Abstract
Significance: Mitochondrial ion channels/transporters and the electron transport chain (ETC) serve as key sensors and regulators for cellular redox signaling, the production of reactive oxygen species (ROS) and nitrogen species (RNS) in mitochondria, and balancing cell survival and death. Although the functional and pharmacological characteristics of mitochondrial ion transport mechanisms have been extensively studied for several decades, the majority of the molecular identities that are responsible for these channels/transporters have remained a mystery until very recently. Recent Advances: Recent breakthrough studies uncovered the molecular identities of the diverse array of major mitochondrial ion channels/transporters, including the mitochondrial Ca2+ uniporter pore, mitochondrial permeability transition pore, and mitochondrial ATP-sensitive K+ channel. This new information enables us to form detailed molecular and functional characterizations of mitochondrial ion channels/transporters and their roles in mitochondrial redox signaling. Critical Issues: Redox-mediated post-translational modifications of mitochondrial ion channels/transporters and ETC serve as key mechanisms for the spatiotemporal control of mitochondrial ROS/RNS generation. Future Directions: Identification of detailed molecular mechanisms for redox-mediated regulation of mitochondrial ion channels will enable us to find novel therapeutic targets for many diseases that are associated with cellular redox signaling and mitochondrial ion channels/transporters. Antioxid. Redox Signal. 21, 987–1006.
Introduction
Over the years, redox signaling has been recognized as an important system for cellular functions in all cell types/tissues (44, 135, 155, 177, 182). Cells maintain redox balance through generation and breakdown of reactive oxygen species (ROS) and nitrogen species (RNS). High levels of ROS and RNS are known to promote cell damage and death. However, recent evidence indicates that the production of low to moderate levels of ROS/RNS is also critical for proper regulation of many essential cellular processes (53, 155, 158, 175, 177, 182). Redox signaling has also gained recognition for its role in mediating diverse tissue-specific cellular functions across a wide range of cell types. For instance, redox signaling regulates muscle contraction/relaxation (145, 155, 169, 178), insulin secretion from pancreatic beta cells (191), metabolic cycles in liver (137), self-renewal and differentiation in stem cells (98), and T-cell homeostasis (67, 100, 120).
Since Szent-Gyorgyi first provided the initial concept in 1967 regarding a possible role for electronic mobility in biological materials as one of the fundamental mechanisms for cellular signaling (170), and the area of redox biology has witnessed a burst of major remarkable discoveries. These include (i) free radical superoxide (O2−) production by cellular enzymes (112), (ii) superoxide dismutase that can catalytically scavenge O2−, blunt the cascade of oxidation, and neutralize oxygen toxicity (113), and (iii) a membrane-bound enzyme complex, nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (11). Since free radicals are highly reactive with cellular lipids, DNA, and proteins and can bring about harmful oxidations, research in the early years concentrated on the chemistry of the individual ROS and the cellular damage that they bring about.
From the late 1970s to the early 1980s, there were several milestone discoveries, which brought the new concept that redox also serves as a signaling molecule to maintain cellular functions. Oberley and Buettner proposed that lower concentrations of O2− or hydrogen peroxide (H2O2) induce cell division (125). Mukherjee et al. showed that H2O2 is involved in the physiological response to insulin in adipocytes (119). These reports first showed that ROS were not simply involved in cellular toxicity. Another important finding is nitric oxide (NO) and its biological action on vascular smooth muscles. Mittal and Murad's group found that NO was capable of activating guanylate cyclase and generating cyclic GMP (10). This critical observation was later followed by the important finding of the identification of endothelial-derived relaxing factor as NO by Ignarro and Kadowitz’ group (76–78) and Moncada's group (133). From these discoveries, redox biology has finally broadened the field into signal transduction research and has drawn major attention from both general biologists and the medical research community.
O2− and NO are the primary ROS and RNS, respectively, that are produced in cells under physiological and pathophysiological conditions, which react with other molecules (and each other) to form a diverse form milieu of additional ROS and RNS such as H2O2 and peroxynitrite (ONOO−) (177, 182). Unlike most other endogenous second messengers for signal transduction, H2O2 and NO are highly active molecules that readily diffuse across cell membranes and cannot be stored inside cellular compartments/organelles. Therefore, their signaling capacity should be tightly controlled at the levels of their local biosynthesis and availability. Indeed, subcellular ROS/RNS concentration is regulated by a diverse subset of enzymes that are localized in each cellular compartment which contributes to their local generation and breakdown. Notably, mitochondria are the most important cellular compartment/organelle for tuning the cellular ROS/RNS concentration, as they contain various ROS/RNS generation systems as well as detoxification enzymes. The electron transport chain (ETC) located in the inner mitochondrial membrane (IMM) is the main cellular source of ROS (Fig. 1). There are a number of studies that suggest the presence of an NO synthase (NOS) in mitochondria (mtNOS), which produces NO in mitochondrial matrix (Fig. 1) (58, 194). However, the existence/function of mtNOS is highly controversial (28, 194) (see Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling and Regulation of ETC Activity by Mitochondrial NO Signaling sections).
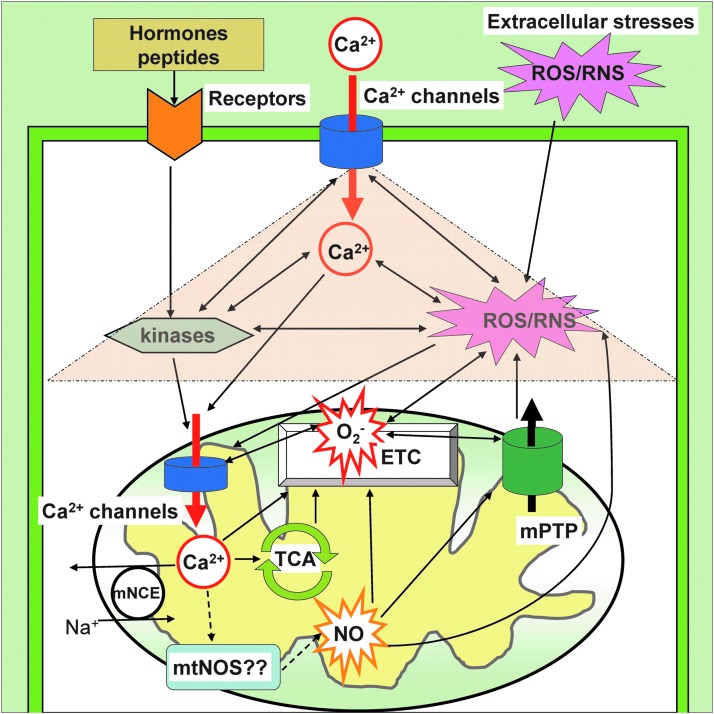
FIG. 1.
Overview of mitochondrial ion channels/transporters and redox signaling. Under physiological conditions, the balance of mitochondrial ROS/RNS is tightly controlled by multiple mitochondrial ion channels/transporters that are located at the IMM and the OMM (OMM structure is abbreviated in this figure). In pathophysiological conditions, plasma membrane receptor (orange) stimulation, extracellular Ca2+ elevation, and/or exogenous ROS/RNS elevation (pink burst) trigger cellular signal transduction via kinase cascades, cytosolic Ca2+ elevation, and cytosolic ROS/RNS generation. These are amplified through reciprocal action (pink triangle) and transmit into mitochondria through redox-dependent PTMs of mitochondrial ion channels/transporters, especially Ca2+ channels at the IMM (blue). Mitochondrial Ca2+ efflux is mainly regulated by an mNCE. The PTM s of mitochondrial ion channels/transporters change the activity of the Ca2+ influx mechanism at IMM and induce Ca2+ accumulation into the mitochondrial matrix, which affects the efficiency of electron flow, ATP production, and O2− generation at ETC. The produced O2− reacts with other molecules (also each other) and forms additional ROS. The existence of mtNOS and the production of NO in matrix are still controversial. The excessive ROS/RNS in mitochondria are released into the cytosol through specific mitochondrial ion channels/transporters such as the mPTP (green) and the IMAC (not shown in the figure). IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; mNCE, mitochondrial Na+/Ca2+ exchanger; mPTP, mitochondrial permeability transition pore; IMAC, inner membrane anion channel; ETC, electron transport chain; mtNOS, mitochondrial nitric oxide synthase; O2−, superoxide; PTM, post-translational modification; ROS, reactive oxygen species; RNS, reactive nitrogen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Under physiological conditions, the balance of mitochondrial ROS/RNS homeostasis is under tight control of mitochondrial ion channels/transporters that are located in IMM and outer mitochondrial membrane (OMM) (Fig. 1). In pathophysiological conditions, intra- or extra-cellular ROS/RNS stress/signaling is transmitted to mitochondria through redox-dependent post-translational modification (PTM) of mitochondrial ion channel/transporters, amplified by mitochondrial ROS/RNS generation systems, which subsequently release excessive ROS/RNS from mitochondria into the cytosol through specific mitochondrial ion channels/transporters such as the mitochondrial permeability transition pore (mPTP) and the inner membrane anion channel (IMAC), which triggers ROS-induced ROS release in neighboring mitochondria (26, 197) and finally, activates cell-death signaling (38) (Fig. 1). Therefore, mitochondrial ion channels/transporters serve as critical sensors and regulators of cellular redox signaling and also in balancing cell survival and death. Especially, the redox-mediated PTMs of mitochondrial ion channels/transporters and ETC serve as key mechanisms for the regulation of mitochondrial ROS/RNS generation (see Overview: Mitochondrial Ion Channels/Transporters and Redox-Dependent PTMs section). Although the functional and pharmacological characteristics of mitochondrial ion transport mechanisms have been well studied for several decades, the majority of the molecular identities that are responsible for the mitochondrial ion channels/transporters have remained a mystery until very recently, owing to the use of updated techniques such as whole genome screening, proteomics, and genetic manipulations (132) These efforts by many researchers finally reached a diverse array of breakthroughs in identifying the coding genes responsible for mitochondrial ion channels/transporters. These include mitochondrial Ca2+ uniporter pore (MCU) (15, 46) and its regulatory proteins (MICU1–3 and MCUR1) (110, 139, 141), mitochondrial Na+/Ca2+ exchanger (mNCE) (134), mPTP (24, 61), and mitochondrial ATP-sensitive K+ channel (mitoKATP) (54). This new information enables us to understand more detailed molecular and functional characterizations of mitochondrial ion channels/transporters and their roles in mitochondrial redox signaling.
In this review, we describe the importance of spatiotemporal control of ROS/RNS at mitochondrial levels and their regulation by mitochondrial ion channels/transporters and ETC, especially focusing on Ca2+-dependent regulation of mitochondrial redox signaling and redox-dependent PTMs. We will introduce recent progress in the molecular identification of mitochondrial ion channels/transporters. We will also discuss the proposed feedback mechanism as to how redox-dependent PTMs of mitochondrial ion channels/transporters and ETC can fine-tune mitochondrial redox signaling.
Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling
As originally proposed by Mitchell and Molye, mitochondria have been well studied as cellular “powerhouse” organelles for producing ATP (92), but recently, they have been recognized as important regulators for multiple signal transduction pathways, including redox signaling. The majority of mitochondrial ATP is produced by oxidative phosphorylation (OXPHOS) through the ETC, which is a concerted series of redox reactions catalyzed by multi-subunit enzymes that are embedded in the IMM (Figs. 1 and 2). Through the slippage of an electron from the ETC to molecular oxygen during OXPHOS, O2− is continuously produced as a primary oxygen-free radical in mitochondria (Figs. 1 and 2). This “constitutive” O2− generation brings mitochondria to the center stage for cellular redox regulation in all cell types/tissues. The ETC also functions as a proton (H+) pump to shuttle electrons within the mitochondrial intermembrane space (IMS) to build up the mitochondrial membrane potential (ΔΨm). Unlike other organelles, mitochondria possess unique double-membrane structures with distinct phospholipids and protein compositions, which enable mitochondria membranes to maintain ΔΨm (39, 92). Generally, to minimize the energy loss, the ion permeability of the IMM is kept low, but the IMM contains a variety of ion channels/transporters (128, 129, 132, 138) that regulate the efficiency of redox reactions at the ETC (29, 129). As summarized in Figure 1, mitochondrial ion channels/transporters receive cell signaling information from the cytosolic side through (i) cytosolic Ca2+ elevation, (ii) phosphorylation by Ca2+- and/or redox-dependent kinases, and (iii) cytosolic ROS/RNS-dependent PTMs (see also Overview: Mitochondrial Ion Channels/Transporters and Redox-Dependent PTMs section) (Fig. 1). These three elements can receive extracellular signals such as plasma membrane receptor stimulation, extracellular Ca2+, and/or ROS/RNS elevation and amplify them through cross-talk between themselves (29, 51). Next, these signal transduction pathways transmit into mitochondrial matrix by changing the function of mitochondrial ion channels/transporters, especially by changing the activities of Ca2+-influx mechanisms at the IMM (Figs. 1 and 2). Ca2+ accumulation into mitochondrial matrix through Ca2+ channels/transporters at the IMM affects the efficiency of electron flow, ATP production, and O2− generation at the ETC either directly (e.g., by changing complex V activity) (174) or indirectly (through changing tricarboxylic acid [TCA] cycle activity and ΔΨm) (63) (Fig. 2). Thus, mitochondrial ion channels/transporters that are responsible for Ca2+ influx and H+ pumps (ETC) at the IMM are important regulators for mitochondrial redox signaling (Figs. 1 and 2). In ETC and Mitochondrial ROS Generation section, we describe the interaction between mitochondrial ROS generation and ETC function. We show the detailed mechanism of how the ETC regulates mitochondrial ROS generation and also of how ROS itself affects ETC function. Next, we summarize updated information of how the mitochondrial Ca2+ influx mechanism regulates mitochondrial ROS generation (see Mitochondrial Ca2+ Influx Mechanism and Mitochondrial ROS Generation section). Here, we also discuss the possibility of whether ROS signaling itself can modulate mitochondrial Ca2+ channels/transporters through redox-dependent PTMs.
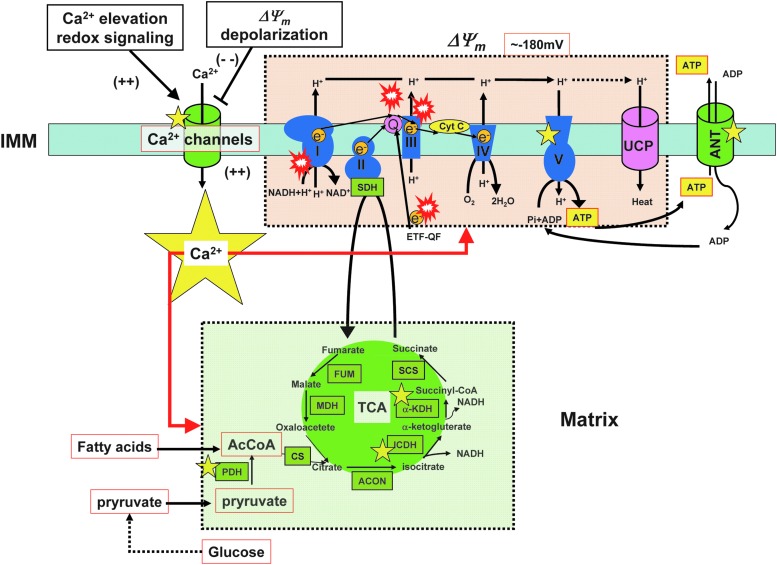
FIG. 2.
Mitochondrial ETC and mitochondrial ROS generation. In a healthy eukaryotic cell, mitochondria generate more than 90% of the total intracellular ATP through the TCA cycle (green area) and OXPHOS (pink area). During OXPHOS, O2− is continuously produced as a primary oxygen free radical in mitochondria. NADH is produced from cytosolic glucose oxidation and the TCA cycle and passes electrons to NADH dehydrogenase (complex I). Then, complex I transfers electrons to CoQ10. CoQ10 can also receive electrons from succinate (complex II) and glycerol-3-phosphate dehydrogenase (NAD+). Electrons from reduced CoQ10 are then transferred to cytC oxidoreductase (complex III). Next, complex III transfers the electrons to cytC and the electron transport continues COX (complex IV) and molecular oxygen. Electron transfer processes through complexes I, III, and IV produce Δp, which, in turn, are used to drive ATP synthase (complex V). When Δp increases, electron transport in complex III is partially inhibited and results in an increased backup of electrons to CoQ10 for binding to molecular oxygen, leading to the generation of O2−. In addition, when the electron-transport efficiency at complex I is suddenly inhibited or complex I activity is changed, this mechanism also generates O2−. Red “explosion” symbols indicate places where O2− production occurs. Yellow star symbols indicate enzymes/channels that are regulated by [Ca2+]mt. ETF-QO, electron transferring flavoprotein-quinone oxidoreductase; UCP, uncoupling protein; ANT, adenine nucleotide translocase; PDH, pyruvate dehydrogenase; CS, citrate synthase; ACON, aconitase; ICDH, isocitrate dehydrogenase; α-KDH, α-ketoglutarate dehydrogenase; SCS, succinyl-CoA synthetase; SDH, succinate dehydrogenase; FUM, fumarase; MDH, malate dehydrogenase; TCA, tricarboxylic acid; OXPHOS, oxidative phosphorylation; [Ca2+]mt, mitochondrial matrix Ca2+ concentration; CoQ10, coenzyme Q10; COX, cytochrome c oxidase; cytC, cytochrome C. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
As briefly mentioned in the Introduction section, not only ROS but also RNS exerts a broad spectrum of functions in mitochondria (28, 29, 33, 58, 195). Compared to O2−, NO has more low reactivity and hydrophobicity, which enables NO to diffuse large distances both within and between cells (176). Therefore, the importance of spatiotemporal control of NO production is highlighted by the localization of NOS isoforms and their subcellular localization-dependent activities (58, 118, 126). Three types of NOSs (neuronal NOS [nNOS], inducible NOS [iNOS], and endothelial NOS [eNOS]) are well characterized, associated with various biological membranes (e.g., plasma membrane, nucleus, Golgi, and sarco/endoplasmic reticulum [SR/ER]), and activated through different mechanisms (58, 118, 126). It is reasonable to assume that NO synthesized at the cytosol can diffuse into mitochondria and modulate their functions. Indeed, cytochrome c oxidase (COX) (complex IV) is one of the main targets of NO, and the inhibition of complex IV by NO binding has been well established (28, 194). In the 1990s, several laboratories proposed that mitochondria also serve as one of the cellular sources for NO production through mtNOS (Figs. 1 and 3), but the existence of mtNOS is still controversial (see also Introduction section). In Regulation of ETC Activity by Mitochondrial NO Signaling section, we will describe the detailed mechanism of how NO regulates mitochondrial ROS generation by changing ETC function and also discuss the controversy of the existence of mtNOS. In Interaction of NO, Mitochondrial Ca2+, and ROS Generation section, we summarize the cross-talk between Ca2+, NO, and ROS in mitochondria.
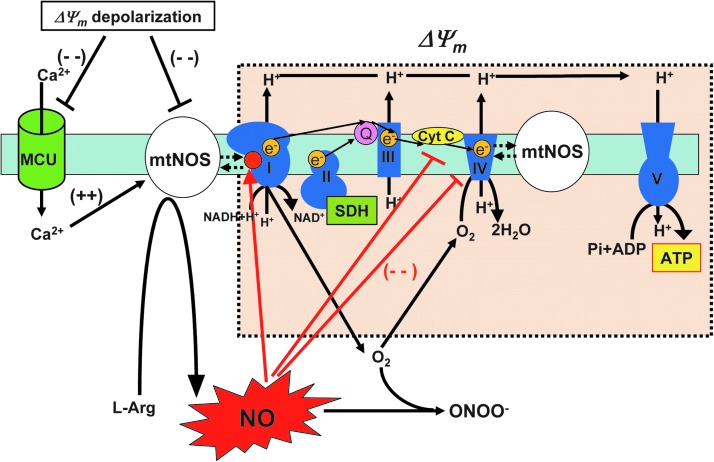
FIG. 3.
Regulation of ETC activity by mitochondrial NO signaling. The existence of mtNOS and the production of NO in matrix are still controversial (see Introduction, Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling, Regulation of ETC activity by mitochondrial NO signaling, and Interaction of NO, mitochondrial Ca2+, and ROS generation sections). NO diffused from cytosol to matrix or produced in mitochondrial matrix by mtNOS can reversibly interact with (complex IV) in competition with oxygen. NO inhibits the electron flow between cytochrome b and cytC at complex III. NADH dehydrogenase (complex I) receives S-nitrosylation (red circle), which shows reversible inhibition of complex I activity. Therefore, mitochondrial NO interferes with ETC activity (electron flow), and excessive mitochondrial NO production results in a decrease of ATP production, depolarization of ΔΨm and an increase in ROS generation. There are several reports showing that this tight functional coupling of NO and complex I or IV is derived from the physical interaction between mtNOS and complex I or IV. Complex I, NADH dehydrogenase; complex II, SDH; complex III, cytochrome bc1 complex; complex IV, COX; complex V, ATP synthase; ΔΨm, mitochondrial membrane potential. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
At the IMM, in addition to Ca2+ channels/transporters, several K+ channels, including mitoKATP and mitochondrial Ca2+-activated K+ channel (mitoKCa), are present. These channels indirectly modulate ROS generation in pathophysiological conditions such as ischemic/reperfusion (I/R) in the heart (36, 124, 127, 128). Opening of mitoKATP and mitoKCa, either by cellular signal transduction or by extracellular stress such as I/R, depolarizes ΔΨm, which reduces the driving force for Ca2+ influx, thereby attenuating mitochondrial Ca2+ overload and excessive ROS generation. Consequently, prevention of matrix Ca2+ overload and ROS over-generation protects the heart against cell death (cardioprotection). In ETC and Mitochondrial Redox Signaling section, we summarize updated information regarding the molecular identities of these K+ channels and discuss the possibility of redox-dependent modulation of mitochondrial K+ channels.
Under pathological conditions, the release of ROS/RNS and proapoptotic proteins from mitochondria act as important amplifiers for accelerating the ROS-induced mitochondrial ROS generation as well as cell death signaling. These redox release channels include mPTP (see Mitochondrial Permeability Transition Pore and Redox Signaling section) and IMAC (see IMAC and Redox Signaling section). Voltage-dependent anion channels (VDAC) are redox-sensitive channels that are located at the OMM and regulate OMM permeability (see VDAC and Redox Signaling section) (64). O2− can trigger VDAC-dependent OMM permeabilization, which subsequently activates apoptotic cascades (99). These channels receive redox-dependent PTMs and often act as an amplifier or positive feedback core to accelerate the pathological processes due to their dual role as sensors/regulators of redox signaling.
