Abstract
BACKGROUND
Frontal-striatal dysfunction has been linked to cognitive impairment in Huntington's disease (HD). The frontal lobes play a role in memory for the temporal order in which items occur in a sequence. However, little is known about temporal order memory in HD or how it may be affected by interference.
OBJECTIVE
The study assessed temporal order memory in patients with manifest HD (n = 20), premanifest gene carriers for HD (Pre-HD; n = 18), and controls (n = 25) using a computerized radial 8-arm maze
METHODS
On the sample phase of each trial, participants viewed a random sequence of circles appearing one at a time at the end of each arm. On the choice phase, participants viewed two sample phase circles and chose the circle occurring earliest in the sequence. Manipulations of the temporal lag (defined as the number of circles occurring in the sample phase sequence between the two choice phase circles) were conducted to systematically vary interference. Temporally proximal lags were hypothesized to generate more interference relative to temporally distal lags.
RESULTS
The Pre-HD group was significantly impaired (p < .05) compared to controls on proximal temporal lags (high interference) but matched controls on distal lags (low interference). HD patients improved as a function of increased lag but demonstrated significant impairments (p < .05) across lags relative to controls.
CONCLUSIONS
Temporal order memory is differentially affected by interference during the premanifest and manifest stages of HD. The study identifies a fundamental, yet relatively unexamined, deficit associated with HD.
Keywords: Sequence, interference, visuospatial, frontal lobes, basal ganglia
INTRODUCTION
Impaired memory for the temporal order of items or events in sequence may have adverse consequences on a number of cognitive functions and may affect the execution of various daily living skills [1]. The prefrontal cortex may play a critical role in memory for sequences of stimuli or events [2]. Studies involving patients with frontal lobe damage [3,4] and neuroimaging studies in healthy adults [5,6] have offered further evidence that the frontal lobes support memory for temporal order. Therefore, neurodegenerative diseases that involve disruption in the frontal systems, such as Huntington's disease (HD), are likely to have adverse effects on temporal order memory.
HD is a progressive neurodegenerative disorder caused by an expansion of CAG repeats on the short arm of chromosome 4 [7]. The disease is characterized by motor abnormalities, psychiatric disturbance, and cognitive dysfunction. Deterioration of the basal ganglia and consequent degeneration of frontal-striatal circuitry [8] is suspected to be a primary cause of the cognitive symptoms in HD [9]. Several neuropsychological studies have detected cognitive changes in individuals who carry the HD CAG expansion but do not meet criteria for a clinical diagnosis of HD, termed premanifest gene carriers.
A study from our laboratory investigated temporal order memory in premanifest gene carriers for HD using a visuospatial temporal order memory task [1]. On the sample phase of each trial, participants viewed a computerized radial 8-arm maze on which a random sequence of circles appeared one at a time at the end of each arm. The sequence was presented over an approximately 30 second period. On the choice phase, participants viewed two circles from the sample phase sequence and were asked to choose the circle occurring earliest in the sequence. Temporal interference was systematically manipulated by varying the temporal lag, defined as the number of arms occurring in the sample phase sequence between the two choice phase arms. Performance on this task has been shown to improve as a function of increased temporal lag in both young and older adults [10]. This is presumed to occur because trials involving smaller temporal lags (i.e. 0 and 2 lags) are hypothesized to be more difficult due to increased temporal interference. However, trials involving large temporal lags (i.e. 6 lag) are easier due to less temporal interference. Premanifest gene carriers for HD within five years of estimated disease onset demonstrated impairments on trials involving temporally proximal lags when temporal interference was high [1]. However, these individuals matched the performance of controls on trials involving temporally distal lags when temporal interference was minimized [1]. To the authors’ knowledge, this was the first published study to demonstrate that temporal order memory was differentially affected by temporal interference in premanifest gene carriers up to five years prior to estimated disease onset. The results suggest that impaired temporal order memory could serve as an early marker of phenoconversion to HD in premanifest gene carriers [1].
