Abstract
Objective
Scientifically-validated food-based interventions are a practical means of addressing the epidemic of metabolic syndrome. An ethanolic extract of Artemisia dracunculus L. (PMI-5011) containing bioactive polyphenols, such as 2′, 4′–dihydroxy-4-methoxydihydrochalcone (DMC-2), improved insulin resistance in vitro and in vivo. Plant polyphenols are concentrated and stabilized when complexed to protein-rich matrices, such as soy protein isolate (SPI), which act as effective food-based delivery vehicles. In this study we compared bioaccessibility, bioavailability and efficacy of polyphenols extracted from A. dracunculus and delivered as PMI-5011 (ethanolic extract alone), formulated with the non-food excipient Gelucire®, (5011- Gelucire), or sorbed to SPI (5011-Nutrasorb®).
Materials and Methods
PMI-5011, 5011-Gelucire or 5011-Nutrasorb each containing 162 μg of DMC-2 was delivered to the TNO Intestinal Model-1 (TIM-1) of the human upper gastrointestinal tract to compare the effect of delivery vehicle on DMC-2 bioaccessibility. C57BL6/J mice were orally administered 5011-Nutrasorb or PMI-5011 to compare effects of polyphenol-protein complexation on acute hypoglycemic activity and bioavailability of DMC-2 in serum.
Results
At 500 mg/kg, 5011-Nutrasorb and PMI-5011 had similar hypoglycemic activity in high fat diet-induced diabetes mouse model despite the fact that 5011-Nutrasorb delivered 15-times less DMC-2 (40 μg/kg vs. 600 μg/kg). This can be partially explained by 8 times greater DMC-2 absorption into serum from 5011-Nutrasorb than from PMI-5011. TIM-1 experiments confirmed higher total bioaccessibility of DMC-2 in vitro when delivered in 5011-Nutrasorb (50.2 %) or Gelucire-5011 (44.4 %) compared to PMI-5011 (27.1 %) (p = 0.08).
Conclusion
Complexation with soy protein makes anti-diabetic A. dracunculus polyphenols more bioavailable and bioaccessible.
Keywords: Artemisia dracunculus, Russian tarragon, metabolic syndrome, type-2 diabetes, TIM-1, polyphenols, chalcones, bioavailability, bioaccessibility, PMI-5011
1. Introduction
Polyphenols found in plant derived foods have demonstrated therapeutic activities in chronic disorders, such as metabolic syndrome and type-2 diabetes [1–3]. Artemisia dracunculus L. (commonly called Russian tarragon) is a perennial herb with a long history of culinary and medicinal use [4]. An ethanolic extract of A. dracunculus, (called PMI-5011) has been evaluated for its anti-diabetic properties. PMI-5011 enhanced insulin receptor signaling in skeletal muscle of KK-A(y) mice [5] and primary human skeletal muscle culture [6], reduced blood glucose in diabetic rodent models, and decreased PEPCK gene expression in rat muscle tissue, a key regulator of hepatic gluconeogenesis [7]. Bioactivity-guided fractionation of PMI-5011 led to isolation of six polyphenols that contribute to the hypoglycemic activity observed in vivo [8–10]. Evidence indicate that chalcone 2′, 4′-dihydroxy-4-methoxydihydrochalcone (DMC-2; Figure 1), the most abundant polyphenol in PMI-5011, is also its main active component, at least partially responsible for down-regulation of PEPCK gene expression and decreased glucose production in H4IIE hepatocytes [10].
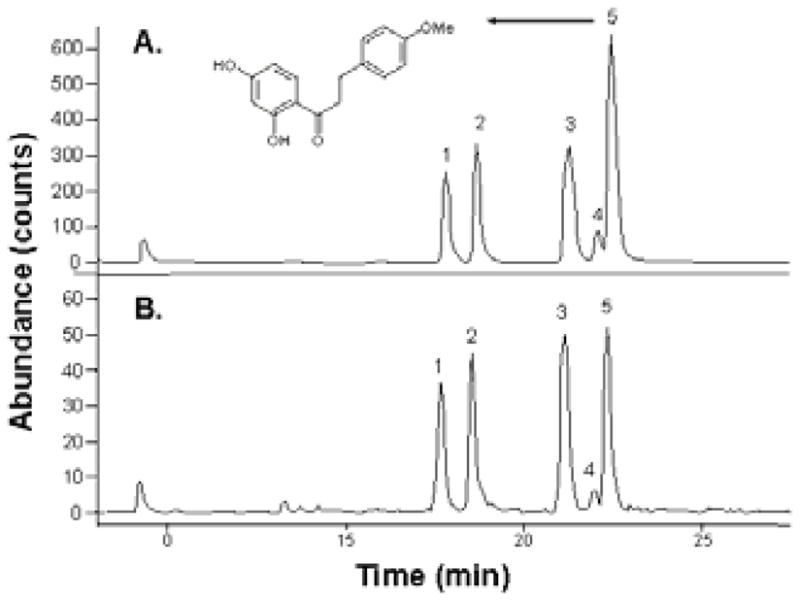
Figure 1. LC-MS chromatograms of polyphenolic compounds in PMI-5011 and 5011-Nutrasorb.
