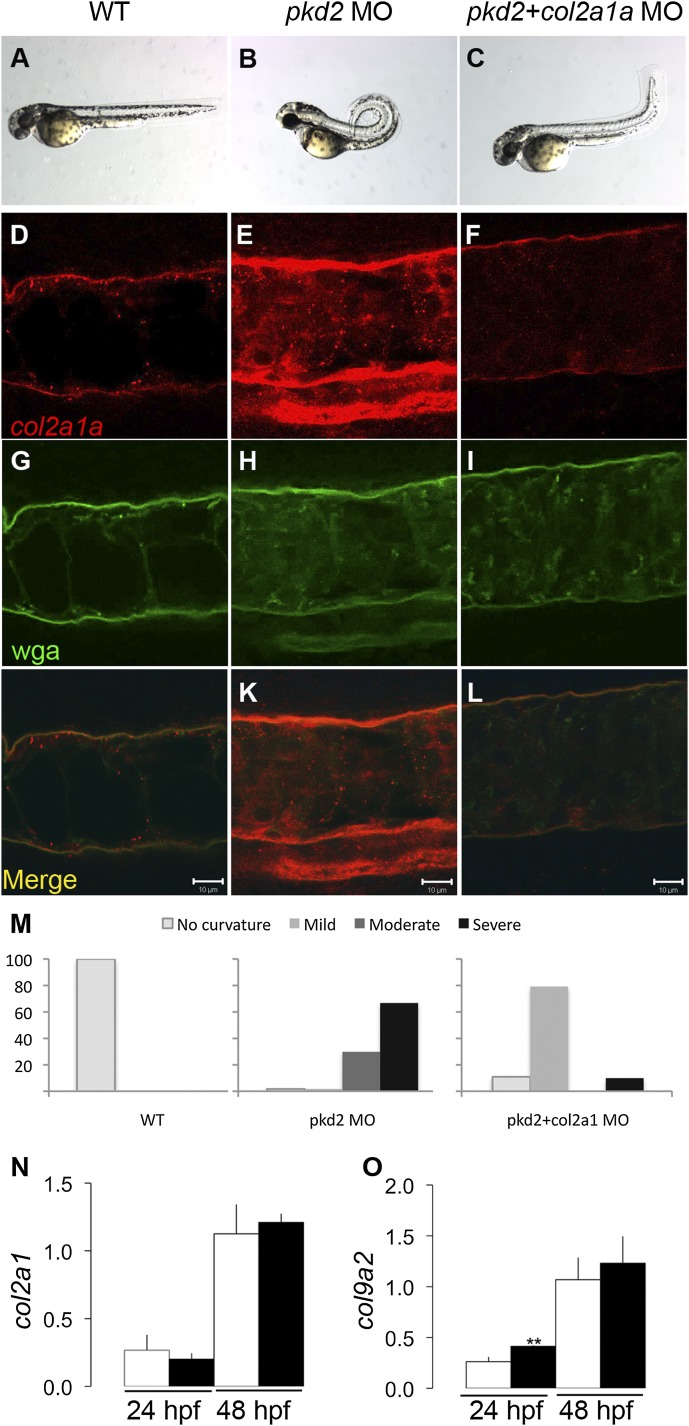
Polycystin 1a/b and polycystin 2 deficiency in zebrafish results in kidney cysts, hydrocephalus, and strong dorsal axis curvature (Figure 1, A and B, Supplemental Figure 1).15,16,18 Dorsal axis curvature is the most penetrant and consistent phenotype, affecting almost 100% of zebrafish pkd2 morphants/mutants, and it is associated with overexpression of notochord sheath collagen type II.18 We have previously shown that complete collagen type II protein knockdown rescued the pkd2 axis curvature phenotype, suggesting that collagen type II overproduction may be causally linked to axis curvature or alternatively, that collagen type II may simply be required to stabilize an altered axial structure induced by other factors in pkd2-deficient embryos. To distinguish between these possibilities, we used lower doses of col2a1a morpholino and attempted to restore collagen type II protein to near wild-type levels in pkd2 morphants. Partial col2a1a knockdown almost completely rescued dorsal axis curvature (Figure 1, A–C). Collagen type II immunostaining revealed that partial col2a1a knockdown successfully restored the amount of collagen type II to wild-type levels in the notochord sheath of pkd2 morphants as shown in longitudinal views of the notochord (Figure 1, D–L). Phenotype quantification showed that nearly all (96.5%) of the pkd2 morphants exhibited moderate or severe dorsal axis curvature at 48 hours postfertilization (hpf), whereas embryos coinjected with col2a1a and pkd2 morpholinos showed only mild or no curvature (Figure 1M). Knockdown of col2a1a in the ventrally curved kidney cyst mutant double bubble19 had no effect on mutant curvature (Supplemental Figure 2). Taken together, these results suggest that matrix overproduction is directly linked to the axis curvature phenotype and that this mechanism is specific to pkd2 deficiency.
Figure 1.
Collagen type II overexpression plays a direct role in dorsal axis curvature in pkd2-deficient embryos. (A) Wild-type embryo and (B) pkd2 morphant (pkd2 MO) at 48 hpf; (C) col2a1a knockdown in pkd2 morphants prevents the dorsal axis curvature. (D–L) Confocal images showing collagen type II immunostaining (red) and wga labeling (green) in the notochord of a (D and G) wild-type embryo, (E and H) pkd2 morphant, and (F and I) col2a1a/pkd2 double morphants (pkd2+col2a1a MO) at 30 hpf. (J–L) Merged images of collagen type II and wga; (D–F) col2a1a partial knockdown in pkd2 morphants reduces the collagen type II level close to wild-type level. Stacks were acquired through the whole notochord, and images from the middle of the notochord were selected. (M) Quantification of the percentage of embryos showing no curvature, mild curvature, moderate curvature, and severe curvature in the different experimental groups from a single representative experiment (of four replicates). Wild-type embryos (n=89); pkd2 morphants (n=57); pkd2+col2a1a morphants (n=101). (N and O) Quantitative RT-PCR measurements of col2a1a and col9a2 mRNA abundance showing no difference in wild-type embryos (white bars) and pkd2 morphants (black bars) at 24 and 48 hpf. Expression is shown relative to the EIF1a mRNA level (n=4 per group). Differences in col2a1a mRNA levels were not statistically significant. A small increase in col9a2 mRNA was significant at 24 hpf (**P=0.003) but not 48 hpf. WT, wild-type. Scale bars, 10 µm.
