Abstract
Objective
To describe the clinical features and imaging characteristics in unilateral acute idiopathic maculopathy (UAIM).
Methods
This is a retrospective review of four patients diagnosed with UAIM. Clinical characteristics (age, symptoms, Snellen visual acuity (VA), and funduscopic features) and images from spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF), fluorescein (FA), and indocyanine green (ICG) angiography were analyzed.
Results
The median age at presentation was 31 years (range 27–52 years). The median interval between symptom onset and presentation was four weeks (range 1–20 weeks). Associated systemic findings included a viral prodrome (50%), orchitis (50%), hand-foot-mouth disease (25%), and positive Coxsackie virus titers (50%). The median presenting VA was 20/400 (range 20/70–1/400), which improved to 20/30 (range 20/20–20/60) at final follow-up. The median follow-up time was 6 weeks (range 0–8 weeks). Early in the disease course, the central macula developed irregular, circular areas of white-grey discoloration. Following recovery, the macula had a stippled retinal pigment epithelium characterized by rarefaction and hyperplasia. FA demonstrated irregular early hyperfluorescence and late subretinal hyperfluorescence. SD-OCT showed a partially reversible disruption of the outer photoreceptor layer. FAF initially revealed stippled autofluorescence that eventually became more hypoautofluorescent. ICG showed “moth-eaten” appearing choroidal vasculature, suggestive of choroidal inflammation.
Conclusions
The imaging characteristics highlight the structural changes during the active and resolution phases of UAIM. The visual recovery correlates with structural changes and suggests that the pathogenesis involves inflammation of the inner choroid, retinal pigment epithelium, and outer photoreceptor complex that is partially reversible.
Unilateral acute idiopathic maculopathy (UAIM) is a rare cause of unilateral, sudden, painless vision loss in young healthy adults. It was originally described by Yannuzzi et al in a series of nine patients in 1991.1 A viral prodrome is common and nearly all patients experience spontaneous visual recovery.1,2 Initially, patients characteristically have unilateral, irregular, pigmentary changes and some have a neurosensory retinal detachment involving the macula.2 Several studies report the fluorescein angiographic findings of an irregular hyperfluorescence and hypofluorescence that originates at the level of the retinal pigment epithelium (RPE). Following resolution of the disease process, most maculae have a bull’s eye pattern of pigmentary disturbance with late staining on fluorescein angiography (FA).1,2 Because this condition is rare and the spectrum of disease is variable, the diagnostic criteria still remain somewhat ill-defined.2–5
With evolving imaging technologies, specific anatomic characteristics of UAIM may help us to better understand the disease process and involved tissues. Prior studies have documented this condition using conventional fundus photography, time domain optical coherence tomography (TD-OCT), and FA. Recently, Day and colleagues described the fundus appearance of a UAIM patient using fundus autofluorescence (FAF), indocyanine green angiography (ICG), and electrophysiology.3 Newer imaging modalities, such as FAF and spectral-domain optical coherence tomography (SD-OCT) are non-invasive methods of evaluating the retinal pigment epithelium (RPE) and specific layers of the retina.6,7 FAF provides topographic mapping of various fluorophores in the RPE, largely lipofuscin and has been used for the evaluation of age-related macular degeneration, inflammatory maculopathies, and retinal dystrophies.8 The axial resolution of most SD-OCT technology is approximately 5 µm, and allows for detailed assessment of the integrity of specific retinal layers and provides insight into pathogenic processes during disease progression and recovery.9 The purpose of this study was to further characterize the features of UAIM using multimodality imaging techniques and to correlate the ultrastructural choroidal, RPE, and retinal changes during the acute and convalescent phases of the disease with the recovery of visual function.
Methods
The Institutional Review Board of Emory University approved this study. We reviewed the medical records of consecutive patients who were diagnosed with UAIM between January 1, 2009 and December 31, 2010. Four patients with clinical features of UAIM were included for analysis. The demographic information, age at presentation, month of presentation, visual and systemic symptoms, past ocular and medical history, family history, and a detailed medication history were recorded for each patient. Snellen visual acuity, funduscopic features, electroretinography findings, and the results of clinical diagnostic imaging (SD-OCT, FAF, FA, and ICG) were also reviewed.
Retinal photography of the macula and fluorescein angiography was performed using a Topcon TRC 50DX retinal camera (Topcon America Corporation; Oakland, New Jersey). Time-domain OCT was performed using the Stratus OCT and SD-OCT images were taken on the Cirrus-HD OCT4000 instrument (Carl Zeiss Meditec; Dublin, CA). High-speed ICG and FAF images were recorded using a modified confocal scanning laser ophthalmoscope (HRA2; Heidelberg Engineering; Dossenheim, Germany). Full-field electroretinography was used in one patient, and results were recorded in accordance with the International Society for Clinical Electrophysiology and Vision (ISCEV) protocol on a Nicolet Bravo system (Madison, Wisconsin).10
Results
Patient characteristics
The clinical characteristics of the four study patients are included in Table 1. All patients were healthy, except patient 4 who had controlled hypertension. The median age at presentation was 31 years (range 27 – 52 years). All patients presented with a complaint of acute-onset, unilateral, painless vision loss. Three patients (75%), all of whom presented shortly after symptom onset, presented during the late summer months (June through September). Patient 4 had amblyopia in the involved eye and presented five months after his initial visual disturbance.