However, no study has compared the performance of individuals in premanifest and manifest stages of HD on this task to examine the relationship between temporal interference and temporal order memory as individuals progress into the manifest stages of the disease. One might expect that temporal order memory would decline in the HD patients compared to the premanifest gene carriers for the disease. However, it is not clear how temporal order memory might be affected by interference in patients with HD. It is possible that HD patients would decline only when interference is high or moderate but may still match controls when interference is low. Alternatively, HD patients may decline under all conditions including when interference is low. Understanding the nature of temporal order memory impairment during the premanifest and manifest stages of HD may have implications for the development of behavioral interventions aimed at reducing interference in the temporal domain and increasing independence in daily living skills that may require intact temporal order memory (e.g., medication management, cooking, scripted events). The present study investigated a fundamental, yet relatively unexamined, processing deficit in temporal order memory that may affect multiple cognitive functions and the execution of various daily living skills in individuals in the premanifest and manifest stages of HD.
MATERIALS AND METHODS
Participants
Study participants included patients diagnosed with manifest HD (n=20), premanifest gene carriers for HD (Pre-HD, n=18) and controls (n=25). Demographic variables are summarized in Table 1. The controls were recruited from a longitudinal study in the laboratory of the principal investigator. Controls were screened for a family history of HD. The Pre-HD group was recruited from the HD Clinical Research Program at the University of California, San Diego. Pre-HD individuals were defined as having greater than 39 CAG repeats with the absence of clinical motor signs as rated on the Unified Huntington's Disease Rating Scale (UHDRS) [11]. The UHDRS was administered by a senior staff neurologist. Based on the UHDRS motor exam, the neurologist assigned a diagnostic confidence rating representing the evaluator's confidence that the presence of motor abnormalities were a manifestation of HD.
TABLE 1.
Mean (standard deviation) demographic variables and Dementia Rating Scale scores for patients with Huntington's disease (HD), premanifest gene carriers for HD (Pre-HD), and controls.
| HD | Pre-HD | Control | F | (df) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 51.20 (12.42) | 40.67 (10.48) | 43.92 (14.57) | 3.43 | (2, 60) | < .05*a |
| Education (years) | 16.55 (3.52) | 15.67 (2.87) | 15.82 (2.41) | .52 | (2, 60) | =.60 |
| DRS Total | 133.10 (7.10) | 140.22 (3.78) | 141.70 (2.26) | 17.75 | (2, 55) | <.001*b |
| Gender (% Female) | 45% | 44% | 42% | |||
Represents statistically significant difference
Newman-Keuls posthoc comparison test revealed no statistically siginificant age difference (p > .05) between the control group and HD group or the control group and the Pre-HD group. The HD group was found to be significantly older (p < .05) than the Pre-HD group.
Newman-Keuls posthoc comparison test revealed no statistically siginificant difference (p > .05) between DRS total scores of the control and the Pre-HD group. The DRS scores from the HD group were significantly lower (p < .05) than the Pre-HD group and the control group.
The mean total motor score for the Pre-HD sample was 2.78 (SE = .71) out of a possible 124. None of the participants in this group met criteria for a diagnosis of manifest HD. The mean (standard error) number of CAG repeats for the Pre-HD group was 42.17 (.49). The mean estimated age of disease onset for the Pre-HD was calculated to be 5.15 (1.73) years [12]. Exclusion criteria for the Pre-HD group included: clinical evidence of an active psychiatric illness (i.e., within the last 6 months); history of other neurological conditions, such as seizures or head injury; and history of alcohol or other substance abuse. The data from a portion of the controls [10] and the 18 premanifest gene carriers [1] were included in previous publications.
Patients with HD also were recruited from the HD Clinical Research Program at the University of California, San Diego. All HD patients included in the present study were rated by a senior neurologist as having unequivocal signs of mild to moderate HD (confidence score of 4 on UHDRS) and CAG repeat length of greater than 39, indicating that all patients carried the fully penetrant genetic mutation for HD. The mean (standard error) UHDRS scores for the HD group were as follows: Total Motor Score 31.40 (2.55); Functional Assessment 19.60 (.41); Independence Scale 76.75 (1.82); Total Functional Capacity Score 8.55 (.29). The mean (standard error) number of CAG repeats for the HD group was 44.15 (.98) and the mean age of disease onset was 44.30 (2.54) years. All procedures were approved by the Institutional Review Boards at San Diego State University and the University of California at San Diego and all participants provided signed consent.