Altered Collagen Type II Accumulation Occurs Post-Transcriptionally
To test whether collagen overaccumulation in the notochord sheath of pkd2 morphants was caused by increased collagen mRNA expression or other mechanisms, we assessed col2a1a and col9a2 expression by quantitative RT-PCR. Surprisingly, expression of col2a1a (Figure 1N) and col9a2 mRNA (Figure 1O) was similar in pkd2 morphants compared with wild-type embryos at 24 and 48 hpf, suggesting a post-transcriptional mechanism for overaccumulation of collagen II protein. Consistent with this idea, low, relatively nontoxic doses of the protein synthesis inhibitor cycloheximide (20 µg/ml) were sufficient to prevent the pkd2 axis curvature phenotype (Supplemental Figure 3). These results suggested that overaccumulation of notochord collagen II protein was most likely linked to enhanced collagen translation, secretion, or reduced degradation.
Collagen Type II Accumulation Is Linked to Increased Expression of COPII Components
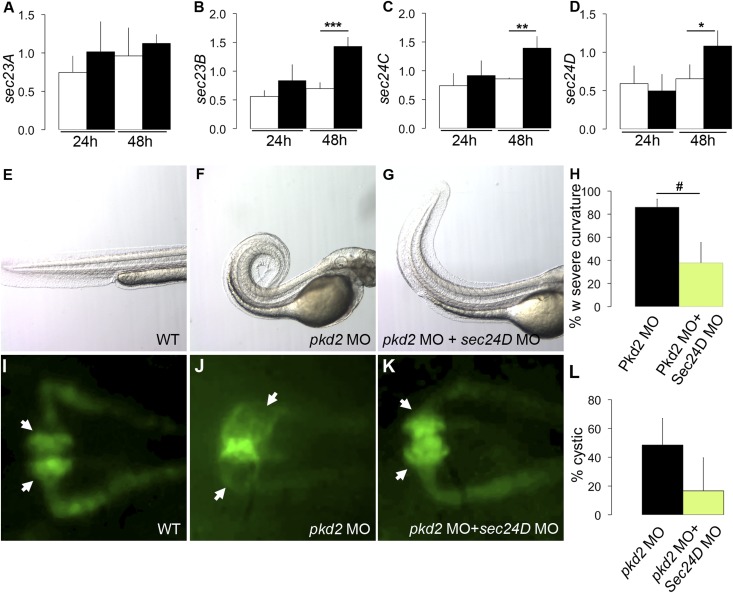
Collagen mRNA translation, fibril assembly, and secretion are subject to multiple processing steps that could affect overall collagen abundance or integrity of the ECM. A critical step of collagen processing is its vesicular trafficking from the ER, where it is synthesized, to the Golgi apparatus for secretion. The COPII complex that consists of multiple Sec proteins mediates ER to Golgi vesicle traffic. Interestingly, quantitative RT-PCR expression analysis of mRNA encoding the COPII components sec23A, sec23B, sec24C, and sec24D indicated that the level of sec23B, sec24C, and sec24D mRNAs (Figure 2, B–D) was significantly increased in pkd2-deficient embryos compared with wild type at 48 hpf, whereas sec23A mRNA expression was not affected (Figure 2A). These data suggest that accumulation of collagen type II could be linked to an increased rate of secretion. Other factors, such as expression of the collagen crosslinking lysyl oxidase genes lox, loxl1, and loxl5b20 (Supplemental Figure 4, A–C) or the crosslinked status of collagen type II protein itself (Supplemental Figure 4, D–F), were not affected. Also, it is unlikely that decreased collagen degradation could account for increased collagen type II accumulation; matrix metalloprotease (MMP) mRNA expression did not decrease in pkd2 morphants (Supplemental Figure 5), and multiple different MMP (GM6001, doxycycline, and ascorbic acid) and proteasome (MG132 and MLN4924) inhibitors did not induce axis curvature in wild-type embryos (data not shown). Increased accumulation of collagen type II protein could, however, be detected in Western blots of pkd2 morphants, confirming our immunostaining results (Supplemental Figure 4, D–F).
Figure 2.
Overexpression of sec genes contributes to dorsal axis curvature and cystogenesis in pkd2-deficient embryos. (A–D) Quantitative RT-PCR measurements of sec genes showing an increased expression of (B) sec23B, (C) sec24C, and (D) sec24D in pkd2 morphants (blacks bars) compared with wild-type embryos (white bars) at 48 hpf (n=4 per group; *P<0.05; **P<0.01; ***P<0.001). (E) Wild-type embryo, (F) pkd2 morphant, and (G) sec24D knockdown in pkd2 morphant at 48 hpf. (H) Quantification of the number of embryos with severe dorsal axis curvature in three independent experiments revealed that sec24D knockdown can prevent the dorsal axis curvature in pkd2 morphants. Wild-type embryos and sec24D morphants never displayed any curvature. Wild type (n=512); sec24D morphants (n=175); pkd2 morphants (n=179); pkd2+sec24D morphants (n=129). #P=0.04. (I–K) Dorsal view of zebrafish pronephros in a (I) control Tg(wt1b:GFP) embryo, (J) Tg(wt1b:GFP) pkd2 morphant, and (K) Tg(wt1b:GFP) sec24D MO in pkd2 morphants at 48 hpf. (L) The quantification of three independent experiments shows that the injection of sec24D morpholino in pkd2 morphants decreases cyst formation compared with pkd2 morphants. Wild type (n=41); sec24D morphants (n=31); pkd2 morphants (n=43); pkd2+sec24D morphants (n=41). MO, morpholino oligo–injected; WT, wild-type.