LC-MS analysis of PMI-5011 (A) and 5011-Nutrasorb (B) was performed using extracted ion monitoring for specific masses of previously identified compounds. Bioactive compounds identified in 5011-Nutrasorb were 1–6-demethoxycapillarisin (1), 2-davidigenin (2), 3-sakuranetin (3), 2′,4-dihydroxy-4′-methoxydihydrochalcone (4), and 2′,4′-dihydroxy-4-methoxydihydrochalcone (DMC-2; 5) and based on the integrated peak areas their concentrations were calculated to be 6.9, 8.9, 7.4, 13.5 and 14.6 times less, respectively, than in PMI-5011. The chemical structure of DMC-2 is shown.
Many bioactive plant polyphenols are poorly bioavailable because of low absorption and/or rapid metabolism and elimination, which can limit their therapeutic activity [11]. Lipid-based excipients are used to improve dissolution and enhance bioavailability of orally administered compounds with poor water solubility and absorption, often resulting in superior efficacy [12, 13]. Examples include Labrasol®, approved for drug formulations, and Gelucire® 44/14, approved for drug and dietary supplement formulations [14–17]. Oral administration of PMI-5011 formulated with Labrasol had significantly better hypoglycemic activity in high fat diet (HFD)-fed mice compared to PMI-5011 alone [18]. Furthermore compounds in PMI-5011 formulated with Labrasol showed increased bioavailability in serum, comparable to gavage with pure DMC-2[18]. These bioavailability-enhancing excipients are, however, not approved or compatible for use in foods.
A food-compatible method of improving bioavailability of dietary polyphenols would be useful for developing scientifically-based and efficacious functional foods. Nutrasorb technology leverages the natural affinity of polyphenols for protein [19], and uses edible protein-based matrices, such as soy protein isolate (SPI) or soy flour, to naturally sorb, concentrate and stabilize polyphenols contained in plant extracts while excluding excess water, sugar and lipid components [20]. Juiced or extracted plant materials, including fruits, spices and pomace, have been used as a source of polyphenols, such as anthocyanins and proanthocyanidins, to produce polyphenol-protein complexes that have anti-diabetic, anti-bacterial and anti-enzymatic activities [20–26]. In a recent clinical trial, metabolomic profiling of blood serum collected from athletes that consumed blueberry and green tea polyphenols complexed to SPI for 17 days, rather than SPI alone, showed elevated 3-hydroxybutyrate and acetoacetate indicative of enhanced ketogenesis and fatty acid oxidation during recovery from 3 days of exercise [27].
The TNO intestinal model (TIM-1) of the human upper gastrointestinal tract simulates the in vivo conditions and kinetic events of the stomach and duodenum, jejunum and ileum compartments of the small intestine [28]. TIM-1 provides information about nutrient/compound transit, release, stability and availability for intestinal absorption or bioaccessibility. Bioaccessibility is defined as the amount of compound (< 5 kDa) released from a food matrix that can pass through membranes of the jejunal and ileal compartments of the TIM-1 system, which is an indicator of intestinal absorption or bioavailability in vivo. The ileal efflux represents what would theoretically be delivered to the colon for further metabolism. TIM-1 coupled with colorimetric quantification of total monomeric anthocyanins was used to compare intestinal absorption/bioaccessibility of blueberry anthocyanins from blueberry juice or blueberry polyphenol-enriched soy flour [29]. Compared to blueberry juice, ileal efflux samples collected after TIM-1 digestion of blueberry polyphenol-soy flour complex contained 2.8-fold more anthocyanins suggesting that the soy flour matrix protected anthocyanins during transit through upper digestive tract for subsequent colonic delivery and metabolism [29]. While TIM-1 is useful for the preliminary assessment of different formulations or food matrices on compound bioaccessibility, in vivo systems must be used to establish whether improved bioavailability of compounds results in enhanced efficacy. This study evaluated the hypoglycemic activity and bioavailability (in C57BL/6 diet-induced diabetes mice) and bioaccessibility (in TIM model) of three different formulations of DMC-2 (PMI-5011, Gelucire-5011 and 5011-Nutrasorb). For all three measured endpoints 5011-Nutrasorb provided the most effective formulation for delivering pharmacologically active doses of DMC-2.
2. Materials and Methods
2.1. Chemicals
Pepsin A from porcine stomach mucosa (2500–3500 units/mg, P-7012), trypsin from bovine pancreas (7500 N-α-benzoyl-L-arginine ethyl ester (BAEE) units/mg, T9201), and α-amylase Type II-A: from Bacillus species (1333 units/mg A-6380) were obtained from Sigma-Aldrich (Stockholm, Sweden). Fresh pig bile was obtained from TNO Zeist, Netherlands. Rhizopus lipase (150, 000 units/mg F-AP-15) was from Amano Enzyme Inc. (Nagoya, Japan). Samples of Labrasol® and Gelucire® 44/14 were provided by Gattefossé, Paramus, New Jersey. DMC-2 was chemically synthesized as previously described [18].