Neuropsychological Measures
Participants were administered a series of neuropsychological tests to assess cognitive function, visuospatial perception, and mood. The participants completed the Dementia Rating Scale (DRS) [13], the Hopkins Verbal Learning Test—Revised (HVLT-R) [14] and the Color-Word Interference and Verbal Fluency subtests of the Delis Kaplan Executive Function System (D-KEFS) [15]. The HD patients also completed the Benton Judgment of Line Orientation Test [16]. Two control participants and one individual in the Pre-HD group did not complete the Color Word Interference Test.
Temporal Order Memory Task
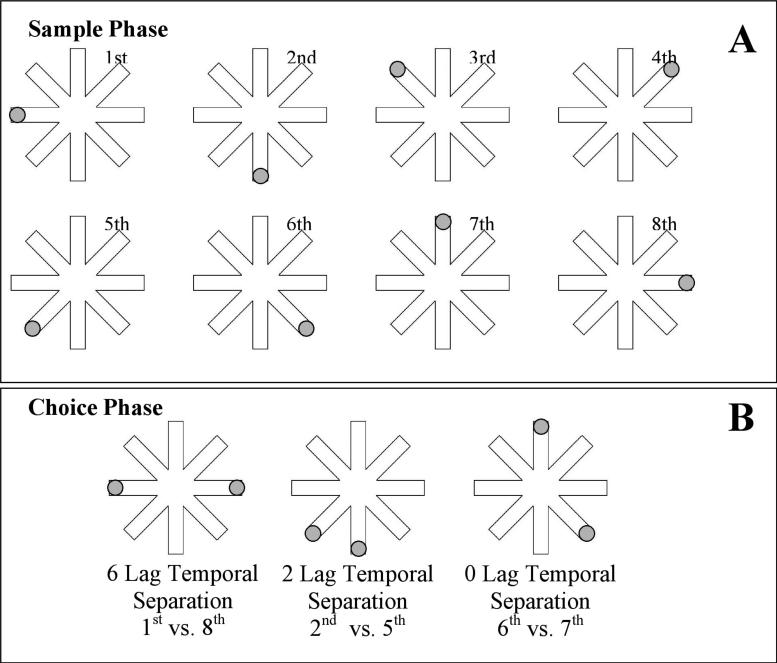
The participants were tested on a visuospatial temporal order task used in previously published studies [1,10]. Participants were seated in a chair approximately 60 cm from a computer monitor. At the beginning of each trial, participants were prompted to focus on the monitor where a computerized version of the radial 8-arm maze was shown. The 8-arm maze consists of 8 arms extending from a center like spokes on a wheel (Figure 1). The maze appeared on the computer screen with a diameter of approximately 30 cm. The participants were told that a circle would appear at the end of each arm, one at a time in a random sequence. The experimenter then instructed participants to remember the sequence in which the circles were presented on the arms. Each trial consisted of a sample phase followed by a choice phase. On the sample phase, a gray circle (3 cm diameter) appeared at the end of a randomly selected arm (see Figure 1A). The circle appeared for 2 s and then the entire display was masked for 2 s by a gray mask to eliminate after image effects. Then another circle appeared at the end of a different randomly selected arm for 2 s followed by a 2 s mask. This continued until a gray circle had been presented at the end of each of the 8 arms once in a random sequence that varied on each trial. During encoding, the participant had to remember the sequence but had no indication regarding which particular circles would be involved in the choice phase.
Figure 1.
A schematic of a sample phase temporal sequence showing locations of the 1st through the 8th arms presented in a sequence (A) and a choice phase (B) consisting a 6 temporal separation lag trial, a 2 temporal separation lag trial, and a 0 temporal separation lag trial.