Table 1.
Clinical characteristics of the study patients with UAIM.
| Patient No./Sex/ Age |
Month of Onset |
Systemic Findings |
FA* | OCT | FAF | ICG | Initial VA |
Final VA |
Total follow-up (weeks) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial* | Final* | Initial* | Final* | ||||||||
| 1/F/27 | Sept. | HFMD; Positive Coxsackie virus A9 | Day 1: Irregular subretinal leakage / central pooling with neurosensory detachment. Week 1: Irregular stippled subretinal staining/leakage | Small subfoveal neurosensory detachment. Week 1: PRL disruption present with preservation of external limiting membrane | Partial restoration of PRL | Stippled HOAF and HRAF | Largely HOAF with granular HRAF | “Moth-eaten” choroidal vasculature | 1/400 | 20/20 | 8 |
| 2/M/30 | Aug. | Viral prodrome; Orchitis | Week 7: Outer circular ring of subretinal staining, middle ring of blockage and central ring of subretinal staining | PRL disruption with preservation of external limiting membrane | Partial restoration of PRL | Mixed HOAF and HRAF | Largely HOAF with granular HRAF | - | 20/70 | 20/30 | 8 |
| 3/M/31 | July | Orchitis; Positive Coxsackie virus B2/B5 | Week 1: Outer circular ring of subretinal staining, middle ring of blockage and central ring of subretinal staining | Mildly noncystic edema of neurosensory retina | - | - | - | - | 2/200 | 20/60 | 13 |
| 4/M/52 | Oct. | None | Month 5: Granular area of subretinal staining and blockage | Atrophic retina | - | Well demarcated HRAF with specks of HOAF | - | - | 20/400 | - | - |
Dates indicate time after onset of symptoms
HOAF: hypoautofluorescence, HRAF: hyperautofluorescence, PRL: photoreceptor layer
Two patients (50%) described a viral prodrome (patients 1 and 2), and one patient (patient 1) described the clinical signs and symptoms of hand-foot-mouth disease. Two patients concomitantly developed orchitis or epididymitis (patients 2 and 3) around the time of vision loss. Additionally, two patients (patients 1 and 3) had positive titers for Coxsackie virus around the time of presentation.
The median visual acuity at presentation to our institution was 20/400 (range 20/70 to 1/400), which improved to 20/30 (range 20/20 to 20/30) at final follow-up. The median followup time was 8 weeks (range 0 to 13 weeks).
Funduscopic features
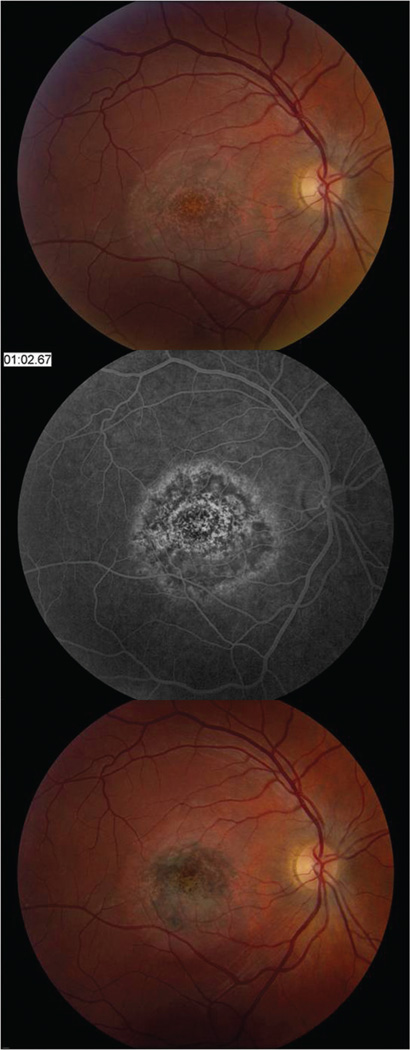
Most patients initially presented with irregular, circular areas of mild white-grey discoloration of the central macula. Patient 1 initially had a subfoveal exudative neurosensory retinal detachment (Figure 1) that resolved one week after symptom onset. Over the initial two to three weeks, the macula developed well-circumscribed areas of RPE atrophy and hyperplasia. Despite the abnormal funduscopic appearance during the disease process, the visual acuity improved dramatically. Figure 2 shows the funduscopic appearance and imaging characteristics one week after symptom onset for patient 1. Most patients showed increased retinal pigment hyperplasia as time progressed (Figure 3).