2.2. Preparation of PMI-5011, 5011-Gelucire and 5011-Nutrasorb
PMI-5011 (ethanolic extract of A dracunculus) was produced as previously described [7, 13, 30]. To produce 5011-Nutrasorb, 2 kg of frozen A. dracunculus was extracted with 10 L of Millipore water (1:5 ratio wt:vol.) at 100 °C for 2 h followed by 15 h at 22 °C. The particulates were removed by filtration through Miracloth. Soy protein isolate (SPI) was added to the extract at 100 g/L and pH was adjusted to 3.5 using 6 N HCl. After 15 min of mixing, liquid was removed using a RA-20 Vx filtering centrifuge fitted with 125 μm polypropylene filter (Rousselet Robatel, Pittsfield, MA). The solids were freeze-dried to yield 800 g of 5011-Nutrasorb powder. Gelucire-5011 was made by mixing PMI-5011 with Gelucire (1:2 w/w) at 60 °C. For biochemical analysis, PMI-5011 was dissolved in 80% ethanol (10 mg/mL) prior to LC-MS analysis. Polyphenols were eluted from 1.0 g of 5011-Nutrasorb three times with 20 mL of 95% ethanol, eluates were pooled and lyophilized to dryness. Dried eluate was dissolved in 95% ethanol (10 mg/mL) prior to LC-MS analysis.
2.3. LC-MS analysis
Samples were separated by UPLC (Dionex® Ultimate 3000 RSLC UPLC system) and detected using a triple quadrupole Varian 1200 L (Varian Inc., Palo Alto, CA) mass spectrometer equipped with electrospray ionization (ESI). Compounds were separated on a Phenomenex® Luna C-8 reverse phase column, size 150 × 2 mm, particle size 3 μm, pore size 100 Å, equipped with a Phenomenex® SecurityGuard™ pre-column. The mobile phase consisted of 2 components: Solvent A (0.5% ACS grade acetic acid in double distilled de-ionized water, pH 3–3.5), and Solvent B (100% Acetonitrile). The mobile phase flow was adjusted at 0.25 ml/min with a gradient from 5% B to 95%B over 35 min.
2.4. DMC-2 bioaccessibility in TIM-1
The dynamic, computer-controlled TIM-1 unit has been described at length [28, 29]. Three independent experiments were performed for each TIM-1 treatment. PMI-5011 (134.5 mg), 5011-Gelucire (403.5 mg) or 5011-Nutraorb (2.0 g), each delivering 162 μg of DMC-2, was mixed with artificial saliva, which consisted of 100 mL electrolyte solution, 30 mL citrate buffer and 11.5 mg amylase. Double-distilled water was added to each mixture up to a final volume of 300 mL. The final mixture was introduced in the gastric compartment of TIM-1 and digestion was initiated. For these studies using fasted state parameters, 100 mL of fresh porcine bile, 3.5 g of pancreatin, 7.5 mg lipase and 6 mg pepsin were introduced to the TIM-1 system. Each TIM-1 digestion experiment was terminated at 240 min (4 h) when approximately 80% of the stomach contents had passed the ileocaecal valve of the model and become the ileal efflux. Jejunum, ileum and ileal efflux samples were collected at 1, 2, 3, and 4 h after initiation of digestion. Residues, which comprised fluids remaining in the stomach, duodenum, jejunum and ileum compartments after the 4 h digestion period, were also collected. A 2 mL aliquot of each TIM-1 sample was diluted with 1 mL of Millipore water, defatted twice with 2 mL hexane and partitioned three times with 2 mL of ethyl acetate. Ethyl acetate was removed by speed vacuum and the dried samples were resuspended in 200 μL of 95% ethanol for LC-MS quantification of DMC-2. DMC-2 bioaccessibility per compartment was calculated as a percentage of input (162 μg). Total bioaccessibility is the amount of DMC-2 absorbed in jejunum and ileum compartments over the 4 h period. The total recovery of DMC-2 or mass balance was calculated from adding the amounts of each DMC-2 in jejunum and ileum samples (representing total bioaccessibility), ileal efflux samples, and the residues collected after each experiment and then dividing by the DMC-2 input.
2.5. Acute hypoglycemic test
Mice
The experimental protocols were approved by Rutgers University Institutional Care and Use Committee and followed Federal, and State laws. Housing conditions and the high fat diet (HFD) protocol used to induce the diabetic profile was previously described [18]. After the initial 12-week period on HFD mice were divided into experimental groups (n= 5–7 per group). On the day of testing mice were food-restricted for 4 h prior to gavage with water (control vehicle), PMI-5011 (500 mg/kg), 5011-Nutrasorb (125, 250 and 500 mg/kg) or SPI (500 mg/kg). Metformin (300 mg/kg) served as a positive control. Blood glucose readings were made before gavage (0 h) and 6 h after treatment using a glucometer (AlphaTRAK® 32004-02, Abbott Animal Health).