On the choice phase, the participants viewed two circles presented simultaneously for 5 s, in two of the arms used in the sample phase (see Figure 1B). One circle was red and the other was blue. The color of the correct circle was randomly determined for each trial. The participants were asked to choose the circle in the location that had appeared earliest in the sequence by identifying the color of the circle. Temporal separations of 0, 2, 4, and 6 lags were randomly selected for each choice phase and represented the number of circles that occurred during the sample phase sequence between the two circles presented simultaneously during the choice phase. For example, on a 6 lag temporal separation, participants were presented with two circles on the choice phase that occurred with six circles between them during the sample phase sequence (e.g., 1st circle presented vs. 8th circle presented). On a 2 lag temporal separation, two circles were presented on the choice phase that occurred with two circles between them during the ssample phase sequence (e.g., 2nd circle presented vs. 5th circle presented). Prior studies using this task have shown that task performance improves as a function of increased temporal lag [1, 10]. This is presumed to occur because trials involving smaller temporal lags (i.e. 0 and 2 lags) are hypothesized to be more difficult due to increased temporal interference. However, trials involving large temporal lags (i.e. 6 lag) are easier due to less temporal interference. Following each sample phase sequence, three choice phases were conducted involving three of the four temporal separations. A total of 16 different sample phase sequences were presented with three choice phases for each sequence. As a result, there were a total of 12 choice phase trials for each of the four temporal separations. A 15 s inter-trial interval was implemented between each trial.
RESULTS
A 3 × 4 analysis of variance (ANOVA) with group (control, Pre-HD, HD) as a between group variable and temporal separation lag (0, 2, 4, 6) as a within group variable was used to analyze the data from the temporal order task. The analysis revealed a statistically significant main effect of group, F(12, 60) = 29.91, p < .0001, η2 = .499 (eta squared). A Newman Keuls posthoc comparison test revealed that controls significantly outperformed (p < .05) both the Pre-HD and the HD groups on the task. The posthoc test also revealed that the Pre-HD significantly outperformed (p < .05) the HD group. The ANOVA revealed a significant main effect of temporal separation lag, F(3, 180) = 34.01 p < .0001, η2 = .333, indicating that performance increased as a function of increased temporal separation lag. Planned polynomial contrasts revealed significant linear effects of temporal lag, F(1, 60) = 105.02, p < .0001, suggesting that performance improved linearly as temporal separation lag increased. Finally, the analysis revealed a significant group x temporal separation lag interaction, F(6, 180) = 2.73, p = .001, η2 = .08.
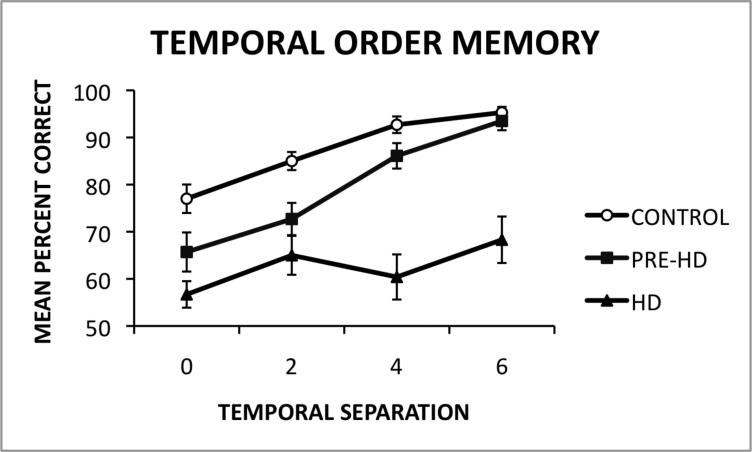
As shown in Figure 2 (see Table 2 for means and standard deviation values), a Newman-Keuls post hoc comparison test of the group x temporal separation lag interaction revealed that the control group signifcantly outperformed (p < .05) the HD group across all temporal separation lags. In addition, the control group signifcantly outperformed (p < .05) the Pre-HD group on 0 and 2 lag trials. However, there were no significant differences between the Pre-HD and control groups on the 6 lag trials and the mean difference between these groups on the 4 lag trials (.066) was slightly below the critical difference cutoff (.067), indicating a significant trend (p = .05). Within the control group, participants performed significantly better on the 6 lag temporal separation trials compared to the 0 and 2 lag temporal separation trials (p < .05). Within the HD group, participants performed significantly better on the 6 temporal separation lag compared to the 0 temporal separation lag (p < .05), indicating that performance in this group improved as a function of temporal separation lag. Within the Pre-HD group, participants performed significantly better on the 4 and 6 lag temporal separation trials compared to the 0 and 2 lag temporal separation trials (p < .05). The effect size for differences between the groups at each lag was calculated using Cohen's d. The effect size for differences between the control group and the Pre-HD group were as follows: zero lag = .69, two lag = 1.00, four lag = .65, and six lag = .24. The effect size for differences between the control group and the HD group were as follows: zero lag = 1.46, two lag = 1.38, four lag = 1.97, and six lag = 1.67.