sec24D Knockdown Prevents the Dorsal Axis Curvature and Cyst Formation in pkd2-Deficient Embryos
To determine if increased Sec gene expression might be linked to the increased collagen production observed in pkd2-deficient embryos, we knocked down sec24D in pkd2 morphants and monitored dorsal axis curvature as a readout of altered ECM. Significantly, the severity of curvature in the pkd2/sec24D double morphants was reduced compared with pkd2 morphants at 48 hpf (Figure 2, E–G). Quantification revealed that 86% of pkd2 morphants had severe curvature compared with 38% of the double morphants, indicating that the secretory pathway plays a crucial role in this phenotype (Figure 2H). To test if increased sec gene expression was also linked to pronephric cyst formation, we monitored kidney cyst formation in the Tg(wt1b:GFP) line after knocking down sec24D in pkd2 morphants. Cyst formation was decreased in double morphants compared with pkd2 knockdown alone (Figure 2, I–K). Cyst quantification revealed that 49% of pkd2 morphants exhibited cysts compared with 17% of the double morphants (Figure 2L). Cyst rescue was not associated with any increase in apoptosis in double morphants (Supplemental Figure 6, A–D).
Brefeldin A Prevents Dorsal Axis Curvature in pkd2 Mutants and Morphants
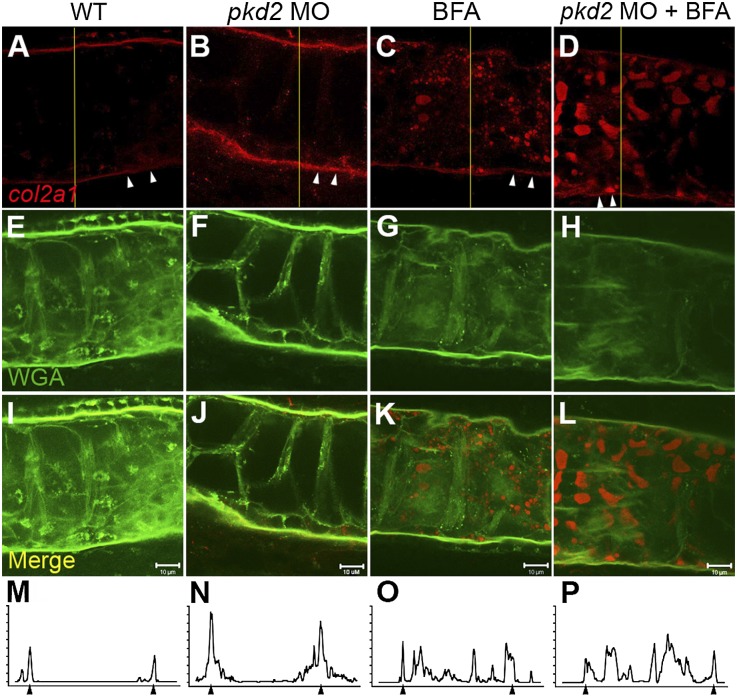
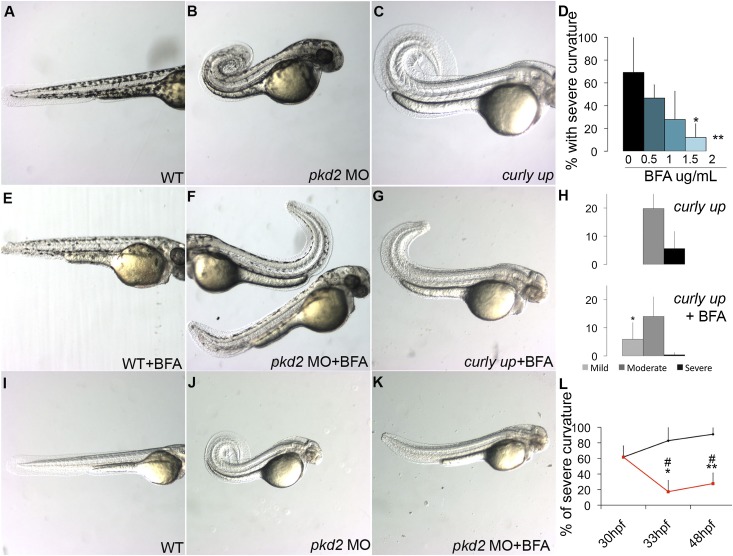
Brefeldin A (BFA) blocks ER to Golgi vesicular traffic by stabilizing the Arf-GDP:ArfGEF intermediate and preventing Arf cycling.21,22 High doses of BFA (10 μg/ml) completely block ER/Golgi traffic in zebrafish embryos, inducing the ER stress response and apoptosis.23 We reasoned that low, nontoxic doses of BFA might reduce the rate of vesicle trafficking without inducing cell stress. We, therefore, treated wild-type zebrafish embryos and pkd2 morphants with low to intermediate doses of BFA (from 0.5 to 2 µg/ml) and monitored collagen accumulation. Collagen type II is primarily localized in the notochord sheath ECM in wild-type embryos and pkd2 morphants at 30 hpf. (Figure 3, A, B, E, F, I, J, M, and N). However, after BFA treatment, collagen accumulated in a vesicular pattern, indicating that low-dose BFA inhibits secretion (Figure 3, C, D, G, H, K, L, O, and P) and can reduce collagen type II accumulation in the ECM of pkd2 morphants to wild-type levels (Figure 3, D, H, L, and P). Notably, collagen II–containing vesicles were always much larger in pkd2 morphants, consistent with an ECM overproduction phenotype (Figure 3D). Wild-type embryos treated with BFA exhibited a shortened body axis, especially at the highest dose tested (2 µg/ml) (Supplemental Figure 7), consistent with previous studies reporting a correlation between disrupted collagen secretion and reduced body length.24,25 Strikingly, early (24 hpf) BFA treatment of pkd2 morphant embryos resulted in a dose-dependent decrease in dorsal axis curvature at 48 hpf (Figure 4, A, B, E, and F), with the highest BFA doses tested nearly completely preventing development of axis curvature (Figure 4D). Another ER/Golgi transport small molecule inhibitor, CI-976, also induced a significant dose-dependent rescue of the pkd2 axis curvature phenotype (Supplemental Figure 8). To confirm the effects of BFA in a genetic model of pkd2 deficiency, we treated embryos from an incross of the zebrafish pkd2 mutant curly up (cup)17 with BFA. Untreated cup embryos from clutches of incrossed heterozygotes exhibited the typical Mendelian ratio (25%) of moderate to severe dorsal axis curvature (Figure 4, C and H, upper panel). BFA treatment eliminated severe axis curvature and induced a significant shift to the mild curvature class (Figure 4, G and H, lower panel). These results confirm that modulation of the secretory pathway can rescue pkd2 mutant phenotypes and further suggest causal links between increased collagen synthesis, increased secretion, and pkd pathology.