2.6. Identification of DMC-2 glucuronide in serum
After a 4 h period of food restriction, the HFD-fed mice were gavaged with 66% Labrasol in water (n= 5) or 500 mg/kg DMC-2 dissolved in 66% Labrasol (n= 10). Mice were sacrificed 2 h post-treatment by CO2 asphyxiation, trunk blood from individual animals was collected into microfuge tubes and allowed to clot followed by centrifugation at 5000 rpm for 10 min. Serum from vehicle (n= 5) or DMC-2 (n= 5) treated mice was hydrolyzed with β-glucuronidase as previously described [18]. To detect the glucuronide conjugate of DMC-2, serum of remaining DMC-2 treated mice (n= 5) was treated with an equal volume of acetonitrile at −20 °C to precipitated proteins. Samples were analyzed by LC-MS with selected ion monitoring with negative ionization for masses corresponding to the glucuronide conjugate of DMC-2 (M.W. 448-1) and DMC-2 (M.W. 272-1).
2.7. Bioavailability of DMC-2 in PMI-5011 and 5011-Nutrasorb
After a 4 h period of food restriction, HFD-fed mice were orally administered 500 mg/kg PMI-5011 (n= 7) or 5011-Nutrasorb (n= 6) suspended in pure water and delivering 600 or 40 μg/kg of DMC-2, respectively. Mice were sacrificed 2 h post-treatment by CO2 asphyxiation, trunk blood from individual animals was collected into microfuge tubes and allowed to clot followed by centrifugation at 5000 rpm for 10 min. Collected serum was hydrolyzed as previously described [18] prior to LC-MS analysis.
2.8. Statistics
Statistics were performed with STATISTICA v.10 (StatSoft). For comparison of differences between three or more groups, one-way ANOVA was performed followed by Tukey or unequal N HSD post-hoc tests. T-tests were performed to compare means before and after treatment (within group) or between two groups.
3. Results
3.1. Biochemical profile of PMI-5011 and 5011-Nutrasorb
LC-MS analysis showed that 5011-Nutrasorb contained the same bioactive compounds previously identified in PMI-5011[6, 9, 10, 18]; however, their abundance was 7–15 times lower in 5011-Nutrasorb (Figure 1). The A. dracunculus compounds have limited water solubility, therefore less are extracted in water compared to ethanol. Based on previous stoichiometry experiments [20], the amount of extracted A. dracunculus polyphenols sorbed to the SPI matrix depends on the concentration of compounds in the extract, the amount of SPI added to the extract, and availability of binding sites provided by the SPI matrix. The concentration of DMC-2 in 5011-Nutrasorb was 81μg/g, 15 times less than in PMI-5011 (1.2 mg/g). DMC-2, the most abundant polyphenol, considered the main bioactive compound in PMI-5011 with glucose lowering activity in vitro [10] and in vivo [18], was used as a marker compounds in comparing various methods for delivering pharmacological effects of A. dracunculus.
3.2. DMC-2 bioaccessibility in TIM-1
PMI-5011, 5011-Gelucire or 5011-Nutrasorb, each containing equivalent amounts of DMC-2 (162 μg), were delivered to TIM-1. Bioaccessibility was quantified based on the amount of DMC-2 (as a percentage of input) that passed through the semi-permeable capillary membranes of the jejunum and ileum compartments. DMC-2 collected in the ileal efflux is expressed as the percentage of compound that would theoretically be delivered to the colon for further metabolism by microbiota. Filtrates from the jejunum and ileum were collected each hour for four consecutive hours. DMC-2 was also measured in TIM-1 residues, which comprised the fluids that remained in all compartments after the 4 h experiment.
Compared to PMI-5011, DMC-2 bioaccessibility (% of input) in the jejunum compartment was higher for 5011-Gelucire and 5011-Nutrasorb at each time point (Figure 2, top) and over the 4 h period (Figure 2, bottom), but differences were statistically significant only at the 4 h time point. DMC-2 bioaccessibility in the ileum was similar for all three formulations whether analyzed as the percentage of DMC-2 absorbed in the ileum compartment each hour (Figure 2, top) or over the 4 h period (Figure 2, bottom). Compared to PMI-5011 (27.1 ± 7.2 %), mean total bioaccessibility of DMC-2 was numerically higher for 5011-Gelucire (50.2 ± 17.3 %) and 5011-Nutrasorb (44.4 ± 13.7 %), but differences were not significant (ANOVA, p= 0.17).
Figure 2. Bioaccessibility of DMC-2 after TIM-1 digestion of PMI-5011, Gelucire-5011, or Nutrasorb-5011.