Overview: Mitochondrial Ion Channels/Transporters and Redox-Dependent PTMs
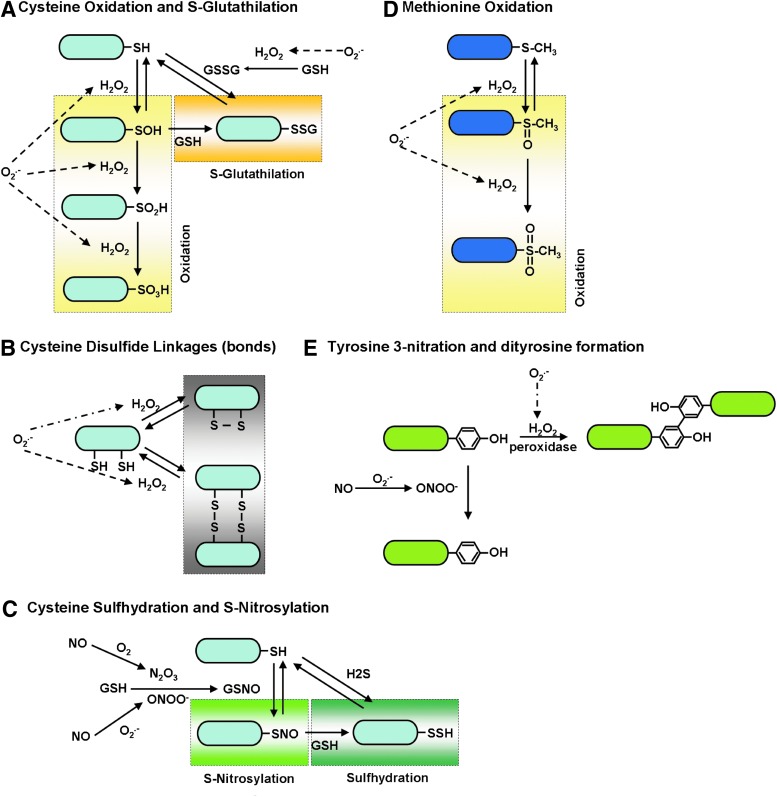
ROS/RNS can alter protein structures/functions, including channel-subunit proteins, by modifying critical amino-acid residues, including (i) cysteine (30, 144) and methionine oxidation (30, 74, 157), (ii) cysteine S-glutathionylation (72, 117), (iii) cysteine disulfide bonds (175), (iv) cysteine surhydration (136), (v) cysteine S-nitrosylation (70, 71, 104), (vi) tyrosine 3-nitration (102), and (vii) dityrosine formation (175) (Fig. 4). In addition, these redox-dependent PTMs are known to change the biophysical properties of ion channels/transporters that are located at plasma membranes or organelle membranes (41, 65, 74, 115) such as their activation/deactivation profiles, Ca2+ or voltage dependency, and interaction affinities between regulatory proteins (Fig. 5). In addition, ion channels/transporters are frequently regulated through phosphorylation by upstream protein kinases (45), whose activities are modulated by redox-dependent PTMs (Fig. 1). For instance, protein kinase A (PKA), protein kinase C (PKC) (60) and protein kinase D (PKD) (169), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (50) are activated either by directly receiving redox-dependent PTMs (for PKA, PKC, and CaMKII) or by the redox-sensitive upstream kinases (for PKC, PKD, and CaMKII), which can ultimately modulate voltage-gated Ca2+ channels at the plasma membrane (186). Therefore, it is conceivable that the mitochondrial ion channels/transporters are also subject to redox-dependent PTMs and phosphorylation through redox-sensitive kinases, which eventually regulate mitochondrial ROS/RNS homeostasis (see Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling section and Fig. 1). Since the molecular identities of the main mitochondrial ion channels/transporters have just been discovered (see Introduction section), investigation of the PTMs of these proteins has just started and the role of mitochondrial redox regulation has begun to emerge (see Mitochondrial Ca2+ Influx Mechanism and Mitochondrial Redox Signaling, Mitochondrial K+ Channels and Mitochondrial Redox Signaling, Mitochondrial Permeability Transition Pore and Redox Signaling, IMAC and Redox Signaling, and DAC and Redox Signaling sections). In addition, the components of the ETC are also targets of redox-dependent PTMs, which influence the efficiency of electron flow, ATP synthesis and ROS production (see ETC and Mitochondrial Redox Signaling section).
FIG. 4.
Redox-dependent PTMs. The redox-dependent PTMs are shown. These include cysteine and methionine oxidation (A, D), cysteine S-glutathionylation (A), cysteine disulfide bonds (B), cysteine surhydration (C), cysteine S-nitorosylation (C), tyrosine 3-nitration (E), and dityrosine formation (E). GSH, glutathione; GSSG, glutathione disulfide; GSNO, S-nitrosoglutathione; H2S, hydrogen sulfide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 5.
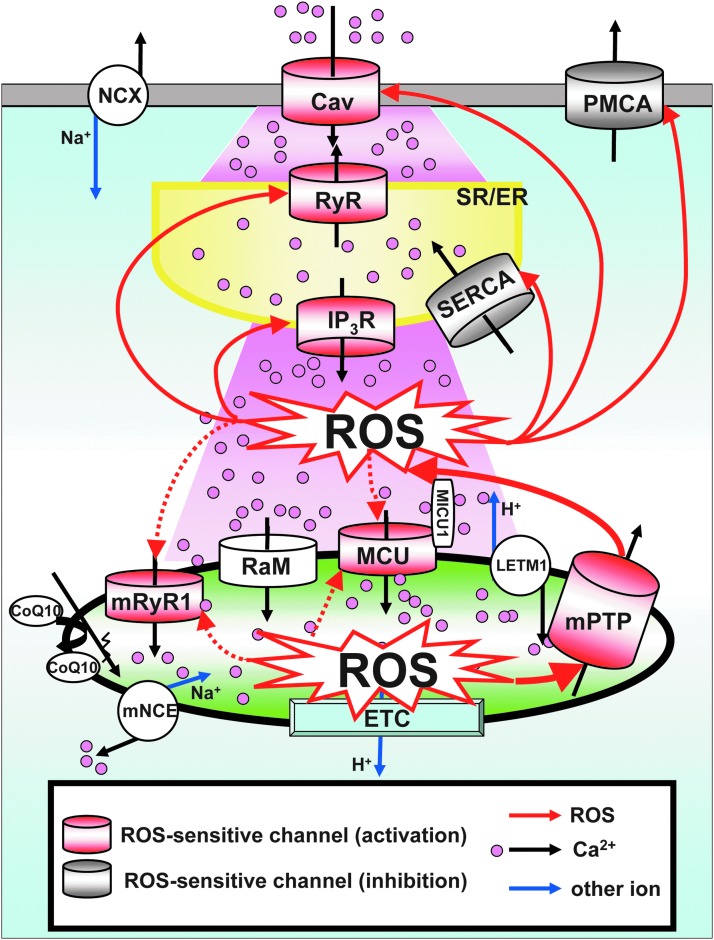
ROS-dependent regulation of cellular Ca2+ handing and ROS-induced ROS generation. Schematic diagram of ROS-dependent regulation of cellular Ca2+ handing and ROS-induced ROS generation. OMM structure is abbreviated in this figure. Red channels/transporters can be activated by the redox-dependent PTM. Gray channels/transporters can be inhibited by the redox-dependent PTMs. The redox modulations of white channels/transporters are unknown (no report) or still controversial. Voltage-gated Ca2+ channels (Cav) at the plasma membrane are phosphorylated by redox-dependent kinases and activated. RyR and IP3R at SR/ER are activated by redox-dependent PTMs, which increase Ca2+ release from SR/ER. SERCA inhibited by irreversible oxidative modifications. PMCA is inhibited by either direct or indirect redox-dependent modifications. Thus, redox signaling generally increases cytosolic [Ca2+]c. This [Ca2+]c elevation feedbacks to mitochondria through an increase in the Ca2+ influx to mitochondrial matrix, which results in a positive feedback loop of ROS-induced ROS generation. mRyR1 and MCU, which are responsible for mitochondrial Ca2+ influx mechanism, are capable of receiving redox-dependent modulation. Excessive ROS/RNS are released through mPTP or IMAC (not shown in this figure) to the cytosol. Mitochondrial Ca2+ efflux is mainly regulated by an mNCE. During chronic heart failure, elevation of cytosolic [Na2+]c accelerates mitochondrial Ca2+ efflux by mNCE and blunted [Ca2+]mt accumulation, followed by an increase in the mitochondrial ROS level through the reduction of the NADPH-dependent antioxdative capacity at the matrix to control the mitochondrial H2O2 level. Thus, elevated [Na+]c and mNCE activity also contributes to the regulation of [Ca2+]mt homeostasis and ROS production, especially during chronic heart failure (see detailed in Mitochondrial Ca2+ Influx Mechanism and Mitochondrial ROS Generation section). PMCA, plasma membrane Ca2+ ATPase; RaM, rapid mode of uptake; LETM1, leucine zipper-EF-hand containing transmembrane protein 1; NCX, Na+/Ca2+ exchanger at plasma membrane; SR/ER, sarco/endoplasmic reticulum; SERCA, SR/ER Ca2+-ATPase; [Ca2+]c, cytosolic Ca2+ concentration; [Na2+]c, Na2+ concentration; H2O2, hydrogen peroxide; MCU, mitochondrial Ca2+ uniporter pore; NADPH, nicotinamide adenine dinucleotide phosphate; RyR, ryanodine receptor; mRyR1, mitochondrial ryanodine receptor type 1; IP3R, IP3 receptors. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
ETC and Mitochondrial Redox Signaling
ETC and mitochondrial ROS generation
ROS are generated by several different cellular sources, which include membrane-associated NADPH oxidase, cytosolic xanthine and xanthine oxidase, and the ETC at the IMM (177) (see Introduction section). Among those, mitochondria are the main source of ROS and play a central role in redox signaling in all cell types/tissues (Figs. 1 and 2). O2− is the primary oxygen free radical via the slippage of an electron from the ETC to molecular oxygen during OXPHOS (177) (see Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling section and Fig. 2).
The most prominent contribution of mitochondria to cellular metabolism is their capacity to generate ATP through the TCA cycle and OXPHOS (Fig. 2). The main driving force of OXPHOS is known as “chemiosmosis” (63). Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient (protonmotive force: Δp), which is determined by both ΔΨm, and ΔpH components across the IMM (Δp=ΔΨm+ΔpH) (92). This large driving force for H+ influx (Δp) is used by the mitochondrial ATP synthase (complex V) to produce ATP (Fig. 2). OXPHOS through the ETC, which is a concerted series of redox reactions, is catalyzed by four multi-subunit enzymes in the IMM (complex I–IV) and two soluble factors, cytochrome c (cytC) and coenzyme Q10 (CoQ10), which function as electron shuttles within the IMS (Fig. 2). In a healthy eukaryotic cell, more than 90% of the total intracellular ATP is generated by mitochondria. The basic assumption of this chemiosmotic theory is derived from the important observation that the IMM is generally impermeable to ions, but it maintains the permeability of H+ by its unique lipid composition of the IMM (39), which also contributes to maintaining the structure of cristae and enhances the efficiency of ETC activity, possibly through the facilitation of forming super complexes of the respiratory chain at the IMM (166) In the ETC, electrons transfer from electron donors such as NADH and CoQ10 to an electron acceptor O2 through a series of redox reactions (Fig. 2). This reaction generates a H+ gradient across IMM and complex I, III, and IV works as H+ pumps. O'Rourke's group showed in cardiomyocytes that the majority of mitochondrial ROS production is from complex III (129), whereas Wei and Dirksen proposed that O2− from complex I triggers transient mPTP opening in cardiac and skeletal muscles (189). As mentioned in Introduction section, it has also been shown that mitochondrial ROS serves as an important signaling platform for T-cell receptor signaling activation and the regulation of T-cell homeostasis [see reviews (67, 120)]. Kaminski and Gulow's group demonstrated a crucial role of complex-I-mediated mitochondrial ROS production for T-cell receptor-mediated PKC θ activation (83, 84). They showed that the ETC switches from an ATP-producing to an oxidative signaling function on T-cell receptor activation, and this mechanism is possibly due to reverse electron transfer by a highly reduced pool of ubiquinone, which is a major mechanism of mitochondrial ROS generation via complex I (85). Meanwhile, Chandel's group demonstrated that ROS production from complex III is specifically required for increasing interleukin-2 expression during T-cell receptor activation (159). Thus, it still remains to be determined which sites (complex I or III) of ROS production dominantly regulate tissue-specific cellular redox signaling and function under physiological or pathophysiological conditions.
As mentioned earlier, the main route for the H+ to flow into mitochondrial matrix, driven by the electrochemical gradient, is through complex V. Another route for the proton inflow is named “proton leak,” attributed by uncoupling proteins, and this process can also modulate the ATP/ADP ratio (163) (Fig. 2). Proton leak is known to significantly contribute to the control of respiration in mitochondria in state 4 and to some extent in state 3. Therefore, uncoupling proteins also play an important role in sustaining proton leak, preventing excessive H+ gradient, and, subsequently, avoiding excessive ROS production (88, 109).