Figure 3.
BFA blocks collagen type II secretion in pkd2 morphants. Confocal images showing (A–D) collagen type II immunostaining (red) and (E–H) wga labeling (green) in the notochord of a (A and E) wild-type embryo, (B and F) pkd2 morphant, (C and G) BFA-treated wild-type embryo, and (D and H) BFA-treated pkd2 morphant at 30 hpf. (I–L) Merged images of collagen type II and wga. (A and B) Collagen type II staining was localized in the notochord sheath in wild-type and pkd2 morphants, with a higher expression in pkd2 morphants, whereas (C and D) it accumulates in a vesicular pattern in BFA-treated embryos, showing that BFA inhibits collagen type II sheath accumulation close to wild-type levels in pkd2 morphants. Arrowheads in A–D designate the notochord sheath ECM. (M–P) Line scan analysis of A–D (at the yellow lines in A–D) shows (N) increased collagen II immunoreactivity in pkd2 morphants and (O and P) reduction in notochord sheath collagen II (arrowheads) in BFA-treated embryos. Confocal stacks were acquired of the whole notochord, and images from the middle of the notochord were selected. Scale bars, 10 µm. MO, morpholino oligo–injected; WT, wild-type.
Figure 4.
BFA treatment prevents dorsal axis curvature in pkd2-deficient embryos. (A) Wild-type embryo, (B) pkd2 morphant, and (C) cup embryo at 48 hpf. (E–G) Treatment with BFA (E) does not induce curvature in wild-type embryos but reduces dorsal axis curvature in (F) pkd2 morphants and (G) cup embryos when added at 2 μg/ml at 24 hpf. (D) Percentage of embryos with severe curvature in pkd2 morphants at 48 hpf (n=3 independent experiments). (H) Percentage of embryos with mild curvature (light gray bars), moderate curvature (dark gray bars), and severe curvature (black bars) in cup embryos (upper panel) and BFA-treated cup embryos (lower panel) at 48 hpf (n=3 independent experiments). pkd2 morphants (n=71); pkd2 morphants+BFA (n=57 at 0.5 μg/ml, n=75 at 1 μg/ml, n=73 at 1.5 μg/ml, n=60 at 2 μg/ml); cup mutants (n=298); cup mutants+BFA (n=222). The increase in pkd2 morphant embryos with mild curvature in the BFA sample was statistically significant (*P=0.05). (I) Wild-type embryo, (J) pkd2 morphant, and (K) BFA-treated pkd2 morphant at 48 hpf. (L) Percentage of embryos with moderate and severe curvature at 48 hpf in pkd2 morphants (black line) and pkd2 morphants treated with BFA at 30 hpf (red line). The quantification of two independent experiments at 33 hpf and three independent experiments at 48 hpf shows that BFA treatment can rescue preexisting curvature in pkd2 morphants. Wild type (n=270 at 30 hpf, n=73 at 33 hpf, n=71 at 48 hpf); pkd2 morphants (n=210 at 30 hpf, n=65 at 33 hpf, n=57 at 48 hpf); wild type+BFA (n=63 at 30 hpf, n=57 at 48 hpf); pkd2 morphants+BFA (n=68 at 33 hpf, n=53 at 48 hpf). *Percentage with severe curvature in BFA treated at 33 and 48 hpf was significantly different from untreated pkd2 morphants at 33 hpf; **percentage with severe curvature in BFA treated at 33 and 48 hpf was significantly different from untreated pkd2 morphants at 48 hpf; #percentage with severe curvature in BFA treated at 33 and 48 hpf was significantly different from the starting time point in both cases. MO, morpholino oligo–injected; WT, wild-type.
To test whether BFA treatment could restore normal morphology after axis curvature had started to form, we initiated BFA treatment at 30 hpf, when more than 60% of pkd2 morphants already exhibit dorsal axis curvature. Strikingly, addition of BFA at 30 hpf reversed axis curvature pathology (Figure 4, I–K), such that after 3 hours of BFA treatment, only 17% of pkd2 morphants exhibited curvature compared with 61% before treatment (Figure 4L). The effect persisted at 48 hpf, when only 27% of pkd2 morphants treated with BFA displayed curvature (Figure 4L). The results show that BFA treatment cannot only prevent development of axis curvature but also, restore a straight axis in previously curved embryos.