PMI-5011 (134.5 mg), Gelucire-5011 (403.5 mg) or Nutrasorb-5011 (2.0 g) delivering equivalent amounts of DMC-2 (162 μg) was fed to TIM-1 under fasted state conditions. Three independent experiments were performed for each treatment. DMC-2 was quantified by LC-MS in samples collected from jejunum, ileum and ileal efflux at 1, 2, 3, and 4 h after start of digestion. DMC-2 bioaccessibility (BA) as a percentage of input was determined in jejunum (black bars) and ileum (grey bars) samples at all time points and represented total BA. DMC-2 in the ileal efflux (white bars) as a percentage of input was also measured and represents the percentage of DMC-2 that could be delivered to the colon. Top: For each treatment, the mean BA of DMC-2 (as % of input) for each compartment and standard deviation (SD) are presented as stacked bar graphs for each time point. PMI-5011, Gelucire-5011, and Nutrasorb-5011 were compared in terms of the % of DMC-2 measured at each time point in each compartment. Absence of letters (a, b) or same letters indicates no difference while different letter subscripts indicate significant differences (ANOVA, Tukey post hoc test, p< 0.05). Bottom: Mean BA of DMC-2 (as % of input) over the 4 h period for each compartment. PMI-5011, Gelucire-5011, and Nutrasorb-5011 were compared in terms of the % of DMC-2 measured for each compartment. Absence of letters (a, b) or same letters indicates no difference while different letter subscripts indicate significant differences (ANOVA, Tukey post hoc test, p< 0.05).
At 1 h and 2 h post-digestion the percentage of DMC-2 in the ileal efflux was numerically higher for 5011-Gelucire and 5011-Nutrasorb, but differences were not statistically significant (Figure 2, top). Over the total 4 h period, the mean percentage of DMC-2 in the ileal efflux of 5011-Nutrasorb (17.8 ± 5.0 %) was similar to that of 5011-Gelucire (14.9 ± 6.7 %), but 3.7-fold higher than PMI-5011 (4.8 ± 0.5 %; Figure 2, bottom), suggesting that when sorbed to the SPI matrix greater amounts of DMC-2 may be delivered to the colon.
The mean percentage of DMC-2 in residues was 13.7 ± 8.3% for PMI-5011, 8.1 ± 2.5 % for 5011-Gelucire and 18.5 ± 3.1 % for 5011-Nutrasorb; differences were not significant. The total percentage of DMC-2 recovered (or mass balance) after TIM-1 digestion of 5011-Nutrasorb (80.7 ± 0.8%) was significantly greater than that of PMI-5011 (45.5 ± 20.4%) and similar to 5011-Gelucire 73.2 ± 11.2%. DMC-2 recovery after TIM-1 digestion of 5011-Gelucire was not significantly better than PMI-5011. The higher percentage of DMC-2 recovered from TIM-digestion of 5011-Nutrasorb suggests that protein-bound DMC-2 is protected during transit through the upper GI tract.
3.3. Hypoglycemic activity of PMI-5011 and 5011-Nutrasorb
In vivo bioactivity is a good indicator of the bioavailability of a formulation. To evaluate effect of protein complexation on the acute hypoglycemic activity of A. dracunculus compounds in HFD-fed C57BL/6 mice, we compared a single administration of PMI-5011 (500 mg/kg), increasing doses of Nutrasorb-5011 (125, 250, 500 mg/kg), SPI (500 mg.kg) and vehicle (Veh; water). Metformin® (Met, 300 mg/kg) was administered as a positive control. Before treatment (T= 0 h) all groups had similar levels of blood glucose; however, significant differences between groups were observed 6 h post-treatment (Figure 3). Posthoc analysis showed that 500 mg/kg of 5011-Nutrasorb delivering 40 μg/kg of DMC-2 had similar hypoglycemic activity to 500 mg/kg of PMI-5011 delivering 600 μg/kg of DMC-2 (Figure 3), suggesting the SPI matrix greatly enhanced DMC-2 efficacy at a 15-times lower dose. Metformin demonstrated robust hypoglycemic activity, while no significant effect was observed for vehicle, SPI alone (500 mg/kg) or the 125 and 250 mg/kg doses of 5011-Nutrasorb (Figure 3).
Figure 3. Hypoglycemic activity of 5011 Nutrasorb and PMI-5011.
(A) Blood glucose levels of C57BL/6 mice before and 6 h after treatment with water (VEH, n= 7 mice), Metformin® (MET, n= 5), SPI (n= 7), 5011-Nutrasorb (n= 7 mice per dose), or PMI-5011 (n= 6). The second row of numbers represent the dose of DMC-2 (μg/kg) delivered in the indicated doses of test material (mg/kg). Each bar represents data mean ± SD. Between group comparisons before treatment (T= 0 h) indicated that all groups had similar blood glucose (ANOVA, p= 0.26). Between group comparisons after treatment (T= 6 h) indicated significant differences (ANOVA, p= 1.0 ×10−5, followed by unequal N HSD post hoc test). Absence of letters (a, b, c) or same letters indicates no difference while different letter subscripts indicate significant differences.