Figure 2.
Mean percent correct performance of HD patients, premanifest gene carriers (Pre-HD) and healthy controls as a function of temporal separation lag on a visuospatial temporal order memory task.
TABLE 2.
Mean percent correct performance (standard error) of HD patients, premanifest gene carriers (Pre-HD), and healthy controls as a function of temporal separation lag on a visuospatial temporal order memory task.
| Temporal Order Memory Task | HD | Pre-HD | Control |
|---|---|---|---|
| Zero Lag Termporal Separation | 56.70 (2.81) | 65.70 (4.15) | 77.00 (3.02) |
| Two Lag Termporal Separation | 65.00 (4.13) | 72.70 (3.42) | 85.00 (1.92) |
| Four Lag Termporal Separation | 60.40 (4.79) | 86.10 (2.70) | 92.70 (1.76) |
| Six Lag Termporal Separation | 68.30 (4.93) | 93.5 (1.97) | 95.30 (1.20) |
Mean scaled scores (standard deviation) and the results from one-way ANOVAs comparing the HD, Pre-HD, and control groups on standardized neuropsychological tests and the temporal order task are shown in Table 3. The effect size for group differences on each test was calculated using Cohen's d. To create a single measure of temporal order task performance for comparison with the standardized tests, the total trials correct across all temporal separation lags were calculated for each individual. The raw data where transformed into z-scores and then a scaled score was calculated for each individual. As summarized in Table 3, the analyses revealed that the Pre-HD group did not differ from the controls on any of the standardized neuropsychological meausres. However, a signficant difference was found between the control and Pre-HD groups on the temporal order task. The HD group was found to differ significantly from both the control and Pre-HD groups on all neuropsychological meausres and the temporal order task. The effects sizes for group differences between the control and HD groups and control and Pre-HD groups were larger for the temporal order task compared to all standardized neuropsychological tests. A one-way ANOVA on the scaled scores from the Benton Judgement of Line Orientation Test did not reveal statistically significant differences, F(1, 35) = 1.94, p = .17, d = .46, between the HD group (Mn= 23.10, SD= 1.08) and separate group of demographically similar contorls not included in the present study (Mn= 25.12, SD= .92). The Benton Judgement of Line Orientation Task was included as a measure of visuospatial perception to examine whether deficits on the visuospatial temporal memory test were due to an impairment in visuospatial perception.
TABLE 3.
Mean scaled scores (standard deviation), results from one-way ANOVAs, and Cohen's d effect size estimates for HD patients, premanifest gene carriers (Pre-HD) and healthy controls on standardized neuropsychological tests and the temporal order task.