BFA Reduces Kidney Cyst Formation in pkd2 Morphants
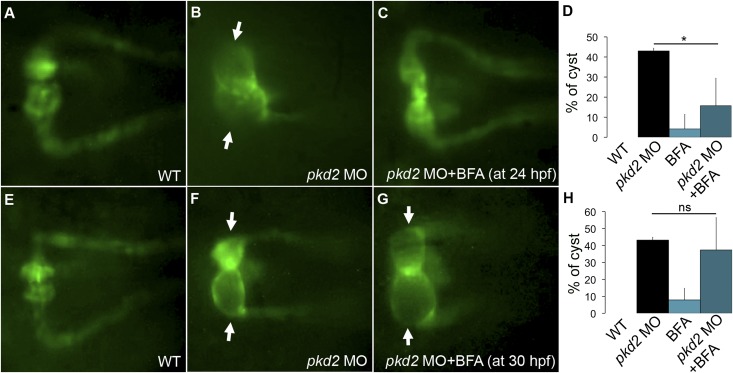
To test whether BFA treatment could also prevent pkd2-associated cystogenesis, we quantified cysts in Tg(wt1b:GFP); pkd2 morphants treated with BFA (2 μg/ml). Similar to the effect of sec24D knockdown, kidney cyst formation was reduced in BFA-treated pkd2 morphants compared with nontreated pkd2 morphants (15.7% versus 43%) (Figure 5, A–D). The dose of BFA used in these experiments was not sufficient to induce general apoptosis26,27 (Supplemental Figure 5), indicating that the reduction in cyst formation was not caused by generally toxicity.
Figure 5.
BFA treatment inhibits cyst initiation in pkd2 morphants. (A) Kidney of a control Tg(wt1b:GFP) embryo, (B) pkd2 morphant with kidney cysts, and (C) kidney of a pkd2 morphant treated with BFA at 24 hpf. (D) Percentage of embryos showing kidney cysts per group. Control Tg(wt1b:GFP) (n=32); Tg(wt1b:GFP) pkd2 morphants (n=28); BFA-treated Tg(wt1b:GFP) embryos (n=22); BFA-treated Tg(wt1b:GFP) pkd2 morphants (n=25). Data represent three independent experiments; pkd2 MO+BFA showed significantly fewer cysts than pkd2 MO alone (*P<0.05). (E) Kidney of a control Tg(wt1b:GFP), (F, arrows) kidney cyst in pkd2 morphant, and (G, arrows) kidney cyst in pkd2 morphant treated with BFA at 30 hpf. (H) Percentage of embryos with kidney cysts at 48 hpf. Control Tg(wt1b:GFP) embryos (n=43); Tg(wt1b:GFP) pkd2 morphants (n=39); BFA-treated Tg(wt1b:GFP) embryos (n=26); BFA-treated Tg(wt1b:GFP) pkd2 morphants (n=44). Data represents three independent experiments. MO, morpholino oligo–injected; WT, wild-type.
The reduction in cyst frequency by early treatment with BFA suggested that modulating the secretory pathway may block critical cyst initiating events or alternatively, that BFA may alter the progression of cyst development. We, therefore, tested the efficacy of BFA in cyst inhibition when it was added at a later stage in development. BFA treatment at 30 hpf did not significantly prevent cystogenesis compared with nontreated pkd2 morphants (Figure 5, E–G). In this experiment, we found that 37% of BFA-treated pkd2 morphants were cystic compared with 43% of nontreated pkd2 morphants (Figure 5H). This result suggests that BFA is most likely acting at the time of cyst initiation and not, for instance, blocking transport of proteins that are required to maintain cystic distension.
Discussion
Despite advances in our understanding of the functions of polycystins, mechanisms underlying ADPKD pathogenesis remain unclear. The polycystin complex is proposed to function in multiple cellular contexts, including the primary cilium as a mechanosensor,6 the ER as a calcium release channel,7,8,28 and the cell membrane as an adhesion complex.29–32 Different features of ADPKD pathology, including failed flow sensing, increased fluid secretion, and cell proliferation, may well involve polycystin function in different subcellular locations. In addition to cystic epithelial pathology in the kidney, pancreas, and liver, ADPKD has been linked to changes in ECM composition in multiple organs.4,33–35 Increased cell adhesiveness, decreased cell migration, and abnormal expression of integrins have also been observed in ADPKD cells.29,36 However, it remains unclear whether these pathologies are primary in disease initiation or a secondary consequence of tissue pathology. Our previous work suggested that the most penetrant zebrafish PKD phenotype, dorsal axis curvature, was caused by notochord deformity induced by abnormal expression of ECM collagen type II.18 Using axis curvature as a readout of pkd2 deficiency, we show here that polycystin2 knockdown in zebrafish causes overproduction of collagen type II protein in the notochord sheath. Moreover, re-establishing wild-type levels of collagen type II protein in the notochord ECM restores a normal axis morphology, supporting the idea that collagen overproduction plays a causal role in zebrafish PKD axis curvature. Collagen overproduction was also associated with increased expression of secretory pathway COPII components. Sec protein knockdown or chemical inhibition of ER to Golgi transport was also sufficient to reverse PKD axis curvature and kidney cyst formation, highlighting the secretory pathway as a potentially novel target for treating PKD.
Increased collagen type II protein production in pkd2-deficient embryos could be caused by several mechanisms, including transcriptional or translational regulation of collagen expression. Whole-embryo quantitative RT-PCR mRNA quantification showed no difference in overall col2a1a or col9a2 mRNA expression between pkd2-deficient and wild-type embryos, suggesting a post-transcriptional mechanism. Recently, we determined that our prior findings of increased col2a1a or col9a2 mRNA expression (by in situ hybridization) in pkd2-deficient embryos could be accounted for by increased embryo sensitivity to proteinase treatment used in the in situ hybridization protocol and enhanced in situ probe penetration (data not shown). These results are consistent with a general ECM defect in pkd2-deficient embryos that renders them more fragile and permeant to probes. Equivalent expression of collagen type II mRNA coupled with increased protein accumulation in pkd-deficient embryos points strongly to differences in translation or post-translational collagen type II processing as the cause of axis curvature in pkd morphant zebrafish. General inhibition of MMPs or the proteasome was not sufficient to induce axis defects, suggesting a principle role for increased collagen production in driving the zebrafish pkd phenotype.