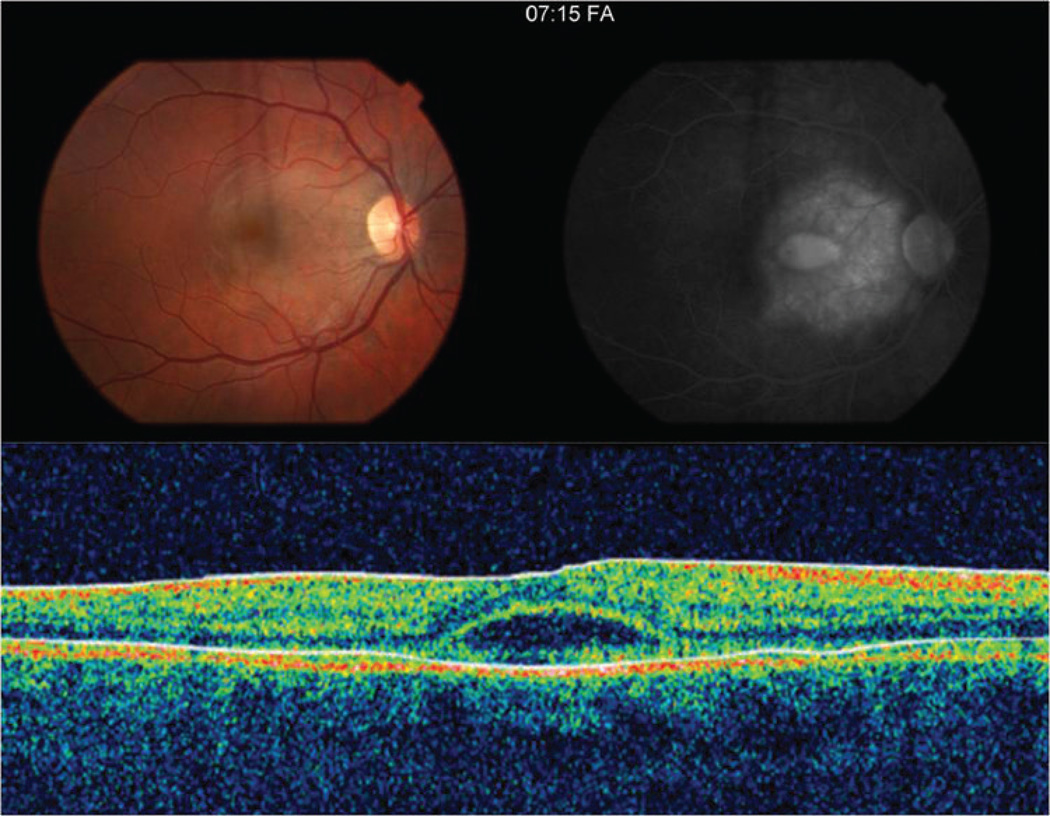
Figure 1.
The color fundus photograph of the affected right eye (A) one day after symptom onset demonstrates an irregular, circular area of grey-white discoloration in the macula. (B) Late-phase fluorescein angiogram image shows a large area of intense subretinal hyperfluorescence consistent with leakage and a smaller central area of pooling coinciding with an exudative detachment of the neurosensory retina. (C) TD-OCT shows a small subfoveal neurosensory detachment with hyperreflective debris at the apical side of the retinal pigment epithelium.
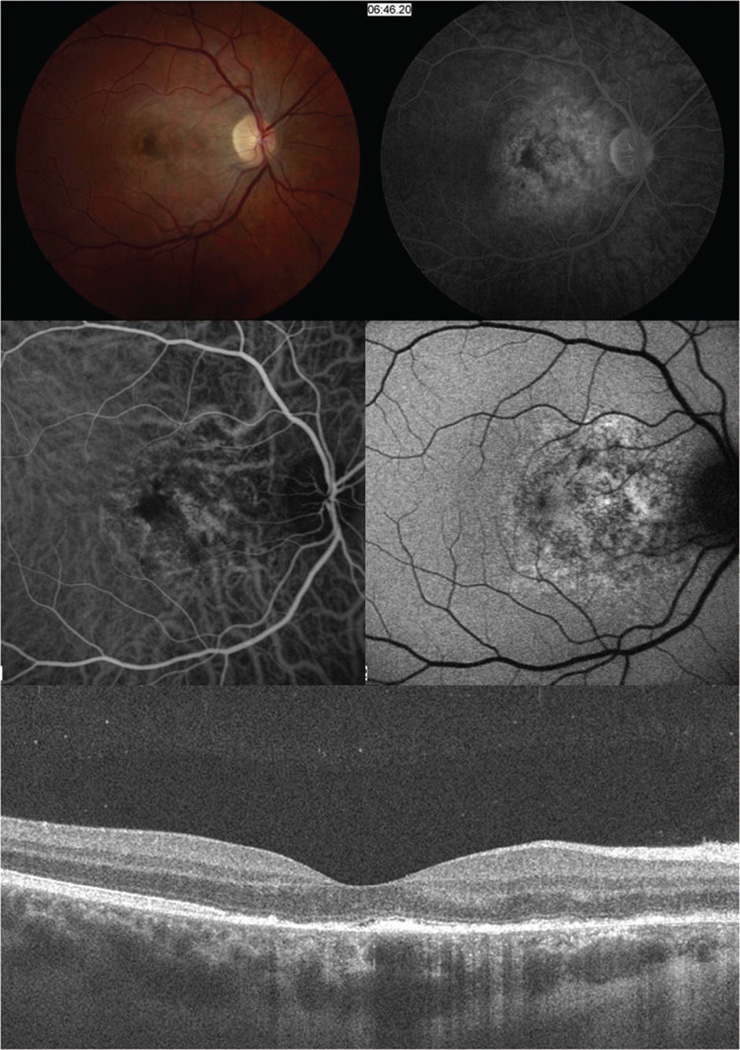
Figure 2.