By using mitochondrial-targeted circularly permuted yellow fluorescent protein, recent studies from our collaborating groups showed a “stochastic” and “transient” O2− burst from either single or restricted clusters of interconnected mitochondria or isolated mitochondria, termed as a “mitochondrial superoxide flash” (mSOF) (188). Interestingly, this mSOF is usually associated with a transient depolarization of ΔΨm (160, 189). mSOF generation is currently proposed as a result of a small increase in constitutive ROS production in mitochondria, which transiently opens a large channel mPTP to evoke transient ΔΨm depolarization and subsequently, stimulates the ETC to produce a burst in O2− production. This idea is supported by the previous observations that the mPTP opens and closes transiently (“flickers”) at its low conductance state (18, 66). Since observed mSOF frequencies vary widely across different cell types and the experimental conditions, our future studies will attempt to use these flashes as a biomarker for the cellular metabolic activity and the oxidative status in physiological and pathophysiological conditions (160). Further studies will also clarify the contribution of altered mSOF activity to ROS overproduction and metabolic dysfunction in a wide range of mitochondrial diseases and oxidative stress-related disorders. It should be also mentioned that the mSOF activity contains a small signal component from mitochondrial pH fluctuation, but much smaller than that from O2− (190). Future studies will also be directed toward inventing a novel O2−-sensitive biomarker to reduce its pH sensitivity.
Regulation of ETC activity by mitochondrial NO signaling
In conjunction with the hydrophobic nature of mitochondria as a cellular compartment, it is a reasonable idea that mitochondria are a significant cellular sink for NO (161) (see Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling section). Indeed, since the discoveries of the biological function of NO (see Overview: Mitochondrial Ion Channels/Transporters, ETC, and Mitochondrial Redox Signaling section), cellular targets of NO have been extensively investigated and reversible binding of NO to COX (complex IV) in mitochondria has been well characterized (28, 194). Through investigation of NO function in mitochondria, it was proposed in the 1990s from several laboratories that mitochondria possess their own isoform of NOS, termed “mtNOS,” which can produce NO in mitochondrial matrix similar to other NOS isoforms observed in the cytosol (Figs. 1 and 3) (13, 14, 56, 59, 91, 143, 172) [see reviews (28, 58, 194)]. However, a number of studies and comments show the conflicting evidence about the existence of mtNOS (28, 43, 96, 97, 173, 184, 185) (see Figs. 1 and 3). We include both types of papers in this review, as they provide important insights into the development of the knowledge of mitochondrial NO functions.
[NO]mt is around nanomolar ranges (3, 4, 58, 179, 194) and at this concentration of NO, it is able to compete with oxygen and interact with COX reversibly (3, 4, 58, 194), interfere with electron flow that results in a decrease in ATP production (62), depolarization of ΔΨm (180, 181), and an increase in ROS generation (142, 156) (Fig. 3). Several studies showed that complex I is also a target for NO and receives S-nitrosylation (Fig. 4C), which causes reversible inhibition of complex I activity (25, 42, 55) (Fig. 3). Recently, Murphy's group demonstrated that the reversible inhibition of complex I by selective S-nitrosylation of Cys39 on the ND3 subunit during the crucial first minutes of the reperfusion of ischemic hearts reduces oxidative damage and tissue necrosis by decreasing ROS production from mitochondria (40). Several groups reported that this tight functional coupling of NO and complex I or IV is derived from the physical interaction between mtNOS and complex I or IV (55, 140) (Fig. 3). Moreover, Poderoso's group reported that NO exerts inhibition of the electron flow between cytochrome b and cytC at complex III in addition to complex IV (142, 143). These findings indicate that NO can regulate ETC activity through direct binding of NO and/or the redox-dependent PTMs, which eventually change the efficiency of mitochondrial ROS generation.
Though the significant role of NO on ETC has been well recognized, there are a number of contradictory reports with regard to the existence and molecular identity of an mtNOS as shown earlier [see reviews (28, 58, 126, 194, 195)]. The diversity of the results is possibly derived from the technical issues used in the earlier studies, including (i) the use of isolated mitochondria and their purity; (ii) the reliability of biochemical and immunohistochemical assays using specific antibodies; and (iii) lack of suitable methods for measurement of mitochondrial NO apart from cytosolic NO. Recently, genetic approaches were also applied to clarify the molecular identity of a putative mtNOS. Through biochemical analysis, eNOS and iNOS are found in mitochondria (79), but the eNOS or iNOS knockout mice still possess NOS activity in mitochondria (86). Meanwhile, no signal was detected in mitochondria from nNOS-knockout mice, suggesting that nNOS is involved in mitochondrial NOS activity (86). This observation is supported by the recent report from the Ritter's group using nNOS-overexpression mice showing that nNOS overexpression causes nNOS translocation into mitochondria, which results in cardioprotection after I/R injury by reducing mitochondrial ROS generation (32). Putzke et al. proposed using nNOSα knockout mice that a splice variant of nNOS is mtNOS (146). However, further studies will be required to clarify whether there is functional nNOS activity in mitochondria in physiological conditions.
Mitochondrial Ca2+ Influx Mechanism and Mitochondrial Redox Signaling
Mitochondrial Ca2+ influx mechanism and mitochondrial ROS generation
As first shown in early studies between the 1960s and 1970s, Ca2+ uptake into the mitochondrial matrix stimulates ATP synthesis (66, 132). Ca2+-dependent ATP generation is explained as follows: (i) Three rate-controlling dehydrogenases of the TCA cycle are activated by Ca2+; (ii) Ca2+-dependent acceleration of TCA cycle increases the production of the primary electron donor for ETC (NADH); and (iii) complex V is activated by Ca2+ (Fig. 2) (63). In addition, regeneration of NADPH-dependent antioxidative capacity is also stimulated by Ca2+, because regeneration of NADPH requires products of the TCA cycle (193). At the resting state, the electrochemical driving force for Ca2+ uptake is provided by ΔΨm across the IMM (see Fig. 2). The mitochondrial Ca2+ influx mechanism was initially considered a result of a single channel/transporter function (MCU) that was proposed more than 30 years ago, principally due to nearly complete inhibition by ruthenium red and lanthanides [see review (66)]. However, updated reports clearly show that additional Ca2+ channels/transporters, whose characteristics are different from the original MCU theory, also exist and, indeed, participate in the mitochondrial Ca2+ influx mechanism, including a rapid mode of uptake (RaM) (31, 168), Leucine zipper-EF-hand containing transmembrane protein 1 (LETM1) (81), CoQ10 (22), and mitochondrial ryanodine receptor (RyR) type 1 (mRyR1) (19, 20, 154) [see also review (132)] (Fig. 5).
If Ca2+ is taken into the mitochondrial matrix down its electrochemical gradient without transport of another ion such as through MCU (37, 90), there is a net transfer of two positive charges into matrix, resulting in a drop of ΔΨm for each Ca2+ influx through MCU. However, Ca2+ accumulation by MCU is counteracted predominantly by mNCE (87, 128, 129, 149) to maintain mitochondrial matrix Ca2+ concentration ([Ca2+]mt). Moreover, the Ca2+-stimulated respiration will not only compensate the loss of ΔΨm by the efflux of H+ through the ETC but also produce a net gain of ATP. Thus, the physiological range of Ca2+ uptake into the mitochondrial matrix stimulates ATP synthesis without loss of ΔΨm. However, if sustained cytosolic Ca2+ concentration ([Ca2+]c) elevation occurs, the [Ca2+]c triggers excessive mitochondrial Ca2+ uptake (i.e., mitochondrial Ca2+ overload), followed by ΔΨm depolarization, a decrease in ATP production, acceleration of ROS generation, and activation of cell death pathways under various pathological conditions; such as under I/R of the heart (29, 101), excitotoxicity of neurons (131), vascular hypoxia (111), and inflammation in endothelial cells (153). Indeed, application of Ca2+ to isolated mitochondria stimulates ROS formation at the level of either complex I (167) or complex III (34) in the ETC, suggesting that the mitochondrial Ca2+-influx mechanism alone can activate mitochondrial ROS generation (Fig. 2). We also reported that blocking mitochondrial Ca2+ uptake by Ru360 can significantly inhibit mitochondrial ROS generation in intact striatal neurons under intense N-methyl-d-aspartate (NMDA) receptor activation (excitotoxicity), indicating that mitochondrial ROS increase requires Ca2+ entry into mitochondria (49). However, it is still not clear (i) which mitochondrial Ca2+ channel(s) is (are) responsible for the Ca2+ influx mechanism and (ii) how the Ca2+ that enters into mitochondria enhances ROS generation [see also reviews (29, 35)]. For the first question, Cheng`s group demonstrated that the knocking down of MCU or its regulatory protein MICU1 markedly diminishes mSOF activity under the cytosolic Ca2+ elevation by hyperosmotic stress (75), suggesting that at least Ca2+ influx through MCU results in O2− generation in mitochondria. Moreover, Rizzuto's group showed that MCU-overexpressing cells were more efficiently killed after treatment with H2O2, indicating that mitochondrial Ca2+ influx through MCU enhances the effect of oxidative stress to mitochondria (46). Therefore, these observations support the initial idea that at least, mitochondrial Ca2+ influx through MCU regulates mitochondrial ROS generation, especially in pathophysiological conditions (Ca2+ overload). Future studies need to address whether Ca2+ influx through other mitochondrial Ca2+ channels/transporters (132) can influence mitochondrial ROS generation. For the second question, it is possible that different levels of [Ca2+]mt (physiological modest increases or pathological excessive increases) lead to different mechanisms for ROS generation (29). For instance, stimulation of the TCA cycle and OXPHOS by physiological Ca2+ increases would simply enhance ROS output by making the metabolic rate faster and consume more O2, which results in more electron leakage from the ETC (Fig. 2). The other possibility is that mitochondrial Ca2+ increases the NO level (e.g., through mtNOS) (see Interaction of NO, Mitochondrial Ca2+, and ROS Generation section), which inhibits ETC efficiency as described in Regulation of ETC Activity by Mitochondrial NO Signaling section (Fig. 3). Finally, [Ca2+]mt overload can lead to mPTP-mediated ROS generation.
In addition to the mechanism of mitochondrial ROS generation through excessive mitochondrial Ca2+ influx, a decrease in mitochondrial Ca2+ uptake can also increase mitochondrial ROS level in certain pathophysiological conditions. A few studies have attempted to show altered mitochondrial Ca2+ uptake during heart failure, but limited evidence suggests that mitochondrial Ca2+ uptake is reduced in failing cardiomyocytes (105, 116). O'Rourke and Maack extensively demonstrated that elevation of cytosolic Na2+ concentration ([Na2+]c) in heart failure dictates mitochondrial ROS generation through a decrease in mitochondrial Ca2+ uptake (93, 105, 107) [see reviews (106, 122, 130)]. Since mitochondrial Ca2+ efflux is mainly regulated by an mNCE as described earlier, [Na+]c accelerates mitochondrial Ca2+ efflux and blunted [Ca2+]mt accumulation. They demonstrated using a guinea pig heart failure model (pressure overload model using aortic banding) that reduced [Ca2+]mt decreases dehydrogenase activity and TCA cycle efficiency (see also Fig. 2), followed by sustained oxidation of NADH and NADPH (93, 105, 107). This, in turn, reduces the NADPH-dependent antioxdative capacity at the matrix to control the mitochondrial H2O2 level (8, 9) and increases the mitochondrial ROS level. They also discussed that a decrease in NADH and NADPH regeneration could also explain a mismatch in energy supply and demand during heart failure (106, 122). Thus, elevated [Na+]c and mNCE activity also critically contributes to the regulation of [Ca2+]mt homeostasis and ROS production especially during heart failure.