The simplest interpretation of our data may be that polycystin 2 activity in the ER either directly or indirectly regulates secreted protein translation or vesicle trafficking. The altered expression of sec23B, sec24C, and sec24D that we observe supports the idea that ER to Golgi secretory traffic may be generally increased in pkd2 morphants. The role of Sec24D in the secretion of collagen type II has already been shown in the zebrafish. Bulldog and feelgood mutants, in which sec24D is disrupted, fail to secrete collagen type II, leading to abnormal cranial morphogenesis and shorter body length, likely because of notochord defects.24,25 Previous studies have reported altered secretion of heparin sulfate proteoglycans12,13 and defective basolateral vesicle trafficking in ADPKD cells.37 Polycystin 2 itself interacts with and is regulated by Syntaxin5, an essential regulator of vesicle trafficking.38 Consistent with a role for polycystin 2 specifically in the ER, expression an ER-retained mutant polycystin 2 protein in zebrafish pkd2 morphants has been shown to rescue the dorsal axis curvature phenotype39 that we have linked to excess collagen production. Could polycystin 2 deficiency directly modify the secretory pathway? PKD mutant cells are known to exhibit reduced agonist-stimulated calcium release from the ER and in some cases, show enhanced amounts of ER calcium.40–42 In localized transport between the ER and Golgi, calcium binding proteins regulate vesicle trafficking. For instance, calcium regulates the binding between Sec31A and Apoptosis-linked gene 2, a penta-EF-hand protein that regulates secretion by stabilizing the COPII complex at the ER.43–46 Additional studies will be required to determine whether altered calcium release from the ER in pkd-deficient embryos could influence vesicle transport rates or the specific vesicle cargo sorting processes required for collagen secretion.47
Regardless of whether pkd2 knockdown directly or indirectly affects secretion, our finding that sec24D knockdown or BFA treatment was sufficient to prevent pkd phenotypes in zebrafish argues strongly that altered protein secretion is one critical component of pkd pathology. BFA treatment resulted in a dose-dependent rescue of dorsal axis curvature in both pkd2 morphants and cup pkd2 mutants and prevented kidney cyst formation in pkd2 morphants. At the concentrations used, BFA did not induce apoptosis or major defects in zebrafish embryos, except a shortened body length. Most importantly, we showed that BFA treatment could straighten embryos that already had a mild dorsal axis curvature before treatment, suggesting that reversal of collagen overproduction could allow realignment of ECM to a normal axial morphology. However, BFA treatment was unable to rescue cyst formation in pkd2 morphants after cysts were formed. The results suggest that ECM defects could play a role in the initiation of cystogenesis and dorsal axis curvature but perhaps, could not play a role in progression of the cystic phenotype.
We have focused here on the secretory pathway as a potential target to reverse collagen overaccumulation in the ECM of Pkd2-deficient embryos. However, it is also clear from our work that collagen overaccumulation must also involve an increased rate of col2a1a mRNA translation. The observed changes in COPII component gene expression could be a compensatory event to support increased secretion. Although the Tor pathway is known to be activated in ADPKD cells48 and could, in principle, signal increased protein synthesis, we did not observe an increase in ribosomal phospho-S6 protein levels (Supplemental Figure 9), a readout of Tor signaling. Although additional studies will be required to determine the mechanism of increased collagen translation, our finding that modulation of the secretory pathway prevented and even reversed some ADPKD phenotypes in zebrafish suggests that the COPII complex may be a new target in the treatment of ADPKD.
Concise Methods
Zebrafish Lines
Wild-type TüAB and Tg(wt1b:GFP) transgenic zebrafish were maintained and raised as described previously.49 Dechorionated embryos were kept at 28.5°C in E3 solution with or without 0.003% 1-phenyl-2-thiourea (Sigma-Aldrich) to suppress pigmentation and staged according to hpf.49
Morpholino Antisense Oligonucleotide Injections
Wild-type (TüAB) or WT1:GFP embryos at the one- to two-cell stages were microinjected with 4.6 nl 0.05 mM col2a1 or 0.25 mM pkd2 or sec24D antisense morpholino oligonucleotide solution (Gene Tools LLC) in 200 mM KCl with 0.1% Phenol Red using a nanoject2000 microinjector (World Precision Instruments). Morpholino sequences were
pkd2 exon 12: 5′-caggtgatgtttacacttggaactc-3′;
col2a1a exon 1: 5′-tgaaaaactccaacttacggtcatc-3′; and
sec24D 5′ untranslated region: 5′-cttactgttatacctctttcattcc-3′.
The control morpholino sequence was 5′-cgtccattgtgtaaaagtgtaacca-3′ (irrelevant sequence).
Quantitative RT-PCR
RNAs were obtained from 24-, 48-, or 72-hpf embryos using the Qiagen RNeasy mini kit. RNAs were treated with DNase (DNase I, RNase-free; Roche Diagnostics) and reverse-transcribed using oligo dT according to the manufacturer's protocols (Superscript III; Invitrogen). Quantitative RT-PCR was performed using SYBR Green. Primer sequences are listed in Table 1. col2a1a, col9a2, lox, loxl1, loxl5b, sec23A, sec23B, sec24C, and sec24D expressions were measured in control and pkd2-deficient embryos (n=4–5 samples per group; each RNA sample is obtained from 10 embryos) by quantitative RT-PCR. Gene expression was normalized with the housekeeping gene EIF1a.
Table 1.