(A) Fundus photography in patient 1 one week after symptom onset showing granular hyperpigmention of the retina and retinal pigment epithelium. (B) Fluorescein angiography demonstrates late staining and less leakage in the nasal macula compared to the patient’s prior angiogram. (C) ICG angiography shows irregular “moth-eaten” choroidal vascular appearance with dilated choroidal vessels underlying the perifoveal region. (D) FAF shows a stippled autofluorescense in the macula areas extending to the optic nerve. E. SD-OCT reveals disruption and irregularity of the photoreceptor layer, subtle hyporeflectivity at the foveal outer segment area, and hyper-reflective material on the apical side of the retinal pigment epithelium.
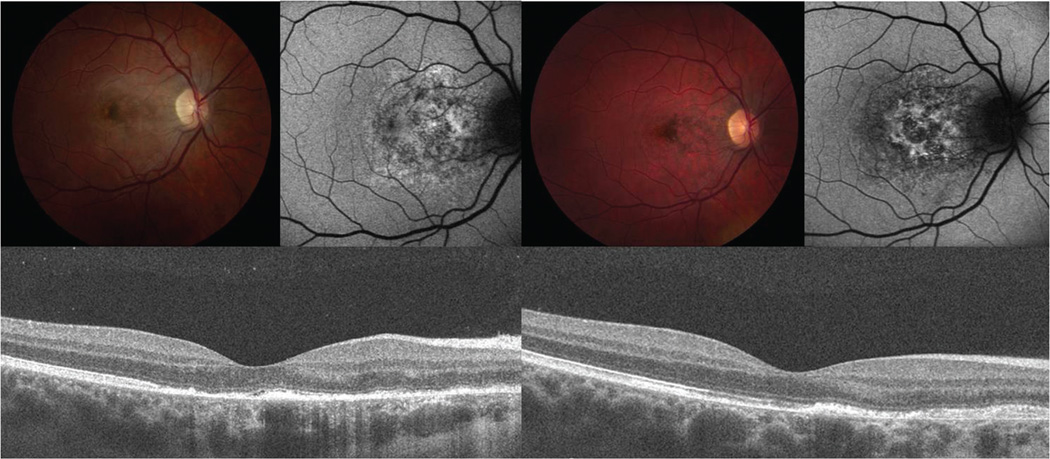
Figure 3.
Color fundus photograph, FAF, and SD-OCT findings in patient 1 at one week (A) and two months (B) after symptom onset. There is a loss of the grey-white discoloration and an increase in retinal pigment hyperplasia later in the disease course. The FAF shows less stippled autofluorescent areas (A, upper right) that evolve to a more stellate shaped autofluorescence with loss of background autofluorescence (B, upper right). SD-OCT shows hyporeflectivity of the outer photoreceptor layer (A, arrows), that are partially restored (B, arrowheads) at the twomonth follow-up visit. Note that the external limiting membrane remains intact in both A and B, yet the apical debris on the RPE has diminished.
Optical coherence tomography
TD-OCT was performed in patients 1, 3, and 4. One patient had subfoveal subretinal fluid documented with TD-OCT shortly after symptom onset. Retinal thinning and irregularity of the outer photoreceptor layer was the most prominent finding in the TD-OCT images from later in the disease course.
SD-OCT was performed in patients 1 and 2. Both had disruption and irregularity of the outer photoreceptor layer early after disease onset. The external limiting membrane was well preserved in each case. Later in the course of this condition, the outer photoreceptor layer appeared to be normalizing (Figure 3).
Fundus autofluorescence imaging
Patients 1, 2, and 4 underwent FAF imaging. The alteration in autofluorescence corresponded to the clinically significant lesion on funduscopic exam. Earlier in the course of the disease, the demarcation line between hypo- and hyper-autofluorescence was distinct. The lesions showed a complex, mixed pattern of hypo- and hyper-autofluorescence, typically involving the fovea or peripapillary region. Later in the disease course, the affected areas displayed decreased hyper-autofluorescence and became more hypo-autofluorescent (Figure 3), suggesting loss of the RPE. The shift from hyper- to hypo-autofluorescence paralleled the visual acuity improvement.