3.4. DMC-2 is glucuronidated in vivo
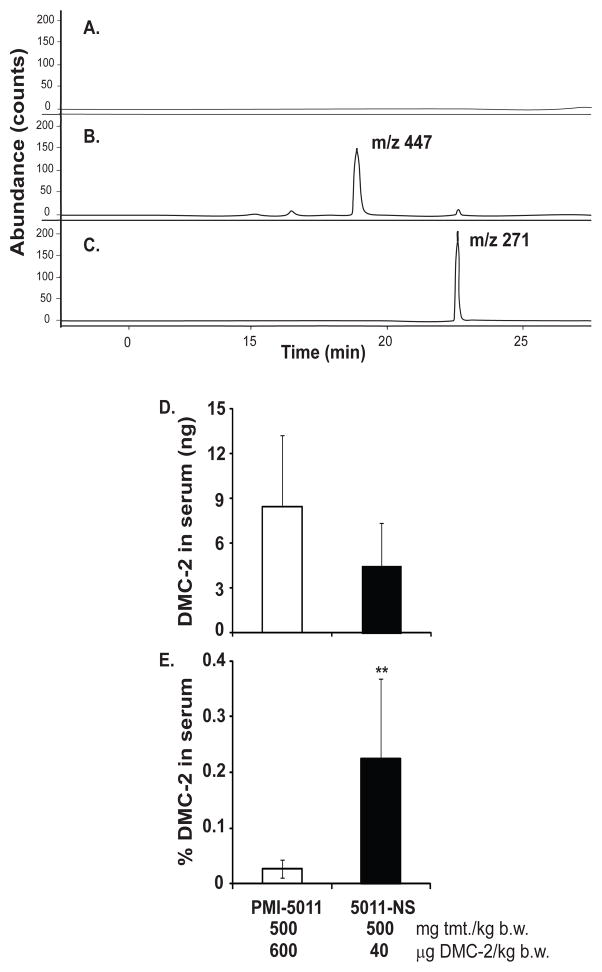
Poorly soluble compounds are commonly glucuronidated to facilitate absorption and circulatory transport. The primary derivative of DMC-2 in unhydrolyzed serum is a glucuronic acid conjugate of DMC-2 (m/z 447, Figure 4B). Serum treated with β-glucuronidase, as previously described [18], lead to loss of glucuronic acid and formation of DMC-2 (m/z 271, Figure 4C). Serum of vehicle-treated mice did not contain DMC-2 or DMC-2-glucuronide (Figure 4A).
Figure 4. DMC-2 from 5011 Nutrasorb is more bioavailable than from PMI-5011.

Representative chromatograms show LC-MC analysis of: (A) serum collected from mouse treated with 66% Labrasol, (B) serum collected from a DMC-2-treated mouse (500 mg/kg in 66% Labrasol) showing the glucuronic acid conjugate of DMC-2 (m/z 447) and, (C) serum collected from a DMC-2-treated mouse (500 mg/kg in 66% Labrasol) hydrolyzed with β-glucuronidase to obtain DMC-2 (m/z 271). To compare bioavailability, C57BL/6 mice were orally administered 500 mg/kg of PMI-5011 (n=7) or Nutrasorb-5011 (n=6). Serum was collected after 2 h, hydrolyzed, and analyzed for DMC-2 content by LC-MS as described in methods. The data is presented as (D) the amount of DMC-2 measured in serum (ng) and (E) percentage of the DMC-2 absorbed in serum relative to the amount dosed. Both doses were comprised of the same amount of treatment material (tmt) with different amounts of DMC-2. Two-tailed t-test **= p< 0.01.
3.5. Bioavailability of DMC-2 delivered in PMI-5011 and 5011-Nutrasorb
We hypothesized that complexation to protein may improve the bioavailability of polyphenols in circulation and explain why 5011-Nutrasorb had similar hypoglycemic activity to PMI-5011 despite delivering 15 times less DMC-2. To test this hypothesis the bioavailability of DMC-2 was compared in mice treated with 500 mg/kg of PMI-5011 (n= 7) or 5011-Nutrasorb (n= 6), delivering 600 or 40 μg/kg of DMC-2, respectively. Serum was hydrolyzed with β-glucuronidase prior to LC-MS quantification of DMC-2. Despite the 15-times greater dose of DMC-2 in PMI-5011 than in 5011-Nutrasorb treatment, there were no significant differences (p= 0.1) in the total amount of DMC-2 found in the serum of both groups (Fig. 4D). The amount of DMC-2 detected in the serum expressed as percentage of the original dose was 8 times higher for 5011-Nutrasorb treatment (0.22 ± 0.14 %) than for the PMI-5011 treatment (0.03 ± 0.02 %; Fig. 4E). These data indicate that DMC-2, and potentially other polyphenols, are more bioavailable when complexed to protein.
4. Discussion
Plants used for food contain an abundance of therapeutically active, but poorly bioavailable polyphenols [31]. Furthermore, dietary polyphenols often coexist with excess sugars, oils, fibers and water that can dilute and/or counteract their health benefits [8, 32]. While PMI-5011 provides concentrated delivery of DMC-2 and other polyphenols from A. dracunculus, they have low water solubility. PMI-5011 has a tar-like consistency and formulation with lipid-based excipients, such as Gelucire or Labrasol, can improve dissolution/absorption and in vivo efficacy [18]; however these bioavailability-enhancers are not food-compatible.