Quantitative RT-PCR primers used
| Gene | Sequence |
|---|---|
| col2a1a | Forward 5′-tctccagcaggtccagtcaa-3′ |
| col2a1a | Reverse 5′-gcgtggtgaaagaggtttcc-3′ |
| col9a2 | Forward 5′-gcaggacaaaacggaagacc-3 |
| col9a2 | Reverse 5′-cacccttaacccccacttca-3′ |
| lox | Forward 5′-tgcacctcccacactcagggtt-3′ |
| lox | Reverse 5′-actccggcacctggtaactggga-3′ |
| loxl1 | Forward 5′-cggtgctccgtatgaccaagtttca-3′ |
| loxl1 | Reverse 5′-ctccacccgtgcctgccgaa-3′ |
| loxl5b | Forward 5′-tccacccggcggtaggacgat-3′ |
| loxl5b | Reverse 5′-ggcacaagatccggcagaccatt-3′ |
| mmp2 | Forward 5′-ggccactcacaccccaccagt-3′ |
| mmp2 | Reverse 5′-tcagccgtcctgaagaggaatctgt-3′ |
| mmp9 | Forward 5′-gatgggctgctggctcacgctt-3′ |
| mmp9 | Reverse 5′-gcgggtttgaatggctggtccag-3′ |
| mmp14 | Forward 5′-cgggctcaggcgattcgctct-3′ |
| mmp14 | Reverse 5′-gcattgccgagagcgtagctgga-3′ |
| sec23A | Forward 5′-aggtggacgtggagcaatac-3′ |
| sec23A | Reverse 5′-cgagaacgtctcggagaaac-3′ |
| sec23B | Forward 5′-atgctgggactgatgaaacc-3′ |
| sec23B | Reverse 5′-tcctgtgtttgggaaagtcc-3′ |
| sec24C | Forward 5′-cagggaagagagtggactgc-3′ |
| sec24C | Reverse 5′-gtcttcagctcctggcaaac-3′ |
| sec24D | Forward 5′-tttgctgacaccaacgagag-3′ |
| sec24D | Reverse 5′-tgattggggaacaggaagag-3′ |
Collagen Crosslinking
Collagen type II was extracted from 500 desiccated wild-type embryos or pkd2 morphants with 4 M guanidine HCl and 0.05 M Tris⋅HCl (pH 7.4) with 2 mM PMSF added as a proteases inhibitor for 48 hours at 4°C. The washed residue was digested with pepsin at 4°C and rocked for 24 hours, and the reaction was then stopped by the addition of pepstatin. Solubilized type II collagen was recovered in the 0.7 M NaCl precipitate. The samples were run on a 6% SDS-PAGE gel and transblotted to a polyvinylidene difluoride membrane for Western blot detection of collagen type II chains with a monoclonal antibody 1C10 that recognizes a sequence-specific epitope in the α1(II) triple helical domain (residues 934–945).50
Drug and Small Molecule Treatments
Collagen crosslinking was inhibited by treating embryos in E3 water with the lysyl oxidase inhibitor β-aminopropionitrile (30 mM) from postgastrulation (6–8 hpf) to 48 hpf. The secretory pathway from the ER to the Golgi apparatus was inhibited using BFA or CI-976. BFA was dissolved in ethanol and used at 0.5, 1, 1.5, and 2 μg/ml in E3 egg water containing 0.25% ethanol. CI-976 was dissolved in DMSO and used at 1–10 μM in E3 egg water containing 0.5% DMSO. Embryos were treated with BFA from 24 or 30 to 48 hpf as indicated or CI-976 from 24 to 30 hpf. Concentrations of MMP inhibitors used (treatment starting at gastrulation) were 10 µM GM6001, 10 µM, 100 µM, and 1 mM doxycycline, and 50, 100, 200, and 500 µM ascorbic acid in E3 water. Proteasome inhibitors MG132 and MLN-4924 were used at 10, 20, and 50 µM in E3 water.
Quantification of Dorsal Axis Curvature
Embryos were sorted as having a curvature of less than 90° (mild), a curvature of more than 90° (moderate), or a curvature with a tail crossing the body axis (severe) at 48 hpf (Supplemental Figure 1).
Statistical Analysis
A t test was performed to compare the different experimental groups (*P<0.05; **P<0.01; ***P<0.001).
Western Blotting
Anti–phospho-S6 rabbit monoclonal (Cell Signaling Technology) was used at 1:2000 dilution and standardized using anti-S6 mouse monoclonal (Cell Signaling Technology) used at 1:500 dilution.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank MaryAnn Weis and Lammy S. Kim for the collagen II analyses and Dr. Ela Knapik for advice and insight on zebrafish sec gene function.
This work was supported by National Institutes of Health Grants AR037318 (to D.E.) and DK070263 (to I.A.D.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101060/-/DCSupplemental.
References
- 1.Sutters M, Germino GG: Autosomal dominant polycystic kidney disease: Molecular genetics and pathophysiology. J Lab Clin Med 141: 91–101, 2003 [DOI] [PubMed] [Google Scholar]
- 2.van Dijk MA, Chang PC, Peters DJ, Breuning MH: Intracranial aneurysms in polycystic kidney disease linked to chromosome 4. J Am Soc Nephrol 6: 1670–1673, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA: Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 319: 907–912, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Hassane S, Claij N, Lantinga-van Leeuwen IS, Van Munsteren JC, Van Lent N, Hanemaaijer R, Breuning MH, Peters DJ, DeRuiter MC: Pathogenic sequence for dissecting aneurysm formation in a hypomorphic polycystic kidney disease 1 mouse model. Arterioscler Thromb Vasc Biol 27: 2177–2183, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA: Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A 97: 1731–1736, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S: Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Wright JM, Qian F, Germino GG, Guggino WB: Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem 280: 41298–41306, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Anyatonwu GI, Ehrlich BE: Calcium signaling and polycystin-2. Biochem Biophys Res Commun 322: 1364–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Jin H, Carone FA, Nakamura S, Liu ZZ, Kanwar YS: Altered synthesis and intracellular transport of proteoglycans by cyst-derived cells from human polycystic kidneys. J Am Soc Nephrol 2: 1726–1733, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Ebihara I, Nakamura T, Takahashi T, Yamamoto M, Tomino Y, Nagao S, Takahashi H, Koide H: Altered extracellular matrix component gene expression in murine polycystic kidney. Ren Physiol Biochem 18: 73–80, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Kovacs J, Carone FA, Liu ZZ, Nakumara S, Kumar A, Kanwar YS: Differential growth factor-induced modulation of proteoglycans synthesized by normal human renal versus cyst-derived cells. J Am Soc Nephrol 5: 47–54, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Liu ZZ, Carone FA, Nakumara S, Kanwar YS: Altered synthesis of proteoglycans by cyst-derived cells from autosomal-dominant polycystic kidneys. Am J Physiol 263: F697–F704, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Charron AJ, Nakamura S, Bacallao R, Wandinger-Ness A: Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J Cell Biol 149: 111–124, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA: Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol 17: 2706–2718, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schottenfeld J, Sullivan-Brown J, Burdine RD: Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134: 1605–1615, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA: The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech 3: 