The next question is whether mitochondrial ROS itself can modify or modulate the mitochondrial ion channels/transporters that are responsible for Ca2+ influx through PTMs. Since the molecular identity of MCU channel pore (15, 46) and its regulatory proteins (110, 139, 141) have just been discovered, the information to answer this question is still quite limited. There is still no report that MCU or its regulatory proteins possess these PTMs through redox signaling and change its channel functions. Anderson's group recently reported that there are two CaMKII phosphorylation candidate motifs at the N-terminus of MCU. These nonphosphorylation mimic MCU mutants do not respond to CaMKII-dependent MCU current activation under mitoplast patch clamp, suggesting that MCU can undergo PTMs by CaMKII (phosphorylation) and play a role in the regulation of MCU function (82). Since the oxidation of methionine residue in CaMKII can activate this kinase (50) (see also Fig. 4D), this report indicates that there is a tight interaction between ROS level and MCU channel function (Fig. 5). They also demonstrated that mitochondrial CaMKII inhibition in the heart is protective against I/R injury, myocardial infarction, and neurohumoral injury, suggesting that MCU current inhibition is beneficial for reducing cell death by certain pathological myocardial oxidative stresses. Further observations will be required to conclude whether oxidized CaMKII can be a direct modulator for MCU through its direct phosphorylation and can regulate mitochondrial ROS levels in heart failure.
The next possible candidate for a redox-sensitive Ca2+ influx (mitochondrial Ca2+ channel) at mitochondria is mRyR1 (19, 20, 154). mRyR1 was discovered as the first mitochondrial Ca2+ influx mechanism with a known molecular identity (19, 20, 154). It is well established that redox-dependent PTMs regulate the activity of RyRs localized at cardiac and skeletal SR, which shows enhanced Ca2+ leak from SR. RyRs (115) at SR received disulfide bond, S-nitrosylation, S-glutathionylation, cysteine oxidation (sulfonic acid), and phosphorylation by oxidized CaMKII (123) (see also Fig. 4). Therefore, it is possible that the redox-dependent PTMs of mRyR1 can contribute to the [Ca2+]mt overload in pathophysiological conditions (Fig. 5). Further studies will be required to determine the PTMs of mRyR1 and to characterize their physiological and pathophysiological relevance to mitochondrial Ca2+ uptake.
Unlike mitochondrial channels, the redox modification of Ca2+ channels in the cytosolic Ca2+ cycling have been well established (Fig. 5) [see reviews (23, 73)]. For instance, voltage-gated Ca2+ channels located at the plasma membrane are phosphorylated by oxidized CaMKII, and Ca2+ influx from the extracellular space to the cytosol increases (164, 165). Ca2+ release channels at SR/ER, RyR, and inositol 1,4,5-trisphosphate receptors (IP3R) are activated by a redox-dependent PTM (73). SR/ER Ca2+-ATPase (SERCA) can be modified by cysteine oxidation or tyrosine nitrosylation (Fig. 4A, E) (155). Low levels of ROS reversibly increase SERCA activity through oxidation, but higher levels of ROS cause its inactivation as a result of irreversible oxidative modifications (Fig. 4A). Plasma membrane Ca2+ ATPase (PMCA) is inhibited through either its direct oxidation (Fig. 4A) or methionine oxidation in its binding partner calmodulin (Fig. 4D) (73). Thus, redox signaling generally increases [Ca2+]c through (i) facilitation of Ca2+ influx through the plasma membrane, (ii) enhancing Ca2+ release from SR/ER, and (iii) inhibiting Ca2+ export from cytosol (Fig. 5). This [Ca2+]c elevation feedbacks to mitochondria through an increase in the Ca2+ influx to mitochondrial matrix, which results in a positive feedback loop of ROS-induced-ROS generation. In addition, as mentioned earlier, a decrease in the mitochondrial Ca2+ influx (by the elevation of [Na+]c and mNCE function) can also increase mitochondrial ROS level through the reduction of NADPH-dependent antioxidative capacity in the matrix.
Therefore, the cross-talk between mitochondrial ROS regulation and cellular Ca2+ cycling determines the amplitude of cellular redox signaling.
Interaction of NO, mitochondrial Ca2+, and ROS generation
As described in Introduction, Overview: Mitochondrial Ion Channels/Transporters, ETC, Mitochondrial Redox Signaling, the existence and functional role of mtNOS is highly controversial. It is still challenging to reach an unequivocal conclusion using currently available approaches and determine whether mitochondria contain NOS, whether this NOS is able to produce physiologically relevant amounts of NO, and whether there are tissue-specific distribution and functions of mtNOS. In this section, we introduce recently published reports that attempt to characterize the interaction between mitochondrial NO level, [Ca2+]mt, and ROS generation on the assumption that NOS activity exists in mitochondria.
Several groups reported that mtNOS has similar characteristics to that of cytoplasmic NOS isoforms. They proposed that the generation of NO by mtNOS is Ca2+ dependent and requires O2 and l-arginine (47, 58, 194). Indeed, blocking the MCU by extra-mitochondrial [Mg2+] (196) or ruthenium red efficiently inhibits the mitochondrial NO level (47, 48), indicating that mitochondrial Ca2+ influx plays a role in maintaining the mitochondrial NO level. However, it is still in question whether this effect is mediated through mtNOS activity. In addition, depolarization of ΔΨm by uncouplers strongly decreases the mitochondrial NO level (48, 86), suggesting a tight regulatory interaction between the mitochondrial NO level and ΔΨm (180), but again, there is no direct observation that ΔΨm can regulate mtNOS activity. Thus, these observations indicate that Ca2+-induced NO elevation, in part, contributes to the Ca2+ influx-induced ROS generation through NO-dependent modification of ETC activity (see ETC and Mitochondrial Redox Signaling and Mitochondrial Ca2+ Influx Mechanism and Mitochondrial ROS Generation sections) (Figs. 2 and 3). However, it should be noted that most of the reports in this section measured mitochondrial NOS activity and/or NO level under cytosolic and mitochondrial Ca2+ overload, suggesting that the interaction of mitochondrial NO and Ca2+ plays some role in mitochondrial ROS production under pathophysiological conditions, such as I/R in the heart. Further studies are required to clarify the role of mitochondrial NO in mitochondrial Ca2+ homeostasis and ROS production under physiological conditions.
Mitochondrial K+ Channels and Mitochondrial Redox Signaling
In cardiomyocytes, both mitoKATP and mitoKCa play key roles in cardioprotection, especially in ischemic preconditioning (IPC) (124, 128). The opening of mitoKATP and mitoKCa depolarizes ΔΨm, which reduces the driving force for Ca2+ influx, thereby attenuating the [Ca2+]mt overload. Consequently, prevention of [Ca2+]mt overload inhibits mitochondrial ROS generation (Figs. 1–3), mPTP opening (see next section: Mitochondrial Permeability Transition Pore and Redox Signaling) and protects against heart cell death. Several endogenous substances such as adenosine and bradykinin can reduce infarct size by activation of mitoKATP channels in a PKC-dependent manner. Adrenomedullin, a potent vasodilator peptide, potentiates the opening of mitoKCa by PKA activation. Treatment with adrenomedullin before ischemia results in the reduction of infarct size via a PKA-mediated activation of mitoKCa channels. Thus, some endogenous substances can confer cardioprotection via PKA- or PKC-mediated activation of mitoKATP or mitoKCa channels (124, 128). As mentioned earlier, since PKA and PKC are redox-sensitive kinases, it is a reasonable idea that the activities of both mitoKATP and mitoKCa can be regulated by redox signaling through phosphorylation by PKA and/or PKC under IPC. Moreover, mitoKATP can receive redox-dependent PTMs, indicating that the redox modulation of mitoKATP is one of the key mechanisms for the cardioprotective effect in IPC (147). The simplest prediction for the molecular identity of mitoKATP was that it is identical to KATP at the plasma membrane: a protein complex with an inward rectifying K+ channel (Kir6.2) as a pore region and a sulfonylurea receptor (SUR2A). Finally, O'Rourke's group has recently identified that one of the splice variants of the renal outer medullary K+ channel (Kir1.1) is a suitable candidate for the pore-forming subunit of mitoKATP using a mitochondrial proteomic approach (54). This novel finding will pave the way for a great opportunity to study the molecular basis of redox-sensitive PTMs in mitoKATP.
The large-conductance Ca2+-activated K+ channels (BK channels) are also biochemically detectable in the IMM of the brain and cardiomyocytes (124, 128). Therefore, the current proposed molecular identity of mitoKCa is identical or similar to the BK channels. BK channels at the plasma membrane possess oxidative regulation (171). However, currently, there are no reports with regard to redox-sensitive PTMs in mitoKCa.
Mitochondrial Permeability Transition Pore and Redox Signaling
mPTP is one of the most widely studied mitochondrial channels/transporters as a critical effector/regulator of cellular redox signaling [see reviews (27, 68, 80, 103, 148)]. The properties of the mPTP are well defined, and mPTP activity is known to be redox, Ca2+, ΔΨm, adenine nucleotide, inorganic phosphate, and pH sensitive (68, 95) (Fig. 6). For instance, under the conditions of [Ca2+]mt overload especially when accompanied by the combination of high levels of ROS and ΔΨmt depolarization, sustained mPTP opening leads to the release of large amounts of Ca2+ and proapoptotic proteins from mitochondria, which subsequently leads to cell death (27, 80). The diameter of the pore is estimated to be large enough to pass molecules of approxiomately 1.5 kDa in their full open state. We and others reported cyclosporine A-sensitive large conductance channel activities (>1 nS single channel conductance) in the IMM (154). This channel also has transient and subconductance (<300 pS) states, and these channel properties contribute to an important physiological or pathological role by preventing [Ca2+]mt overload working as a Ca2+ efflux mechanism (66) and/or by regulating mitochondrial ROS generation as described as an mSOF (188, 190). On mPTP opening, the IMM no longer maintains a barrier to protons, which leads to depolarization of ΔΨm, followed by the inhibition of ATP production. Due to its pore size, mPTP opening also results in equilibration of cofactors and ions across the IMM, including the release of accumulated Ca2+. This will not only lead to the disruption of metabolic gradients between the mitochondria and the cytosol, but also influx of water concomitantly occurs and results in swelling of the mitochondria until the OMM eventually ruptures. The OMM rupture also facilitates the release of proapoptotic proteins and mitochondrial ROS, which potentially lead to apoptotic and necrotic cell death.
FIG. 6.
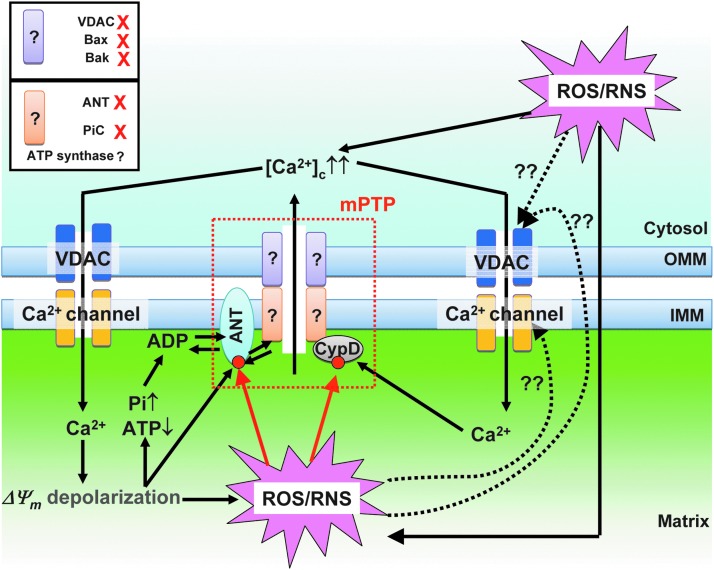
Ca2+- and redox-dependent regulation of mPTP. A hypothetical model of mPTP structure is shown at the middle (red dot line area). CypD and ANT are currently recognized as regulatory proteins of mPTP. CypD and ANT receive redox-dependent PTMs (red circles). CypD, cyclophilin D. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Despite numerous efforts, the exact molecular identity of mPTP is not conclusive (Fig. 6). Based on previous biochemical and pharmacological studies, the classical hypothetical model of mPTP is the supra molecular structure that is composed of (i) the VDAC in OMM; (ii) mitochondrial creatine kinase in IMS; and (iii) the adenine nucleotide translocase (ANT) in IMM and cyclophilin D (CypD) in the matrix. However, recent genetic studies using knockout mouse modes reveal that VDAC and ANT are not essential for pore opening and CypD potently sensitizes the pore opening to Ca2+ (12). Therefore, these proteins have been currently established as regulatory rather than pore-forming components of the mPTP (94). Halestrap's group has recently proposed the mitochondrial phosphate carrier (PIC) as a pore component of the mPTP (68). However, PIC knockdown using siRNA in HeLa cells (65%–80% reduction of PIC) could not abolish mPTP opening, and an essential role for the PIC in mPTP formation still remains controversial (183). Finally, two recent publications have identified the mitochondrial ATP synthase as a possible molecular identity of the pore-forming structure of the mPTP (24, 61). Future studies will address the detailed molecular components of mPTP, including ATP synthase, and identify the critical site(s) for the direct redox-dependent PTMs of mPTP under physiological and pathophysiological conditions. Since recent publications showed that oxidation of α-subunits of ATP synthase at cysteine 294 can modulate its activity (187), this single cysteine will become a primary candidate site for investigating the PTM of mPTP.