Nutrasorb technology leverages the natural affinity of dietary proteins and polyphenols to produce polyphenol-protein complexes. SPI was used to sorb polyphenols from an aqueous extract of A. dracunculus and LC-MS analysis demonstrated that the resulting 5011-Nutrasorb contained the same bioactive found in PMI-5011. Protein-polyphenol interactions occur through reversible electrostatic interactions, mainly van der Waals forces strengthened by hydrophobic and hydrogen bonding [19]. As DMC-2 and other Artemisia polyphenols have low water solubility, they likely interact with non-polar surfaces of SPI particles. Although 5011-Nutrasorb has 15 times less of the main bioactive DMC-2, both showed similar hypoglycemic activity in the HFD-fed C57BL/6 mouse model. This finding is consistent with previous data where single-dose administration of blueberry polyphenol-enriched soy flour that delivered just 0.45 to 1.8 mg/kg of anthocyanins had similar acute hypoglycemic effects in HFD-fed C57BL/6 mice [20] as column-purified anthocyanin-enriched fraction delivering 250 mg/kg of anthocyanins [33].
We hypothesized that sorption to the protein-rich matrix improved the bioavailability of DMC-2 and, possibly, other A. dracunculus polyphenols, allowing hypoglycemic effects to be reproduced at much lower doses. DMC-2 and other polyphenols in PMI-5011 have limited water solubility and TIM-1 experiments showed that greater DMC-2 bioaccessibility and recovery could be obtained when PMI-5011 was formulated with the lipid-based non-food, water dispersible surfactant, Gelucire (5011-Gelucire). SPI was at least as effective as Gelucire with respect to enhancing DMC-2 bioaccessibility and recovery in the TIM-1 system. The TIM-1 data suggest that SPI serves as carrier for hydrophobic Artemisia polyphenols and protects it from degradation during GI transit, while still allowing DMC-2 to be released for intestinal absorption. This data is consistent with previous TIM-1 studies that showed higher recovery and bioaccessibility of blueberry anthocyanins sorbed to soy flour compared to unbound anthocyanins in blueberry juice [29]. While TIM-1 is a good tool for preliminary comparison of DMC-2 intestinal absorption from the three A. dracunculus formulations, it does not reproduce all physiological processes of digestion and true DMC-2 bioavailability must be evaluated in vivo.
Using C57BL/6 mice gavaged with of DMC-2, we demonstrated that DMC-2 is present in serum as a glucuronide conjugate and must be hydrolyzed to recover DMC-2. Whether the conjugated or free form of the DMC-2 is responsible for anti-diabetic activities is presently unknown; however, some glucuronidated compounds have been reported to be biologically active [34]. LC-MC analysis of serum from C57BL/6 mice dosed with 5011-Nurtasorb (500 mg/kg) indicated more DMC-2 was absorbed in circulation compared to animals that received PMI-5011 (500 mg/kg), even though the amount of DMC-2 contained in 5011-Nutrasorb (40 μg/kg) was 15 times less than in PMI-5011 (600 μg/kg). Protein-bound polyphenols are thus markedly more bioavailable than extracted, unbound polyphenols. This provides a compelling explanation for the comparable hypoglycemic activity of the much lower doses of DMC-2 and other polyphenols in 5011-Nutrasorb compared to PMI-5011. The exact mechanisms whereby DMC-2 and other A. dracunculus polyphenols produce in vivo biological effects are unclear. However, protein-mediated protection of polyphenols during GI transit may allow more compounds to remain protected and, possibly, active within the small intestine or allow delivery of more polyphenols to the colon for microbial metabolism to other active metabolites. We suggest that polyphenol-protein complexes, such as 5011-Nutrasorb, provide a food-compatible solution for improving the bioavailability and efficacy of dietary polyphenols, such as DMC-2 and may be useful in formulating health-promoting functional foods.
Acknowledgments
The authors thank Rob Havenaar, Susann Bellman, Mans Minekus and Mark Jelier from TNO (Zeist, Netherlands) for providing the TIM-1 unit and support, Gattefossé (Paramus, New Jersey) for providing Labrasol and Gelucire, and Archer Daniels Midland Company (Decatur, IL) for supplying soy protein isolate.
Funding
This project was supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), which funds the Botanical Research Center and the New Jersey Agricultural Experiment Station at Rutgers University.
Abbreviations
- SPI
soy protein isolate
- TIM-1
TNO Intestinal Model-1
- DMC-2
2′, 4′–dihydroxy-4-methoxydihydrochalcone
Footnotes
Disclosure statement
IR and DER have equity in Nutrasorb, LLC.