Fluorescein and indocyanine green angiography
All four patients underwent fluorescein angiography and patient 1 had an ICG angiogram. Patient 1, who presented very early in the disease course, showed subretinal fluid leakage with pooling in the central macula that resolved one week later. In the subacute and chronic phases of the disease, the macula from each patient showed a well-demarcated, speckled pattern of subretinal fluorescein staining (Figures 4 and 5). Patient 1 underwent ICG angiography approximately 2 weeks after symptom onset with an irregular “moth-eaten” appearance of the choroidal vasculature throughout the involved area (Figure 2). There were no areas of abnormal hyperfluorescence or definite leakage.
Figure 4.
Six weeks after symptom onset in patient 2, there is increased retinal pigment hyperplasia (A) and late subretinal staining on FA (B and C). FAF (D) shows a stippled pattern of autofluorescence, as well as loss of background autofluorescence. The SD-OCT (E) reveals disruption and irregularity of the outer photoreceptors and debris on the apical side of the RPE.
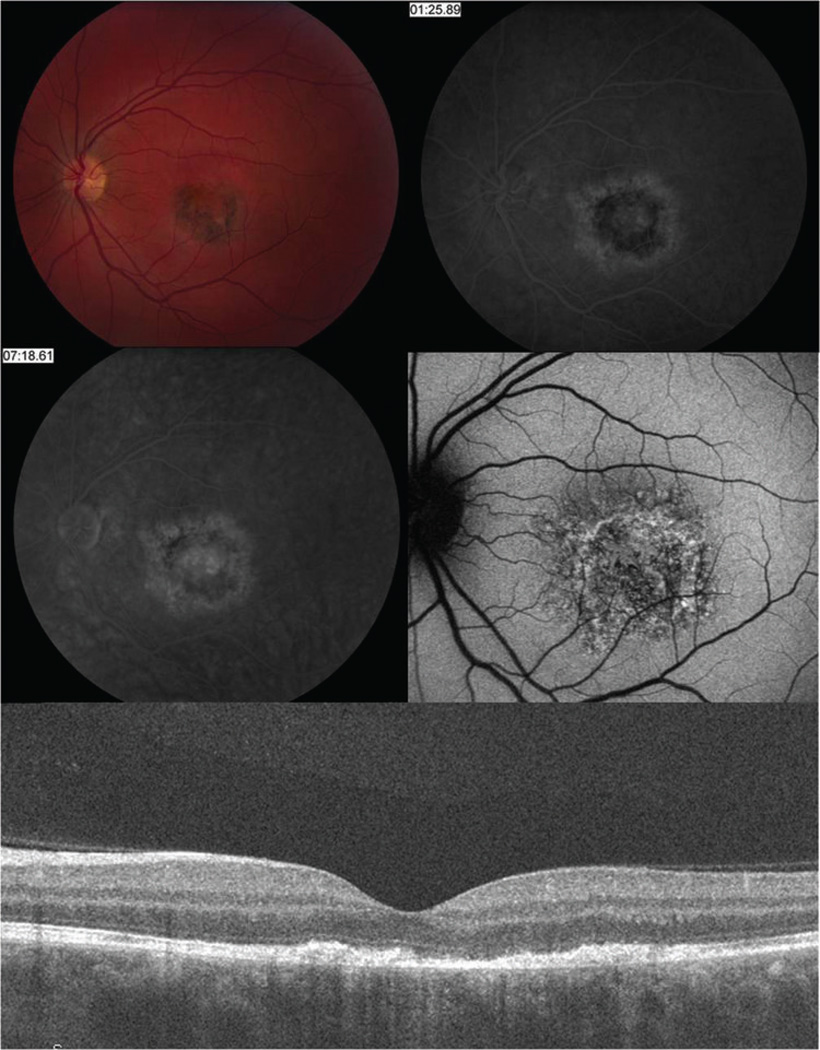
Figure 5.
Fundus imaging in patient 3 one week after symptom onset reveals a grayish discoloration of the retina within the macula (A) and subretinal staining on FA (B). Seven weeks after symptom onset, there is increased retinal pigment hyperplasia (C).
Electroretinography
The full-field electroretinogram (ERG) performed on patient 1, approximately two weeks after symptom onset, showed mildly diminished 30 Hz flicker amplitudes, suggesting mild cone dysfunction, and was otherwise unremarkable.