There are several reports showing the redox-dependent PTM of mPTP regulatory proteins, CypD and ANT, but it is not well understood whether or how these modifications affect mPTP activity (for VDAC see VDAC and Redox Signaling section): Critical thiols on the matrix side of ANT, at Cys57, Cys160, and Cys257 regulate the bindings of ADP or CypD to ANT (114); the S-nitrosylation of Cys203 in CypD is necessary for the redox stress-induced activation of mPTP (121) (Fig. 6).
IMAC and Redox Signaling
IMAC is also an important sensor and regulator of ROS signaling [see review (129)]. Early studies of mitochondrial swelling showed that an anion-selective channel is present in IMM and later, it was named IMAC (57). IMAC is a benzodiazepine-sensitive channel and it shows ∼100 pS single channel conductance (89). This channel is activated by matrix Mg2+ depletion or alkalization. We also reported that single channel recordings of the heart IMM show a variety of anion selective conductance and the most prominent one is the centum picosiemen conductance channel, which matches the channel properties of IMAC (154). The single channel current-voltage curve of IMAC exhibits a linear relation, but the open probability of IMAC is higher in positive voltages, thereby showing an outward rectifying whole-mitoplast current (154). IMAC has slight anion selectivity and small molecules such as chloride, bicarbonate, phosphate, succinate, citrate, and ATP can pass through the IMAC pore (57).
The physiological role of IMAC is still unknown, because IMAC only appears to conduct ions under alkaline matrix conditions. However, this anion efflux through IMAC enables mitochondria to restore their normal volume after pathological swelling. The role of IMAC in pathophysiological conditions was well studied, especially in cardiomyocytes, by O'Rourke's group [see reviews (2, 5, 21, 192)]. They showed that photoactivation of single mitochondrion in cardiomyocytes using two-photon microscopy induces whole-cell wide oscillation of ΔΨm and ROS generation (7). In this experimental model, they found that the O2− leaked through IMAC at the photoactivated mitochondrion and stimulated the opening of IMAC at the neighboring mitochondria. This series of reactions (regenerative ROS-induced IMAC opening in the neighboring mitochondria) through the mitochondrial network can reach a critical point that evokes whole-cell-wide oscillation of ΔΨm and ROS generation. Moreover, this metabolic instability (or metabolic oscillation) induces an oscillatory activation of sarcolemmal KATP channel, which results in periodic shortening of the cardiac action potential (7). In an ex vivo whole heart model, this mechanism provides a “metabolic sink” in the I/R regions to induce abnormal propagation of cardiac action potential and cause arrhythmias (1). A benzodiazepine receptor antagonist, 4-cholorodiazepam abolishes metabolic oscillation, mitochondrial ROS generation, and cardiac arrhythmia induced by reoxygenation, indicating that redox-dependent modulation of IMAC plays critical roles in ischemia/reperfusion-induced arrhythmia.
The current major limitation in the research in IMAC functions is that the molecular identity of this channel has not yet been identified. Recently, the families of voltage-dependent Cl− channels and/or Cl− intracellular channel 4 (CLIC4) are proposed as candidates for IMAC (52). Further investigation will be required for confirming that the CLIC4 is, indeed, responsible for IMAC activity. Though the molecular identity of IMAC is still unknown, functional observation suggests that this channel contains thiol groups (16). Pretreatment of thiol reactive agents, N-ethylmaleimide, mersalyl, and p-chloromercuribenzenesulfonate increases the IC50 values of IMAC inhibitors (e.g., H+, Mg2+, and cationic amphiphiles) (17). Matrix glutathione level seems to be critical for IMAC activity, because the inhibition of mitochondrial glutathione uptake, the NADPH-dependent glutathione reductase, or the NADH/NADPH transhydrogenase triggers IMAC opening (6). These observations support the idea that there are redox-dependent regulations of IMAC activity. Future studies will be required to understand the IMAC molecular identity as well as the precise patterns of the redox-dependent PTM of this channel.
VDAC and Redox Signaling
VDAC is the most abundant protein in the OMM, and it serves as the primary route of entry and exit for metabolites and ions across the OMM [see review (64)]. VDAC controls the diffusion of O2− from the IMS to the cytosol (69). Moreover, VDAC itself is a putative target of O2−-, and O2− can modulate its function (108).
VDAC channels have a highly conserved homology with bacterial porins and can exist in a variety of functional states that differ in their ability to pass nonelectrolytes and conduct ions. VDAC gating has a voltage-dependent profile (162). VDAC shows ∼3 nS in 1 M NaCl in the full conductance “open” state (152). In the open state, it shows a significant preference for anions and especially favors metabolic anions. The closed state favors cations. The permeability of VDAC to small cations by free diffusion includes K+, Na+, and Ca2+ (see also Fig. 6). Ca2+ ion permeates through both the open and closed states of VDAC, because the ion selectivity at the open state is rather poor (152). Nonelectrolytes (<6 kDa) can also pass through the open channel, enabling the passage of metabolites such as ATP, ADP, and inorganic phosphate (150).
As mentioned in Mitochondrial Permeability Transition Pore and Redox Signaling section, VDAC is not essential for the opening of mPTP, but its opening or closure is one of the important factors for the regulation of cell death signaling (Fig. 6). VDAC interacts with the apoptosis regulators, Bcl2-family members, including Bak, Bad, tBid, and Bcl-xL, which can change the VDAC channel activity (128, 152). For instance, Bax interaction can induce a novel high-conductance state of VDAC that permits cytC to escape from the IMS. Madesh and Hajnoczky found another mechanism for the VDAC-dependent permeabilization of the OMM by O2−. They showed in permeabilized hepatocytes that O2− treatment can elicit the proapoptotic protein cytC to be released from mitochondria through VDAC, whereas H2O2 cannot. This O2−-induced cytC release through VDAC is an independent phenomenon of Ca2+-dependent mPTP opening. Moreover, O2− triggers apoptosis via VDAC-dependent OMM permeabilization without apparent contribution of proapoptotic Bcl2-family proteins. However, the detailed mechanism of how O2− modulates VDAC function remains an open question. There are two possible mechanisms: (i) O2− directly adds PTMs to VDAC when released to the cytosol through this channel (69); and (ii) ROS, including O2−, targets VDAC via lipid oxidation by indirectly altering the lipid environment of VDAC (151). However, there is still no evidence to support these ideas.
Closing Remarks
Research conducted on the mitochondrial ion channels/transporters has been widely recognized as an important field in cell biology, even though their progress is relatively slow mostly due to the fact that their molecular identities remain unknown for a long period of time. Recent advances in the cloning of various mitochondrial ion channels/transporters have provided essential information for investigating the regulatory mechanisms underlying redox-dependent PTMs of the structure and function of these channels/transporters. This new knowledge is crucial for our understanding of the fundamental roles of mitochondrial ion channels/transporters in cell metabolism and signaling under healthy and diseased states. Furthermore, it will lead us to design or discover more specific inhibitors/activators to each channel/transporter that will have the potential to turn into therapeutic drugs for treating diseases caused by mitochondria-mediated oxidative stress. Meanwhile, we need to keep in mind that the molecular identities of the majority of mitochondrial ion channels/transporters are still unknown, which will limit our understanding of the whole picture of their roles as sensors and regulators of cellular redox signaling.
In conclusion, cardiac mitochondrial ion channels/transporters are crucial sensors and regulators for mitochondrial and cellular redox signaling. Revealing the molecular identities and biophysical and biochemical properties of mitochondrial ion channels/transporters will provide us with new strategies for antioxidation therapies targeting various human diseases.
Abbreviations Used
- α-KDH
α-ketoglutarate dehydrogenase
- ΔΨm
mitochondrial membrane potential
- ACON
aconitase
- ANT
adenine nucleotide translocase
- BK channel
large-conductance Ca2+-activated K+ channel
- [Ca2+]c
cytosolic Ca2+ concentration
- [Ca2+]mt
mitochondrial matrix Ca2+ concentration
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CLIC4
Cl− intracellular channel 4
- CoQ10
coenzyme Q10
- COX
cytochrome c oxidase
- CS
citrate synthase
- CypD
cyclophilin D
- cytC
cytochrome C
- eNOS
endothelial NOS
- ER
endoplasmic reticulum
- ETC
electron transport chain
- ETF-QO
electron transferring flavoprotein-quinone oxidoreductase
- FUM
fumarase
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- GSSG
glutathione disulfide
- H+
proton
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- ICDH
isocitrate dehydrogenase
- IMAC
inner membrane anion channel
- IMM
inner mitochondrial membrane
- IMS
mitochondrial intermembrane space
- iNOS
inducible NOS
- IP3R
inositol 1,4,5-trisphosphate receptors
- IPC
ischemic preconditioning
- I/R
ischemic/reperfusion
- LETM1
leucine zipper-EF-hand containing transmembrane protein 1
- MCU
mitochondrial Ca2+ uniporter pore
- MDH
malate dehydrogenase
- mitoKATP
mitochondrial ATP-sensitive K+ channel
- mitoKCa
mitochondrial Ca2+-activated K+ channel
- mNCE
mitochondrial Na+/Ca2+ exchanger
- mPTP
mitochondrial permeability transition pore
- mRyR1
mitochondrial ryanodine receptor type 1
- mSOF
mitochondrial superoxide flash
- mtNOS
mitochondrial nitric oxide synthase
- [Na2+]c
cytosolic Na2+ concentration
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCX
Na+/Ca2+ exchanger at plasma membrane
- NMDA
N-methyl-d-aspartate
- nNOS
neuronal NOS
- NO
nitric oxide
- NOS
NO synthase
- O2−
superoxide
- OMM
outer mitochondrial membrane
- ONOO−
peroxynitrite
- OXPHOS
oxidative phosphorylation
- PDH
pyruvate dehydrogenase
- PIC
mitochondrial phosphate carrier
- PKA
protein kinase A
- PKC
protein kinase C
- PKD
protein kinase D
- PMCA
plasma membrane Ca2+ ATPase
- PTM
post-translational modification
- RaM
rapid mode of uptake
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SCS
succinyl-CoA synthetase
- SDH
succinate dehydrogenase
- SERCA
SR/ER Ca2+-ATPase
- SR
sarcoplasmic reticulum
- TCA
tricarboxylic acid
- UCP
uncoupling protein
- VDAC
voltage-dependent anion channel
Acknowledgments
This research was supported by Irisawa Memorial Promotion Award, the Physiological Society of Japan (to J.O.-U.), SRC program (2010-0029394) through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (to S.-Y. R.), the Korean Health Technology R&D project (HI13C1096), the Ministry of Health and Welfare (to S.-Y. R.), NIH training grant (5T32AA007463-26) (to S.H), and NIH grants (RO1HL-033333, RO1HL-093671, and R21HL-110371) (to S.-S.S.).