354–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC: Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655–4667, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Gansner JM, Mendelsohn BA, Hultman KA, Johnson SL, Gitlin JD: Essential role of lysyl oxidases in notochord development. Dev Biol 307: 202–213, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson JG, Finazzi D, Klausner RD: Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360: 350–352, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL: Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: Involvement of specific residues of the Sec7 domain. Mol Cell 3: 275–285, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Pyati UJ, Gjini E, Carbonneau S, Lee JS, Guo F, Jette CA, Kelsell DP, Look AT: p63 mediates an apoptotic response to pharmacological and disease-related ER stress in the developing epidermis. Dev Cell 21: 492–505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melville DB, Montero-Balaguer M, Levic DS, Bradley K, Smith JR, Hatzopoulos AK, Knapik EW: The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis. Dis Model Mech 4: 763–776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW: Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS One 5: e10367, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao RG, Shimizu T, Pommier Y: Brefeldin A is a potent inducer of apoptosis in human cancer cells independently of p53. Exp Cell Res 227: 190–196, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Lee SA, Kim YJ, Lee CS: Brefeldin a induces apoptosis by activating the mitochondrial and death receptor pathways and inhibits focal adhesion kinase-mediated cell invasion. Basic Clin Pharmacol Toxicol 113: 329–338, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Santoso NG, Cebotaru L, Guggino WB: Polycystin-1, 2, and STIM1 interact with IP(3)R to modulate ER Ca release through the PI3K/Akt pathway. Cell Physiol Biochem 27: 715–726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson PD, Geng L, Li X, Burrow CR: The PKD1 gene product, “polycystin-1,” is a tyrosine-phosphorylated protein that colocalizes with alpha2beta1-integrin in focal clusters in adherent renal epithelia. Lab Invest 79: 1311–1323, 1999 [PubMed] [Google Scholar]
- 30.Boca M, D’Amato L, Distefano G, Polishchuk RS, Germino GG, Boletta A: Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Mol Biol Cell 18: 4050–4061, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A: A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell 15: 1334–1346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streets AJ, Newby LJ, O’Hare MJ, Bukanov NO, Ibraghimov-Beskrovnaya O, Ong AC: Functional analysis of PKD1 transgenic lines reveals a direct role for polycystin-1 in mediating cell-cell adhesion. J Am Soc Nephrol 14: 1804–1815, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Joly D, Berissi S, Bertrand A, Strehl L, Patey N, Knebelmann B: Laminin 5 regulates polycystic kidney cell proliferation and cyst formation. J Biol Chem 281: 29181–29189, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ramasubbu K, Gretz N, Bachmann S: Increased epithelial cell proliferation and abnormal extracellular matrix in rat polycystic kidney disease. J Am Soc Nephrol 9: 937–945, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Wilson PD, Hreniuk D, Gabow PA: Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol 150: 360–369, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Daïkha-Dahmane F, Narcy F, Dommergues M, Lacoste M, Beziau A, Gubler MC: Distribution of alpha-integrin subunits in fetal polycystic kidney diseases. Pediatr Nephrol 11: 267–273, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Charron AJ, Bacallao RL, Wandinger-Ness A: ADPKD: A human disease altering Golgi function and basolateral exocytosis in renal epithelia. Traffic 1: 675–686, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Geng L, Boehmerle W, Maeda Y, Okuhara DY, Tian X, Yu Z, Choe CU, Anyatonwu GI, Ehrlich BE, Somlo S: Syntaxin 5 regulates the endoplasmic reticulum channel-release properties of polycystin-2. Proc Natl Acad Sci U S A 105: 15920–15925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu X, Wang Y, Schetle N, Gao H, Pütz M, von Gersdorff G, Walz G, Kramer-Zucker AG: The subcellular localization of TRPP2 modulates its function. J Am Soc Nephrol 19: 1342–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguiari G, Banzi M, Gessi S, Cai Y, Zeggio E, Manzati E, Piva R, Lambertini E, Ferrari L, Peters DJ, Lanza F, Harris PC, Borea PA, Somlo S, Del Senno L: Deficiency of polycystin-2 reduces Ca2+ channel activity and cell proliferation in ADPKD lymphoblastoid cells. FASEB J 18: 884–886, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Weber KH, Lee EK, Basavanna U, Lindley S, Ziegelstein RC, Germino GG, Sutters M: Heterologous expression of polycystin-1 inhibits endoplasmic reticulum calcium leak in stably transfected MDCK cells. Am J Physiol Renal Physiol 294: F1279–F1286, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Hooper KM, Boletta A, Germino GG, Hu Q, Ziegelstein RC, Sutters M: Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am J Physiol Renal Physiol 289: F521–F530, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Yamasaki A, Tani K, Yamamoto A, Kitamura N, Komada M: The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol Biol Cell 17: 4876–4887, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibata H, Suzuki H, Yoshida H, Maki M: ALG-2 directly binds Sec31A and localizes at endoplasmic reticulum exit sites in a Ca2+-dependent manner. Biochem Biophys Res Commun 353: 756–763, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Bentley M, Nycz DC, Joglekar A, Fertschai I, Malli R, Graier WF, Hay JC: Vesicular calcium regulates coat retention, fusogenicity, and size of pre-Golgi intermediates. Mol Biol Cell 21: 1033–1046, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshibori M, Yorimitsu T, Sato K: Involvement of the penta-EF-hand protein Pef1p in the Ca2+-dependent regulation of COPII subunit assembly in Saccharomyces cerevisiae. PLoS One 7: e40765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgärtel C, Schekman R, Rape M: Ubiquitin-dependent regulation of COPII coat size and function. Nature 482: 495–500, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westerfield M: The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), Eugene, OR, University of Oregon Press, 2007 [Google Scholar]
- 50.Eyre DR, Pietka T, Weis MA, Wu JJ: Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J Biol Chem 279: 2568–2574, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.