Author contributions
DMR designed experiments. DER and DMR wrote the manuscript. AP conducted LC-MS analysis. PK conducted animal experiments. DER performed statistical analysis. AO prepared plant extracts, ran TIM-1 experiments and prepared TIM-1 and serum samples for analysis. WTC and IR provided experimental guidance and editorial comments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cefalu WT, et al. Botanicals and the metabolic syndrome. Am J Clin Nutr. 2008;87(2):481S–7S. doi: 10.1093/ajcn/87.2.481S. [DOI] [PubMed] [Google Scholar]
- 2.Graf BL, et al. Plant-derived therapeutics for the treatment of metabolic syndrome. Curr Opin Investig Drugs. 2010;11(10):1107–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Visioli F, et al. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. 2011;51(6):524–46. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 4.Obolskiy D, et al. Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem. 2011;59(21):11367–84. doi: 10.1021/jf202277w. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZQ, et al. An extract of Artemisia dracunculus L. enhances insulin receptor signaling and modulates gene expression in skeletal muscle in KK-A(y) mice. Journal of Nutritional Biochemistry. 2011;22(1):71–8. doi: 10.1016/j.jnutbio.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZQ, et al. Bioactives of Artemisia dracunculus L enhance cellular insulin signaling in primary human skeletal muscle culture. Metabolism. 2008;57(7 Suppl 1):S58–64. doi: 10.1016/j.metabol.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribnicky DM, et al. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550–7. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, et al. A natural history of botanical therapeutics. Metabolism. 2008;57(7 Suppl 1):S3–9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logendra S, et al. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry. 2006;67(14):1539–46. doi: 10.1016/j.phytochem.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Govorko D, et al. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab. 2007;293(6):E1503–10. doi: 10.1152/ajpendo.00420.2007. [DOI] [PubMed] [Google Scholar]
- 11.Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012;52(10):936–48. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 12.Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Advanced Drug Delivery Reviews. 1997;25(1):103–128. [Google Scholar]
- 13.Ribnicky DM, et al. Evaluation of botanicals for improving human health. Am J Clin Nutr. 2008;87(2):472S–5S. doi: 10.1093/ajcn/87.2.472S. [DOI] [PubMed] [Google Scholar]
- 14.Koga K, Kawashima S, Murakami M. In vitro and in situ evidence for the contribution of Labrasol (R) and Gelucire 44/14 on transport of cephalexin and cefoperazone by rat intestine. European Journal of Pharmaceutics and Biopharmaceutics. 2002;54(3):311–318. doi: 10.1016/s0939-6411(02)00116-9. [DOI] [PubMed] [Google Scholar]
- 15.Svensson A, Neves C, Cabane B. Hydration of an amphiphilic excipient, Gelucire (R) 44/14. International journal of pharmaceutics. 2004;281(1–2):107–118. doi: 10.1016/j.ijpharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, et al. Enhancing effect of Labrasol on the intestinal absorption of ganciclovir in rats. Drug Development and Industrial Pharmacy. 2011;37(12):1415–1421. doi: 10.3109/03639045.2011.582874. [DOI] [PubMed] [Google Scholar]
- 17.Yuksel N, et al. Enhanced bioavailability of piroxicam using Gelucire 44/14 and Labrasol: in vitro and in vivo evaluation. European Journal of Pharmaceutics and Biopharmaceutics. 2003;56(3):453–459. doi: 10.1016/s0939-6411(03)00142-5. [DOI] [PubMed] [Google Scholar]
- 18.Ribnicky DM, et al. Improved absorption and bioactivity of active compounds from an anti-diabetic extract of Artemisia dracunculus L. Int J Pharm. 2009;370(1–2):87–92. doi: 10.1016/j.ijpharm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandyopadhyay P, Ghosh AK, Ghosh C. Recent developments on polyphenol-protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012;3(6):592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- 20.Roopchand DE, et al. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chemistry. 2012;131:1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roopchand DE, et al. Biochemical Analysis and in Vivo Hypoglycemic Activity of a Grape Polyphenol-Soybean Flour Complex. J Agric Food Chem. 2012 doi: 10.1021/jf300232h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roopchand DE, et al. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol Res. 2013;68(1):59–67. doi: 10.1016/j.phrs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng DM, et al. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chemistry. 2012;135(4):2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grace MH, et al. Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J Agric Food Chem. 2013;61(28):6856–64. doi: 10.1021/jf401627m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plundrich N, et al. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. International Journal of Cosmetic Science. 2013;35:394–401. doi: 10.1111/ics.12057. [DOI] [PubMed] [Google Scholar]
- 26.Roopchand DE, et al. Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chemistry. 2013;141(4):3664–9. doi: 10.1016/j.foodchem.2013.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieman DC, et al. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. PLoS One. 2013;8(8):e72215. doi: 10.1371/journal.pone.0072215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minekus M, et al. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl Microbiol Biotechnol. 1999;53(1):108–14. doi: 10.1007/s002530051622. [DOI] [PubMed] [Google Scholar]
- 29.Ribnicky DM, et al. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1) Food Chemistry. 2014;142:349–57. doi: 10.1016/j.foodchem.2013.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribnicky DM, et al. Toxicological evaluation of the ethanolic extract of Artemisia dracunculus L. for use as a dietary supplement and in functional foods. Food and Chemical Toxicology. 2004;42(4):585–98. doi: 10.1016/j.fct.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Manach C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 32.Raskin I, Ripoll C. Can an apple a day keep the doctor away? Current Pharmaceutical Design. 2004;10(27):3419–3429. doi: 10.2174/1381612043383070. [DOI] [PubMed] [Google Scholar]
- 33.Grace MH, et al. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–15. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stachulski AV, Meng X. Glucuronides from metabolites to medicines: a survey of the in vivo generation, chemical synthesis and properties of glucuronides. Nat Prod Rep. 2013;30(6):806–48. doi: 10.1039/c3np70003h. [DOI] [PubMed] [Google Scholar]