References
- 1.Akar FG, Aon MA, Tomaselli GF, and O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akar FG. and O'Rourke B. Mitochondria are sources of metabolic sink and arrhythmias. Pharmacol Ther 131: 287–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antunes F, Boveris A, and Cadenas E. On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. Proc Natl Acad Sci U S A 101: 16774–16779, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes F. and Cadenas E. The mechanism of cytochrome C oxidase inhibition by nitric oxide. Front Biosci 12: 975–985, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aon MA, Cortassa S, Akar FG, Brown DA, Zhou L, and O'Rourke B. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol 41: 1940–1948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aon MA, Cortassa S, Maack C, and O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282: 21889–21900, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aon MA, Cortassa S, Marban E, and O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Aon MA, Cortassa S, and O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta 1797: 865–877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, and Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold WP, Mittal CK, Katsuki S, and Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A 74: 3203–3207, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babior BM, Kipnes RS, and Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52: 741–744, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol 104: 181–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates TE, Loesch A, Burnstock G, and Clark JB. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem Biophys Res Commun 213: 896–900, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Bates TE, Loesch A, Burnstock G, and Clark JB. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochem Biophys Res Commun 218: 40–44, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, and Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavis AD. The mitochondrial inner-membrane anion channel possesses two mercurial-reactive regulatory sites. Eur J Biochem 185: 511–519, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Beavis AD. N-ethylmaleimide and mercurials modulate inhibition of the mitochondrial inner membrane anion channel by H+, Mg2+ and cationic amphiphiles. Biochim Biophys Acta 1063: 111–119, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Bernardi P. and von Stockum S. The permeability transition pore as a Ca(2+) release channel: new answers to an old question. Cell Calcium 52: 22–27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutner G, Sharma VK, Giovannucci DR, Yule DI, and Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem 276: 21482–21488, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, and Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta 1717: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Biary N, Xie C, Kauffman J, and Akar FG. Biophysical properties and functional consequences of reactive oxygen species (ROS)-induced ROS release in intact myocardium. J Physiol 589: 5167–5179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogeski I, Gulaboski R, Kappl R, Mirceski V, Stefova M, Petreska J, and Hoth M. Calcium binding and transport by coenzyme Q. J Am Chem Soc 133: 9293–9303, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, and Niemeyer BA. Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium 50: 407–423, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, and Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12: 674–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borutaite V, Budriunaite A, and Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta 1459: 405–412, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Brady NR, Hamacher-Brady A, Westerhoff HV, and Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal 8: 1651–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Brenner C. and Moulin M. Physiological roles of the permeability transition pore. Circ Res 111: 1237–1247, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion 3: 187–204, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Brookes PS, Yoon Y, Robotham JL, Anders MW, and Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Brosnan JT. and Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr 136: 1636S–1640S, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Buntinas L, Gunter KK, Sparagna GC, and Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta 1504: 248–261, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Burkard N, Williams T, Czolbe M, Blomer N, Panther F, Link M, Fraccarollo D, Widder JD, Hu K, Han H, Hofmann U, Frantz S, Nordbeck P, Bulla J, Schuh K, and Ritter O. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation 122: 1588–1603, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Burwell LS. and Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal 10: 579–599, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Cadenas E. and Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J 188: 31–37, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, and Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol 291: C1082–C1088, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Cardoso AR, Queliconi BB, and Kowaltowski AJ. Mitochondrial ion transport pathways: role in metabolic diseases. Biochim Biophys Acta 1797: 832–838, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Chaudhuri D, Sancak Y, Mootha VK, and Clapham DE. MCU encodes the pore conducting mitochondrial calcium currents. Elife 2: e00704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Azad MB, and Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16: 1040–1052, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Chicco AJ. and Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292: C33–C44, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, and Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung HS, Wang SB, Venkatraman V, Murray CI, and Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. Circ Res 112: 382–392, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clementi E, Brown GC, Feelisch M, and Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A 95: 7631–7636, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csordas A, Pankotai E, Snipes JA, Cselenyak A, Sarszegi Z, Cziraki A, Gaszner B, Papp L, Benko R, Kiss L, Kovacs E, Kollai M, Szabo C, Busija DW, and Lacza Z. Human heart mitochondria do not produce physiologically relevant quantities of nitric oxide. Life Sci 80: 633–637, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Csordas G. and Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta 1787: 1352–1362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai S, Hall DD, and Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev 89: 411–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Stefani D, Raffaello A, Teardo E, Szabo I, and Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dedkova EN. and Blatter LA. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J Physiol 587: 851–872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dedkova EN, Ji X, Lipsius SL, and Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am J Physiol Cell Physiol 286: C406–C415, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Duan Y, Gross RA, and Sheu SS. Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol 585: 741–758, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson JR, He BJ, Grumbach IM, and Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev 91: 889–915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feissner RF, Skalska J, Gaum WE, and Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci 14: 1197–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Salas E, Suh KS, Speransky VV, Bowers WL, Levy JM, Adams T, Pathak KR, Edwards LE, Hayes DD, Cheng C, Steven AC, Weinberg WC, and Yuspa SH. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol Cell Biol 22: 3610–3620, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster DB, Ho AS, Rucker J, Garlid AO, Chen L, Sidor A, Garlid KD, and O'Rourke B. Mitochondrial ROMK channel is a molecular component of mitoKATP. Circ Res 111: 446–454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franco MC, Antico Arciuch VG, Peralta JG, Galli S, Levisman D, Lopez LM, Romorini L, Poderoso JJ, and Carreras MC. Hypothyroid phenotype is contributed by mitochondrial complex I inactivation due to translocated neuronal nitric-oxide synthase. J Biol Chem 281: 4779–4786, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Frandsen U, Lopez-Figueroa M, and Hellsten Y. Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Commun 227: 88–93, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Garlid KD. and Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta 853: 187–204, 1986 [DOI] [PubMed] [Google Scholar]
- 58.Ghafourifar P. and Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26: 190–195, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Ghafourifar P. and Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett 418: 291–296, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Giorgi C, Agnoletto C, Baldini C, Bononi A, Bonora M, Marchi S, Missiroli S, Patergnani S, Poletti F, Rimessi A, Zavan B, and Pinton P. Redox control of protein kinase C: cell- and disease-specific aspects. Antioxid Redox Signal 13: 1051–1085, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, and Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110: 5887–5892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giulivi C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem J 332 (Pt 3): 673–679, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glancy B. and Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51: 2959–2973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goncalves RP, Buzhysnskyy N, and Scheuring S. Mini review on the structure and supramolecular assembly of VDAC. J Bioenerg Biomembr 40: 133–138, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez DR, Beigi F, Treuer AV, and Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A 104: 20612–20617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunter TE. and Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta 1787: 1291–1308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haas R, Marelli-Berg F, and Mauro C. In the eye of the storm: T cell behavior in the inflammatory microenvironment. Am J Clin Exp Immunol 2: 146–155, 2013 [PMC free article] [PubMed] [Google Scholar]
- 68.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Han D, Antunes F, Canali R, Rettori D, and Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Hess DT, Matsumoto A, Kim SO, Marshall HE, and Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Hess DT. and Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem 287: 4411–4418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill BG. and Bhatnagar A. Protein S-glutathiolation: redox-sensitive regulation of protein function. J Mol Cell Cardiol 52: 559–567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hool LC. and Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal 9: 409–435, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Hoshi T. and Heinemann S. Regulation of cell function by methionine oxidation and reduction. J Physiol 531: 1–11, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hou T, Zhang X, Xu J, Jian C, Huang Z, Ye T, Hu K, Zheng M, Gao F, Wang X, and Cheng H. Synergistic triggering of superoxide flashes by mitochondrial Ca2+ uniport and basal reactive oxygen species elevation. J Biol Chem 288: 4602–4612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ignarro LJ, Buga GM, Wood KS, Byrns RE, and Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84: 9265–9269, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ignarro LJ, Byrns RE, Buga GM, and Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 61: 866–879, 1987 [DOI] [PubMed] [Google Scholar]
- 78.Ignarro LJ, Harbison RG, Wood KS, and Kadowitz PJ. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther 237: 893–900, 1986 [PubMed] [Google Scholar]
- 79.Jagnandan D, Sessa WC, and Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol 289: C1024–C1033, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Javadov S, Karmazyn M, and Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther 330: 670–678, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Jiang D, Zhao L, and Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326: 144–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, and Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature 491: 269–273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaminski M, Kiessling M, Suss D, Krammer PH, and Gulow K. Novel role for mitochondria: protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol Cell Biol 27: 3625–3639, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaminski MM, Roth D, Sass S, Sauer SW, Krammer PH, and Gulow K. Manganese superoxide dismutase: a regulator of T cell activation-induced oxidative signaling and cell death. Biochim Biophys Acta 1823: 1041–1052, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Kaminski MM, Sauer SW, Kaminski M, Opp S, Ruppert T, Grigaravicius P, Grudnik P, Grone HJ, Krammer PH, and Gulow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2: 1300–1315, 2012 [DOI] [PubMed] [Google Scholar]
- 86.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, and Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A 98: 14126–14131, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim B, Takeuchi A, Koga O, Hikida M, and Matsuoka S. Mitochondria Na(+)-Ca (2+) exchange in cardiomyocytes and lymphocytes. Adv Exp Med Biol 961: 193–201, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Kim-Han JS. and Dugan LL. Mitochondrial uncoupling proteins in the central nervous system. Antioxid Redox Signal 7: 1173–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, and Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci U S A 90: 1374–1378, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirichok Y, Krapivinsky G, and Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Kobzik L, Stringer B, Balligand JL, Reid MB, and Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophys Res Commun 211: 375–381, 1995 [DOI] [PubMed] [Google Scholar]
- 92.Kocherginsky N. Acidic lipids, H(+)-ATPases, and mechanism of oxidative phosphorylation. Physico-chemical ideas 30 years after P. Mitchell's Nobel Prize award. Prog Biophys Mol Biol 99: 20–41, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M, O'Rourke B, and Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 121: 1606–1613, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, and Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427: 461–465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kroemer G, Galluzzi L, and Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 96.Lacza Z, Horn TF, Snipes JA, Zhang J, Roychowdhury S, Horvath EM, Figueroa JP, Kollai M, Szabo C, and Busija DW. Lack of mitochondrial nitric oxide production in the mouse brain. J Neurochem 90: 942–951, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Lacza Z, Snipes JA, Zhang J, Horvath EM, Figueroa JP, Szabo C, and Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med 35: 1217–1228, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, and Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8: 59–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Le Bras M, Clement MV, Pervaiz S, and Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol 20: 205–219, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Leavy O. T cells: mitochondria and T cell activation. Nat Rev Immunol 13: 224, 2013 [DOI] [PubMed] [Google Scholar]