| HD | Pre-HD | Control | F | (df) | p | Posthoc | HD d | Pre-HD d | |
|---|---|---|---|---|---|---|---|---|---|
| Temporal Order Testa | 2.60 (2.96) | 6.28 (4.04) | 9.44 (2.86) | 20.26 | (2, 60) | <.001* | Con > Pre-HD > HD | 2.67 | 1.42 |
| Verbal Fluency | |||||||||
| Letter Fluency | 7.25 (3.52) | 11.06 (4.39) | 12.72 (3.12) | 12.82 | (2, 60) | <.001* | Con = Pre-HD > HD | 1.65 | .44 |
| Category Fluency | 5.05 (2.84) | 10.61 (3.22) | 11.68 (3.02) | 29.18 | (2, 60) | <.001* | Con = Pre-HD > HD | 2.26 | .34 |
| Color Word Interference Test | 5.75 (3.71) | 10.76 (3.99) | 12.52 (2.04) | 24.08 | (2, 57) | <.001* | Con = Pre-HD > HD | 2.26 | .55 |
| Hopkins Verbal Learning Test | |||||||||
| Total Recall | 4.50 (4.06) | 10.50 (3.70) | 12.04 (2.69) | 28.14 | (2, 60) | <.001* | Con = Pre-HD > HD | 2.19 | .57 |
| Delayed Recall | 4.90 (4.28) | 10.67 (2.28) | 10.84 (2.84) | 22.42 | (2, 60) | <.001* | Con = Pre-HD > HD | 1.64 | .07 |
Represents statistically significant difference
Total correct across all spatial separation lags
DISCUSSION
On the present task, it was hypothesized that as temporal separation lag decreased (i.e. choice phase circles were closer together in time during the sample phase sequence), interference was likely to increase resulting in poorer temporal order memory. In support of this hypothesis, the results demonstrate that the performance of all groups improved as a function of increased temporal separation lag and decreased temporal interference. However, controls were found to outperform the Pre-HD group on temporal separations with high and moderate temporal interference (e.g. 0 and 2 lags), but no group differences were detected on separations with low interference (e.g. 6 lag). In contrast, controls were found to outperform HD patients across temporal separations with high, moderate, and low temporal interference. The data demonstrate that temporal order memory for a sequence of visuospatial stimuli is impaired in individuals with manifest HD. However, temporal order memory is only impaired during the premanifest stages of HD when termporal intereference is high.
Although the HD group was found to be impaired across all temporal separations, it is important to note that temporal order memory did improve in HD patients with decreased interference. The data indicated that HD participants performed significantly better on 6 lag temporal separation trials compared to 0 lag temporal separation trials. This finding provides some evidence that the present deficits on the temporal order task are not due solely to a general cognitive deficit in working memory, attention, or visuospatial perception. If the present deficits were the result of a more general cognitive impairment, then performance would not be expected to change as a function of temporal separation. The cognitive demands during the encoding of each sample sequence are identical. During encoding, the participant has to remember the sequence but has no indication regarding which particular circles will be involved in the choice phase. Similarly, for each choice phase trial the participant is asked to remember which circle appeared earliest in the sequence. The only aspect of the task that differs from trial to trial is how many circles were presented in the sequence between the two choice phase circles (i.e. the temporal separation). This manipulation was designed to assess memory for temporal order, while keeping all other cognitive aspects of the task identical. Although we cannot completely rule out the possibility that general cognitive decline contributed to the present deficits, the data provide some evidence (i.e. change in performance as a function of lag) to indicate a deficit in temporal order memory in the HD group rather than a general cognitive deficit. In addition, the HD group did not differ significantly from a demographically similar control sample on the Benton Judgement of Line Orientation test. This finding suggests that the temporal order deficits observed in individuals with HD were not due solely to impaired visuospatial perception.
As mentioned previously, a prior study from our laboratory examined the performance of premanifest gene carriers for HD on the present task [1]. The study reported that premanifest gene carriers within five years of estimated disease onset demonstrated significant deficits in task performance on 0 and 2 lag separation trials when temporal interference was high. However, performance increased on 4 lag trials and matched the level of controls on 6 lag trials when temporal interference was reduced. In the present study, the data from these gene carriers [1] was reexamined. The Pre-HD group as a whole was compared to a larger control sample, without separating the Pre-HD group based on estimated years to disease onset. The current findings in the Pre-HD group are consistent with what was reported in those within five years of estimated disease onset in our previous study [1]. The present data provide novel insight into the relationship between temporal interference and temporal order memory during the premanifest and manifest stages of the disease. In addition, the findings demonstrate that temporal order memory is worse once individuals convert to manifest HD even when temporal interference is low.