Discussion
In this series of UAIM patients, we found that multimodality diagnostic imaging was helpful in identifying the structural changes that occur and evolve during the early phases of this disease process. These findings provide insight related to the pathogenesis of this rare maculopathy. FAF signal, which is primarily derived from lipofuscin deposition within the RPE,8 was particularly revealing. Specifically, the abnormal stippled hyper-autofluorescent pattern observed in the acute phase of UAIM evolved into a more stellate pattern in the late phase of the disease while concomitant loss of the surrounding of background autofluorescence suggested a fibrotic process with loss of normal RPE cells in the foveal and perifoveal region. In some cases, the changes follow along the distribution of the papillomacular bundle. High-speed ICG angiography demonstrating dilated choroidal vessels and neighboring “moth-eaten” vessels were suggestive of active choroidal inflammation and disturbance of the RPE layer. The absence of retinal vascular and optic disc hyperfluorescence suggests that disease activity appeared localized at the inner choroid, RPE and outer retina. However, we do note that papillitis has been previously reported.2
Early in the disease process, OCT imaging confirmed the presence of a subfoveal neurosensory detachment, possibly resulting from underlying choroidal vasculitis or choroidal congestion and resultant RPE injury. SD-OCT demonstrated that outer segment photoreceptor disruption and injury occurs during the acute phase of UAIM. Interestingly, the anatomic disruption in this layer appears to be at least partially reversible. The photoreceptor layer was partially restored in patients 1 and 2; however, anatomic improvement lagged behind the visual recovery. Furthermore, each case seems to have a hyperreflective material during the acute phase of UAIM, which may represent photoreceptor outer segments. Multimodal imaging later in the disease course demonstrates at least partial resolution of this material.
The integrity of the outer photoreceptor layer, specifically studying the inner segment /outer segment (IS/OS) junction, has been a topic of increasing interest with the evolution of more sophisticated imaging modalities. Inoue and colleagues showed that the persistent disruption of the IS/OS junction correlated to poor visual outcome in patients who had epiretinal membrane (ERM) surgery.12 The authors speculated that postoperative inflammation may lead to reversible damage to the photoreceptors in some patients. Several other studies also suggest a similar correlation between IS/OS junction disruption and visual potential.13,14 In patients with diabetic macular edema, Maheshwary et al. reported that the integrity of the IS/OS junction is an important predictor of visual acuity.15 In the current study, the IS/OS junction was significantly disrupted during the acute phase of the illness, yet there was partial restoration of the photoreceptor integrity two to three months after symptom onset in some patients. The relatively good visual recovery and OCT findings in these UAIM patients suggest that the outer photoreceptor layer injury is partially reversible. Preservation of the external limiting membrane (ELM) may indicate that the inner retina, cell nuclei, and cellular structures necessary for outer segment regeneration remain intact. Furthermore, it is possible that restoration of RPE pump function occurs following resolution of the acute inner choroidal inflammatory process with secondary resolution of the subretinal fluid and photoreceptor debris. The inflammation may be either directly or indirectly related to coxsackievirus infection.
The precise mechanism of coxsackievirus tissue injury in UAIM is still unclear. Multiple reports have documented the association of Hand-Foot-Mouth Disease (HFMD) with UAIM.2,5 HFMD, originally described by Robinson in 1958, occurred as an outbreak of vesicular and ulcerative stomatitis associated with a maculopapular rash and vesicles on the hands and feet.21,22 HFMD is usually a self-limited, benign condition in children under 10 years of age, but adults may also be affected.22 HFMD is most frequently associated with coxsackievirus serotype A16 or enterovirus 71, but multiple serotypes including A2, A5, A7, A9, A10, B1, B2, B3, B4, B6 have been reported in association with HFMD.5, 23–25 In this study, one patient had clinical signs of HFMD and developed vision loss in temporal proximity with the evolution of the skin rash and also had an elevated coxsackie A9 viral titer. While HFMD associated with coxsackievirus A16 has a more benign course, outbreaks of enterovirus 71 have been associated with serious clinical findings including encephalitis, fatal pulmonary edema and myocarditis.24 In addition to HFMD, the Coxsackie virus has been associated with orchitis, epididymitis, as well as other ophthalmic pathologies.5, 25–33
Uveitis associated with coxsackie virus has been reported in several cases.25–29 One patient with a serotype B4 infection developed iridocyclitis and an occlusive retinal vasculitis.28 Additionally, Coxsackie virus B3 and B4 serotypes have been implicated in posterior segment inflammatory conditions with lesions similar to those seen in UAIM.26–29 Coxsackievirus A16 and B6 antibodies were also found to be elevated in a case report of a 30 year old woman with UAIM.5 Among the positive serotypes found in our patients (A9, B2, B5), none had previously been reported in the English literature to be associated with ocular inflammation.
In this series, a viral prodrome was reported in the majority of UAIM patients. A comprehensive medical history and review of systems was especially helpful in raising our clinical suspicion of UAIM. Specifically, two patients reported viral prodromes and two had positive coxsackievirus antibodies. One patient was diagnosed with HFMD and two developed orchitis or epididymitis prior to the visual loss. Interestingly, three of our four patients (75%) presented during the late summer to early fall months. Given that coxsackievirus transmission is highest during these months, it is feasible that patients with UAIM may present more frequently during this season.