- 101.Lemasters JJ, Theruvath TP, Zhong Z, and Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leon L, Jeannin JF, and Bettaieb A. Post-translational modifications induced by nitric oxide (NO): implication in cancer cells apoptosis. Nitric Oxide 19: 77–83, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Leung AW. and Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta 1777: 946–952, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Lima B, Forrester MT, Hess DT, and Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu T. and O'Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res 103: 279–288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu T. and O'Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J Bioenerg Biomembr 41: 127–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, and O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 99: 172–182, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Madesh M. and Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155: 1003–1015, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mailloux RJ. and Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51: 1106–1115, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, and Madesh M. MCUR1 is an essential component of mitochondrial Ca(2+) uptake that regulates cellular metabolism. Nat Cell Biol 14: 1336–1343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mark Evans A. and Ward JP. Hypoxic pulmonary vasoconstriction—invited article. Adv Exp Med Biol 648: 351–360, 2009 [DOI] [PubMed] [Google Scholar]
- 112.McCord JM. and Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 243: 5753–5760, 1968 [PubMed] [Google Scholar]
- 113.McCord JM. and Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055, 1969 [PubMed] [Google Scholar]
- 114.McStay GP, Clarke SJ, and Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J 367: 541–548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium 35: 621–628, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, Wahlers T, and Hoppe UC. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation 119: 2435–2443, 2009 [DOI] [PubMed] [Google Scholar]
- 117.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, and Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moody BF. and Calvert JW. Emergent role of gasotransmitters in ischemia-reperfusion injury. Med Gas Res 1: 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mukherjee SP, Lane RH, and Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol 27: 2589–2594, 1978 [DOI] [PubMed] [Google Scholar]
- 120.Murphy MP. and Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity 38: 201–202, 2013 [DOI] [PubMed] [Google Scholar]
- 121.Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, and Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem 286: 40184–40192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nickel A, Loffler J, and Maack C. Myocardial energetics in heart failure. Basic Res Cardiol 108: 358, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Niggli E, Ullrich ND, Gutierrez D, Kyrychenko S, Polakova E, and Shirokova N. Posttranslational modifications of cardiac ryanodine receptors: Ca(2+) signaling and EC-coupling. Biochim Biophys Acta 1833: 866–875, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nishida H, Sato T, Ogura T, and Nakaya H. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: mitochondrial ion channels and cardioprotection. J Pharmacol Sci 109: 341–347, 2009 [DOI] [PubMed] [Google Scholar]
- 125.Oberley LW. and Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res 39: 1141–1149, 1979 [PubMed] [Google Scholar]
- 126.Oess S, Icking A, Fulton D, Govers R, and Muller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J 396: 401–409, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res 94: 420–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O'Rourke B. Mitochondrial ion channels. Annu Rev Physiol 69: 19–49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O'Rourke B, Cortassa S, and Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 20: 303–315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.O'Rourke B. and Maack C. The role of Na dysregulation in cardiac disease and how it impacts electrophysiology. Drug Discov Today Dis Models 4: 207–217, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Orrenius S, Zhivotovsky B, and Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003 [DOI] [PubMed] [Google Scholar]
- 132.O-Uchi J, Pan S, and Sheu SS. Perspectives on: SGP symposium on mitochondrial physiology and medicine: molecular identities of mitochondrial Ca2+ influx mechanism: updated passwords for accessing mitochondrial Ca2+-linked health and disease. J Gen Physiol 139: 435–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palmer RM, Ferrige AG, and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 134.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, and Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 107: 436–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paravicini TM. and Touyz RM. Redox signaling in hypertension. Cardiovasc Res 71: 247–258, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Paul BD. and Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012 [DOI] [PubMed] [Google Scholar]
- 137.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, and Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peixoto PM, Ryu SY, and Kinnally KW. Mitochondrial ion channels as therapeutic targets. FEBS Lett 584: 2142–2152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, and Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467: 291–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Persichini T, Mazzone V, Polticelli F, Moreno S, Venturini G, Clementi E, and Colasanti M. Mitochondrial type I nitric oxide synthase physically interacts with cytochrome c oxidase. Neurosci Lett 384: 254–259, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, and Mootha VK. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One 8: e55785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, and Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 328: 85–92, 1996 [DOI] [PubMed] [Google Scholar]
- 143.Poderoso JJ, Peralta JG, Lisdero CL, Carreras MC, Radisic M, Schopfer F, Cadenas E, and Boveris A. Nitric oxide regulates oxygen uptake and hydrogen peroxide release by the isolated beating rat heart. Am J Physiol 274: C112–C119, 1998 [DOI] [PubMed] [Google Scholar]
- 144.Poole LB, Karplus PA, and Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 44: 325–347, 2004 [DOI] [PubMed] [Google Scholar]
- 145.Powers SK, Talbert EE, and Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 589: 2129–2138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Putzke J, Seidel B, Huang PL, and Wolf G. Differential expression of alternatively spliced isoforms of neuronal nitric oxide synthase (nNOS) and N-methyl-D-aspartate receptors (NMDAR) in knockout mice deficient in nNOS alpha (nNOS alpha(Delta/Delta) mice). Brain Res Mol Brain Res 85: 13–23, 2000 [DOI] [PubMed] [Google Scholar]
- 147.Queliconi BB, Wojtovich AP, Nadtochiy SM, Kowaltowski AJ, and Brookes PS. Redox regulation of the mitochondrial K(ATP) channel in cardioprotection. Biochim Biophys Acta 1813: 1309–1315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ricchelli F, Sileikyte J, and Bernardi P. Shedding light on the mitochondrial permeability transition. Biochim Biophys Acta 1807: 482–490, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Rizzuto R, Bernardi P, Favaron M, and Azzone GF. Pathways for Ca2+ efflux in heart and liver mitochondria. Biochem J 246: 271–277, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rostovtseva T. and Colombini M. ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J Biol Chem 271: 28006–28008, 1996 [DOI] [PubMed] [Google Scholar]
- 151.Rostovtseva TK, Kazemi N, Weinrich M, and Bezrukov SM. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J Biol Chem 281: 37496–37506, 2006 [DOI] [PubMed] [Google Scholar]
- 152.Rostovtseva TK, Tan W, and Colombini M. On the role of VDAC in apoptosis: fact and fiction. J Bioenerg Biomembr 37: 129–142, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, Bhattacharya S, and Bhattacharya J. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest 121: 1986–1999, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ryu SY, Beutner G, Kinnally KW, Dirksen RT, and Sheu SS. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J Biol Chem 286: 21324–21329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Santos CX, Anilkumar N, Zhang M, Brewer AC, and Shah AM. Redox signaling in cardiac myocytes. Free Radic Biol Med 50: 777–793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sarkela TM, Berthiaume J, Elfering S, Gybina AA, and Giulivi C. The modulation of oxygen radical production by nitric oxide in mitochondria. J Biol Chem 276: 6945–6949, 2001 [DOI] [PubMed] [Google Scholar]
- 157.Schoneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer's disease. Biochim Biophys Acta 1703: 111–119, 2005 [DOI] [PubMed] [Google Scholar]
- 158.Sena LA. and Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, and Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38: 225–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sheu SS, Wang W, Cheng H, and Dirksen RT. Superoxide flashes: illuminating new insights into cardiac ischemia/reperfusion injury. Future Cardiol 4: 551–554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shiva S, Brookes PS, Patel RP, Anderson PG, and Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci U S A 98: 7212–7217, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, and Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31: 227–285, 2010 [DOI] [PubMed] [Google Scholar]
- 163.Sluse FE. Uncoupling proteins: molecular, functional, regulatory, physiological and pathological aspects. Adv Exp Med Biol 942: 137–156, 2012 [DOI] [PubMed] [Google Scholar]
- 164.Song YH, Cho H, Ryu SY, Yoon JY, Park SH, Noh CI, Lee SH, and Ho WK. L-type Ca(2+) channel facilitation mediated by H(2)O(2)-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol 48: 773–780, 2010 [DOI] [PubMed] [Google Scholar]
- 165.Song YH, Choi E, Park SH, Lee SH, Cho H, Ho WK, and Ryu SY. Sustained CaMKII activity mediates transient oxidative stress-induced long-term facilitation of L-type Ca(2+) current in cardiomyocytes. Free Radic Biol Med 51: 1708–1716, 2011 [DOI] [PubMed] [Google Scholar]
- 166.Soubannier V. and McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 1793: 154–170, 2009 [DOI] [PubMed] [Google Scholar]
- 167.Sousa SC, Maciel EN, Vercesi AE, and Castilho RF. Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett 543: 179–183, 2003 [DOI] [PubMed] [Google Scholar]
- 168.Sparagna GC, Gunter KK, Sheu SS, and Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem 270: 27510–27515, 1995 [DOI] [PubMed] [Google Scholar]
- 169.Sumandea MP. and Steinberg SF. Redox signaling and cardiac sarcomeres. J Biol Chem 286: 9921–9927, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Szent-Gyorgyi A. Charge transfer and electronic mobility. Proc Natl Acad Sci U S A 58: 2012–2014, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, and Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol 117: 253–274, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tatoyan A. and Giulivi C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J Biol Chem 273: 11044–11048, 1998 [DOI] [PubMed] [Google Scholar]
- 173.Tay YM, Lim KS, Sheu FS, Jenner A, Whiteman M, Wong KP, and Halliwell B. Do mitochondria make nitric oxide? no? Free Radic Res 38: 591–599, 2004 [DOI] [PubMed] [Google Scholar]
- 174.Territo PR, French SA, Dunleavy MC, Evans FJ, and Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, and light scattering. J Biol Chem 276: 2586–2599, 2001 [DOI] [PubMed] [Google Scholar]
- 175.Thannickal VJ. and Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 176.Thomas DD, Liu X, Kantrow SP, and Lancaster JR., Jr.The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A 98: 355–360, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, and Huang P. Redox regulation of cell survival. Antioxid Redox Signal 10: 1343–1374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, and Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol 90: 713–738, 2012 [DOI] [PubMed] [Google Scholar]
- 179.Valdez LB, Alvarez S, Arnaiz SL, Schopfer F, Carreras MC, Poderoso JJ, and Boveris A. Reactions of peroxynitrite in the mitochondrial matrix. Free Radic Biol Med 29: 349–356, 2000 [DOI] [PubMed] [Google Scholar]
- 180.Valdez LB. and Boveris A. Mitochondrial nitric oxide synthase, a voltage-dependent enzyme, is responsible for nitric oxide diffusion to cytosol. Front Biosci 12: 1210–1219, 2007 [DOI] [PubMed] [Google Scholar]
- 181.Valdez LB, Zaobornyj T, and Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim Biophys Acta 1757: 166–172, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, and Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 183.Varanyuwatana P. and Halestrap AP. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 12: 120–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Venkatakrishnan P, Nakayasu ES, Almeida IC, and Miller RT. Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria. J Biol Chem 284: 19843–19855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Venkatakrishnan P, Nakayasu ES, Almeida IC, and Miller RT. Arginase activity in mitochondria—an interfering factor in nitric oxide synthase activity assays. Biochem Biophys Res Commun 394: 448–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wagner S, Rokita AG, Anderson ME, and Maier LS. Redox regulation of sodium and calcium handling. Antioxid Redox Signal 18: 1063–1077, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang SB, Murray CI, Chung HS, and Van Eyk JE. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc Med 23: 14–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, and Cheng H. Superoxide flashes in single mitochondria. Cell 134: 279–290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wei L. and Dirksen RT. Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies. J Gen Physiol 139: 425–434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wei-Lapierre L, Gong G, Gerstner BJ, Ducreux S, Yule DI, Pouvreau S, Wang X, Sheu SS, Cheng H, Dirksen RT, and Wang W. Respective contribution of mitochondrial superoxide and pH to Mt-cpYFP flash activity. J Biol Chem 288: 10567–10577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wiseman DA. and Thurmond DC. The good and bad effects of cysteine S-nitrosylation and tyrosine nitration upon insulin exocytosis: a balancing act. Curr Diabetes Rev 8: 303–315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie C. and Akar FG. Mitochondrial dysfunction: a new frontier in the search for elusive arrhythmia mechanisms. Front Physiol 1: 163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10: 179–206, 2008 [DOI] [PubMed] [Google Scholar]
- 194.Zaobornyj T. and Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. Am J Physiol Heart Circ Physiol 303: H1283–H1293, 2012 [DOI] [PubMed] [Google Scholar]
- 195.Zemojtel T, Kolanczyk M, Kossler N, Stricker S, Lurz R, Mikula I, Duchniewicz M, Schuelke M, Ghafourifar P, Martasek P, Vingron M, and Mundlos S. Mammalian mitochondrial nitric oxide synthase: characterization of a novel candidate. FEBS Lett 580: 455–462, 2006 [DOI] [PubMed] [Google Scholar]
- 196.Zenebe WJ, Nazarewicz RR, Parihar MS, and Ghafourifar P. Hypoxia/reoxygenation of isolated rat heart mitochondria causes cytochrome c release and oxidative stress; evidence for involvement of mitochondrial nitric oxide synthase. J Mol Cell Cardiol 43: 411–419, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zorov DB, Juhaszova M, and Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]