Prior studies have reported motor sequence learning impairments in individuals with premanifest and manifest HD [17]. However, prior to our previously published study in premanifest gene carriers [1], only two other studies to the authors’ knowlegde have examined non-motor sequence learning deficits in premanifest or manifest HD using a picture sequencing task from the WAIS-R [18,19]. These studies suggest that cognitive changes associated with HD may impair sequence learning, even on tasks that do not involve a motor component. The present study is the first to directly manipulate temporal interference in a temporal sequence task in individuals with manifest HD. The current data coupled with the findings from our prior study investigating temporal order memory in premanifest gene carriers for HD [1] provide a unique perspective on the role of interference on temporal order memory during the premanifest and manifest stages of HD.
The prefrontal cortex is thought to support processes that organize sequences of stimuli or events [2]. Disruption of these processes may lead to impairments in cognitive domains that rely on goal directed behavior, such as executive functions. Executive dysfunction is a prominent cognitive feature of HD [9] that may occur even prior to disease diagnosis [20,21]. Temporal order memory deficits in HD may be associated with deficits in executive function, which is a cognitive domain essential to the execution of many activities of daily living tasks. Impaired temporal order memory also may play a role in other mnemonic functions such as episodic memory, where a series of linked elements must be remembered in a spatial and temporal context. Therefore, impaired temporal order memory may contribute to episodic memory deficits in individuals with HD.
To facilitate comparisons between performance on the standardized neuropsychological tests and the temporal order memory test, the raw data were converted into scaled scores and the effect size (Cohen's d) for group differences was calculated for each test. The analyses revealed that the largest effect size for group differences between the Control and HD groups and the Control and Pre-HD groups was found on the temporal order task. Although these findings offer some insight into the relative magnitude of temporal order memory impairment in patients with HD and premanifest gene carriers, additional research is needed to further examine the nature of temporal order memory deficits in relation to other well characterized cognitive impairments associated with HD. In particular, it is important to base future comparisons on a larger normative sample for the temporal order task.
In conclusion, these present results indicate that visuospatial temporal order memory is impaired during the premanifest and manifest stages of HD. In addition, the data provide insight into how temporal order memory is affected by temporal interference. The findings identify a processing deficit that may affect multiple cognitive functions and the execution of various daily living skills in individuals with HD. However, as discussed previously, the present study is not without limitations. Future research studies involving larger samples are needed so that statistical approaches can be used to test the incremental value of the temporal order memory task beyond standard measures of cognitive function using a wider battery of tests. In addition, future cross-sectional or longitudinal studies are needed to examine temporal order memory impairment across different stages of the disease from the premanifest stage to the various stages of manifest HD. These analyses also would provide the opportunity to examine how age interacts with disease stage and how these factors affect task performance. Furthermore, future studies are needed to examine the ecological validity of temporal order memory tasks for predicting declines in everyday functioning skills (e.g., cooking, medication management). The identification of temporal order memory deficits in individuals with HD may result in behavioral interventions that structure daily living tasks to mitigate interference in the temporal domain with the goal of increasing functional independence and improving quality of life in those with HD.
ACKNOWLEDGMENTS
We would like to thank all of the participants for their contributions to this study. A portion of the research was supported by a National Institutes of Health Grant (AG034202) from the National Institute on Aging awarded to Paul E. Gilbert. Savanna M. Tierney was supported by a National Institutes of Health/National Institute of General Medical Sciences Grant (2R25GM058906-09A3) and the SDSU MBRS/IMSD Program.
This study was supported by university funds from SDSU to Dr. Gilbert.
Ms. Tierney is funded by NIH grant #2R25GM058906 09A3 and the SDSU MBRS/ IMSD Program.
Dr. Corey-Bloom receives clinical trial support from Medivation Inc and Neurosearch Inc. Dr. Corey-Bloom also is a consultant for Biogen Idec Inc and Teva Inc.
Dr. Gilbert is funded by NIH grant #AG034202 and is the principal investigator on NSF fellowship DGE-0738622.
Footnotes
CONFLICT OF INTEREST
Mrs. Nicoll reports no disclosures.
Dr. Pirogovsky reports no disclosures.