The strong association of UAIM with coxsackievirus, viral prodromal illness and systemic comorbidities including orchitis and epididymitis, suggests that two broad categories of disease mechanism may be involved. First, UAIM may result from direct viral infection. Second, UAIM may also be due to an autoimmune response in the setting of the viral infection. Indeed, coxsackievirus B3 is capable of infecting RPE cells in vitro, and it is possible that hematogenous spread to the RPE may occur during coxsackievirus-associated viremia.34 Another category of disease mechanism relates to immune-mediated damage, as typified by coxsackievirus-associated myocarditis.35 Specifically, molecular mimicry from coxsackievirus proteins may lead to activation of the host immune response and the failure of autoreactive Tcells to distinguish between non-self (i.e. coxsackievirus) and self-antigen (e.g. choroidal, RPE or outer retina). These processes may subsequently result in local tissue inflammation and structural damage. Although the role of local or systemic immunosuppression has not been explored, the majority of patients with UAIM experience improvement in vision and spontaneous resolution of serous retinal detachments. The role of corticosteroids in this condition could potentially expedite visual recovery by limiting tissue damage. However, with the potential to worsen a direct viral-mediated process, we do not currently recommend corticosteroid use in this setting.
In summary, we have described the clinical features of UAIM using multimodality clinical imaging, highlighting disease evolution its ultra-structural characteristics. Abnormalities on FA, ICG angiography and FAF suggest the critical role of inflammation at the level of the inner choroid, RPE, and outer retinal layers. SD-OCT was especially valuable in highlighting the partially reversible disruption of the outer photoreceptor layer. Specifically, the disruption of IS/OS junction during acute illness was correlated with visual loss, while its partial restoration during the convalescent phase of illness corresponded to visual recovery. Although the majority of patients experience a spontaneous improvement in visual acuity, a better understanding of the precise relationship between viral infection and inflammation is needed to target therapies that minimize tissue damage in patients with UAIM.
Acknowledgements
Support: Supported in part by a grant to Emory University Eye Center from the Research to Prevent Blindness, Inc.
Footnotes
Financial Disclosures: The authors have no financial or proprietary interest in any product mentioned herein.
Conformity of Information: The Emory University School of Medicine Institutional Review Board approved this study and all work pertaining to this project maintained HIPAA compliance.
Access to Data: The corresponding author, Dr. Steven Yeh, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Yannuzzi LA, Jampol LM, Rabb MF, Sorenson JA, Beyrer C, Wilcox LM. Unilateral acute idiopathic maculopathy. Arch Ophthalmol. 1991;109:1411–1416. doi: 10.1001/archopht.1991.01080100091049. [DOI] [PubMed] [Google Scholar]
- 2.Freund KB, Yannuzzi LA, Barile G, Spaide RF, Milewski SA, Guyer DR. The expanding clinical spectrum of unilateral acute idiopathic maculopathy. Arch Ophthalmol. 1996;114:555–559. doi: 10.1001/archopht.1996.01100130547007. [DOI] [PubMed] [Google Scholar]
- 3.Day AC, Rotsos T, Holder GE, Tufail A, Robson AG. Electrodiagnostic and two-wavelength fundus autofluorescence imaging investigations in acute idiopathic maculopathy. Doc Ophthalmol. 2010;121:155–160. doi: 10.1007/s10633-010-9235-0. [DOI] [PubMed] [Google Scholar]
- 4.Haruta H, Sawa M, Saishin Y, Ohguro N, Tano Y. Clinical findings in unilateral acute idiopathic maculopathy. Int Ophthalmol. 2010;30:199–202. doi: 10.1007/s10792-009-9299-6. [DOI] [PubMed] [Google Scholar]
- 5.Beck AP, Jampol LM, Glasser DA, Florissant M, Pollack JS. Is coxsackievirus the cause of unilateral acute idiopathic maculopathy? Arch Ophthalmol. 2004;122:121–123. doi: 10.1001/archopht.122.1.121. [DOI] [PubMed] [Google Scholar]
- 6.Yeh S, Forooghian F, Faia L, et al. Fundus autofluorescence changes in cytomegalovirus retinitis. Retina. 2010;30:42–50. doi: 10.1097/IAE.0b013e3181bfbdb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi Y, Nishimura A, Higashide T, et al. Unilateral choroidal excavation in the macula detected by spectral-domain optical coherence tomography. Acta Ophthalmol. 2010;88:87–91. doi: 10.1111/j.1755-3768.2010.01895.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 9.Sull A, Vuong LN, Price LL, et al. Comparison of spectral/fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina. 2010;30:235–245. doi: 10.1097/IAE.0b013e3181bd2c3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmor MF, Fulton AB, Holder GE, et al. ISCEV standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA, Ober MD, Slakter JS, et al. Ophthalmic fundus imaging: today and beyond. Am J Opthalmol. 