Ms. Collazo reports no disclosures.
REFERENCES
- 1.Pirogovsky E, Goldstein J, Peavy G, Jacobson M, Corey-Bloom J, Gilbert PE. Temporal order memory deficits prior to clinical diagnosis in Huntington's disease. J Int Neuropsychol Soc. 2009 Sep;15(5):662–70. doi: 10.1017/S1355617709990427. [DOI] [PubMed] [Google Scholar]
- 2.Fuster JM. The prefrontal cortex—An update: Time is of the essence. Neuron. 2001 May;30(2):319–33. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 3.Daum I, Mayes AR. Memory and executive function impairments after frontal or posterior cortex lesions. Behav Neurol. 2000;12(4):161–73. doi: 10.1155/2000/327304. [DOI] [PubMed] [Google Scholar]
- 4.Shimamura AP. Memory and the prefrontal cortex. Ann NY Acad Sci. 1995 Dec;769:151–59. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. J Cogn Neurosci. 2000 Jan;12(1):197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- 6.Knutson KM, Wood JN, Grafman J. Brain activation in processing temporal sequence: an fMRI study. Neuroimage. 2004 Dec;23(4):1299–307. doi: 10.1016/j.neuroimage.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's chromosomes. Cell. 1993 Mar 26;72(6):971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 8.Vonsattel J, Myers R, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985 Nov;44(6):559–77. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Brandt J, Butters N. Neuropsychological characteristics of Huntington's disease. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. 2nd ed. Oxford University Press; Oxford, NY: 1997. pp. 312–41. [Google Scholar]
- 10.Tolentino JC, Pirogovsky E, Luu TT, Toner CK, Gilbert PE. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn Mem. 2012 May 21;19(6):251–55. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huntington Study Group The Unified Huntington's Disease Rating Scale: Reliability and consistency. Mov Disord. 1996 Mar;11(2):136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 12.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004 Jul 13;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 13.Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellac L, Katsu TB, editors. Geriatric psychiatry: A handbook for psychiatrists and primary care physicians. Grune and Stratton; New York, NY: 1976. pp. 77–121. [Google Scholar]
- 14.Brandt J, Benedict RHB. Hopkins Verbal Learning Test—Revised. Psychological Assessment Resources, Inc.; Lutz, FL: 1997. [Google Scholar]
- 15.Delis D, Kaplan E, Kramer JH. Delis-Kaplan executive functioning system. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 16.Benton A, Sivan A, Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment: A clinical manual. 2nd ed. Oxford University Press; New York, NY: 1994. [Google Scholar]
- 17.Schneider SA, Wilkinson L, Bhatia KP, Henley SM, Rothwell JC, Tabrizi SJ, et al. Abnormal explicit but normal implicit sequence learning in premanifest and early Huntington's disease. Mov Disord. 2010 Jul 30;25(10):1343–9. doi: 10.1002/mds.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foroud T, Simers E, Kleindorfer D, Bill DJ, Hodes ME, Norton JA. Cognitive scores in carriers of Huntington's disease gene compared to noncarriers. Ann Neurol. 1995 May;37(5):657–64. doi: 10.1002/ana.410370516. [DOI] [PubMed] [Google Scholar]
- 19.Snowden JS, Craufurd D, Thompson J, Neary D. Psychomotor, executive, and memory function in preclinical Huntington's disease. J Clin Exp Neuropsychol. 2002 Apr;24(2):133–45. doi: 10.1076/jcen.24.2.133.998. [DOI] [PubMed] [Google Scholar]
- 20.Farrow M, Churchyard A, Chua P, Bradshaw JL, Chiu E, Georgiou-Karistianis N. Attention, inhibition, and proximity to clinical onset in preclinical mutation carriers for Huntington's disease. J Clin Exp Neuropsychol. 2007 Apr;29(3):235–46. doi: 10.1080/13803390600657693. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen JS, Zhao H, Stout JC, Brinkman RR, Guttman M, Ross CA, et al. Clinical markers of early disease in persons near onset of Huntington's disease. Neurology. 2001 Aug 28;57(4):658–62. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]