2004;137(3):511–524. doi: 10.1016/j.ajo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Morita S, Watanabe Y, et al. Inner segment/outer segment junction assessed by spectral-domain optical coherence tomography in patients with idiopathic epiretinal membrane. Am J Ophthalmol. 2010;150(6):834–839. doi: 10.1016/j.ajo.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147:473–480. doi: 10.1016/j.ajo.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Oster SF, Mojana F, Brar M, et al. Disruption of the photoreceptor inner segment/outer segment layer on spectral domain-optical coherence tomography is a predictor of poor visual acuity in patients with epiretinal membrane. Retina. 2010;30:713–718. doi: 10.1097/IAE.0b013e3181c596e3. [DOI] [PubMed] [Google Scholar]
- 15.Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150:63–67. doi: 10.1016/j.ajo.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh resolution optical coherence tomography. Arch Ophthalmol. 2003;121:696–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 18.Kiernan DF, Mieler WF, Hariprasad SM. Spectral-domain optical coherence tomography: a comparison of modern high-resolution retinal imaging systems. Am J Ophthalmol. 2010;149:18–31. doi: 10.1016/j.ajo.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan DF, Hariprasad SM, Chin EK, et al. Prospective comparison of Cirrus and Stratus optical coherence tomography for quantifying retinal thickness. Am J Ophthalmol. 2009;147:267–275. doi: 10.1016/j.ajo.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Watanabe Y, Arakawa A, et al. Spectral-domain optical coherence tomography images of inner/outer segment junctions and macular hole surgery outcomes. Graefes Arch Clin Exp Ophthalmol. 2009;247:325–330. doi: 10.1007/s00417-008-0999-9. [DOI] [PubMed] [Google Scholar]
- 21.Robinson CR, Doane FW, Rhodes AJ. Report of an outbreak of febrile illness with pharyngeal lesions and exanthema: Toronto, summer 1957; isolation of group A coxsackie virus. Can Med Assoc J. 1958;79:615–621. [PMC free article] [PubMed] [Google Scholar]
- 22.Buchner A. Hand, foot, and mouth disease. Oral Surg Oral Med Pathol. 1976;41(3):333–337. doi: 10.1016/0030-4220(76)90147-x. [DOI] [PubMed] [Google Scholar]
- 23.Willems WR, Hornig C, Bauer H, Klingmüller V. Orchitis caused by coxsackie A9. The Lancet. 1977:1350. doi: 10.1016/s0140-6736(77)90388-9. [DOI] [PubMed] [Google Scholar]
- 24.Tsao K, Chang P, Ning H, et al. Use of molecular assay in diagnosis of hand, foot, and mouth disease caused by enterovirus 71 or coxsackievirus A 16. J Vir Methods. 2002;102:9–14. doi: 10.1016/s0166-0934(01)00376-7. [DOI] [PubMed] [Google Scholar]
- 25.Haamann P, Kessel L, Larsen M. Monofocal outer retinitis associated with hand,foot, and mouth disease caused by coxsackievirus. Am J Ophthalmol. 2000;129:552–553. doi: 10.1016/s0002-9394(99)00440-7. [DOI] [PubMed] [Google Scholar]
- 26.Forster W, Bialasiewicz AA, Busse H. Coxsackievirus B3-associated panuveitis. Br J Ophthalmol. 1993;77:182–183. doi: 10.1136/bjo.77.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadrmas EF, Buzney SM. Coxsackievirus B4 as a cause of adult chorioretinis. Am J Ophthalmol. 1999;127:347–349. doi: 10.1016/s0002-9394(98)00322-5. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi M, Sakai J, Usui M. Coxsackievirus B4 associated uveoretinitis in an adult. Br J Ophthalmol. 2003;87(4):501–502. doi: 10.1136/bjo.87.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirakata K, Oshima T, Azyma N. Chorioretinitis induced by coxsackievirus B4 infection. Am J Ophthalmol. 1990;109:225–227. doi: 10.1016/s0002-9394(14)75993-8. [DOI] [PubMed] [Google Scholar]
- 30.Willems WR, Hornig C, Bauer H, Klingmüller V. Orchitis caused by coxsackie A9. The Lancet. 1977:1350. doi: 10.1016/s0140-6736(77)90388-9. [DOI] [PubMed] [Google Scholar]
- 31.Babić K, Lisić M, Kruzić V, Marton E. Orchitis caused by coxsackie virus type B. Lijec Vjesn. 1978;100(1):25–28. [PubMed] [Google Scholar]
- 32.Willems WR, Hornig C, Bauer H, Klingmüller V. A case of coxsackie A9 virus infection with orchitis. J Med Virol. 1978;3(2):137–140. doi: 10.1002/jmv.1890030207. [DOI] [PubMed] [Google Scholar]
- 33.Craighead JE, Mahoney EM, Carver DH, et al. Orchitis due to coxsackie virus group B, type 5. Report of a case with isolation of virus from the testis. N Engl J Med. 1962;267(6):498–500. doi: 10.1056/NEJM196209062671008. [DOI] [PubMed] [Google Scholar]
- 34.Huemer HP, Larcher C, Kirchebner W, Klingenschmid J, Göttinger W, Irschick EU. Susceptibility of human retinal pigment epithelial cells to different viruses. Graefes Arch Clin Exp Ophthalmol. 1996;234:177–185. doi: 10.1007/BF00462030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2008;155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]