Abstract
Background: We present a clinical trial establishing the feasibility of a control-to-range (CTR) closed-loop system informed by heart rate (HR) and assess the effect of HR information added to CTR on the risk for hypoglycemia during and after exercise.
Subjects and Methods: Twelve subjects with type 1 diabetes (five men, seven women; weight, 68.9±3.1 kg; age, 38±3.3 years; glycated hemoglobin, 6.9±0.2%) participated in a randomized crossover clinical trial comparing CTR versus CTR+HR in two 26-h admissions, each including 30 min of mild exercise. The CTR algorithm was implemented in the DiAs portable artificial pancreas platform based on an Android® (Google, Mountainview, CA) smartphone. We assessed blood glucose (BG) decline during exercise, the Low BG Index (LBGI) (a measure of hypoglycemic risk), number of hypoglycemic episodes (BG <70 mg/dL) and overall glucose control (percentage time within the target range 70 mg/dL≤BG≤180 mg/dL).
Results: Using HR to inform the CTR algorithm reduced significantly the BG decline during exercise (P=0.022), indicated marginally lower LBGI (P=0.3) and fewer hypoglycemic events during exercise (none vs. two events; P=0.16), and resulted in overall higher percentage time within the target range (81% vs. 75%; P=0.2). LBGI and average BG remained unchanged overall, during recovery, and overnight.
Conclusions: HR-informed closed-loop control can be implemented in a portable artificial pancreas. Although closed loop has been shown to reduce hypoglycemia, adding HR signal may further limit the risk for hypoglycemia during and immediately after exercise. The most prominent effect of adding HR information is reduced BG decline during exercise, without deterioration of overall glycemic control.
Introduction
People with type 1 diabetes mellitus (T1DM) are at continual risk for hypoglycemia, which is recognized as one of the principal impediments to optimal glycemic control.1–3 Physical activity in T1DM has been associated with an imbalance between hepatic glucose production and glucose disposal into muscle,4 increased insulin sensitivity related to glucose transporter type 4 translocation up-regulation,5,6 and impaired counterregulatory hormonal response.5,7 Therefore T1DM could increase the risk for exercise-induced hypoglycemia.3 In the absence of sufficient insulin reduction and/or carbohydrate supplementation, hypoglycemia often occurs during exercise as well as during early and late recovery. Despite a growing awareness of exercise benefits, fear of hypoglycemia often results in avoidance of exercise8 or overcompensatory treatment behaviors associated with worsened metabolic control.9,10 Exercise has also been shown to mask hypoglycemic symptoms, facilitating repeated exposure to unrecognized hypoglycemia and potentially causing hypoglycemia-associated autonomic failure7 with all its negative consequences.11,12
Recent closed-loop control artificial pancreas (AP) studies have shown reduction in the risk for hypoglycemia in T1DM subjects, increase of the time in near-normoglycemia (70–180 mg/dL), and reduction in average glucose level, both in the hospital and in home-like settings, with and without exercise.13–22 However, although overnight hypoglycemia can be reduced severalfold, preventing hypoglycemia during and immediately after exercise remains a hurdle, likely due to the inherent delay of the glycemic response to exercise coupled with delays due to subcutaneously injected insulin.13 Informing insulin dosing of physical activity could theoretically decrease these risks.23–25
In this pilot feasibility study we assess whether heart rate (HR)—an easy-to-measure marker of physical activity3—could inform the AP algorithm and thereby improve protection against hypoglycemia during exercise. To do so, we present results comparing a previously tested13 control-to-range (CTR) AP system with the same system enhanced with the HR signal (CTR+HR) in a randomized crossover design.
Research Design and Methods
Participants
Thirteen adults with T1DM were enrolled in a randomized crossover study at the University of Virginia Clinical Research Unit (Charlottesville, VA); 12 subjects finished the study with a body weight of 68.9±3.1 kg, age of 38±3.3 years, glycated hemoglobin level of 6.9±0.2%, and T1DM duration of 23.6±4.4 years (one screen failure). All subjects were experienced insulin pump users (>6 months) actively using a bolus calculator with predefined parameters (carbohydrate ratio, insulin sensitivity factor, and blood glucose [BG] target). Subjects with a glycosylated hemoglobin level of >10.5%, with recent diabetic ketoacidosis or severe hypoglycemia (<3 months), or at increased cardiovascular risk during exercise were excluded. Use of a medication that significantly lowers HR was also excluded (e.g., β-blocker).
Protocol
The study was approved by the University of Virginia Human Subjects Research-Institutional Review Board and the Food and Drug Administration. After written informed consent was obtained, subjects were randomized to determine the order of the control (CTR) and experimental (CTR+HR) admissions (Fig. 1A). No washout period was imposed between admissions. Each admission lasted up to 26 h (24 h of closed loop) and included an afternoon (approximately 3–4 p.m.) mild exercise bout consisting of 30 min on a cycle ergometer at a rate of perceived exertion of 9–10 on the Borg scale,26 with the workload adjusted every 5 min to maintain the rating of perceived exertion. Three meals were given at fixed times: a light breakfast at 8 a.m. (20±5 g of carbohydrate), an early lunch at 11 a.m. (46±17 g of carbohydrate), and dinner at 7 p.m. (42±20 g of carbohydrate), identical for both admissions (Fig. 1B). This design was chosen to increase the risk of hypoglycemia during and shortly after exercise and to mimic late afternoon physical activity. The plasma glucose level was measured at least every 30 min (model YSI 2300/2700; Yellow Springs Instruments, Yellow Springs, OH), and more frequently around exercise (5–10 min). Hypoglycemia was defined as a YSI reading below 70 mg/dL or the occurrence of hypoglycemic symptoms and was treated with glucose tablets (the amount was left to the physician's discretion) until the BG level recovered above 80 mg/dL.
FIG. 1.
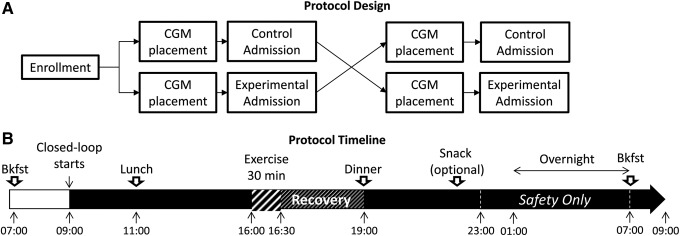
Study and protocol design. (A) Subjects were admitted twice at the University of Virginia clinical research center for 24 h to test one of the artificial pancreas systems (heart rate–enhanced or standard) in a randomized order. (B) Both admissions followed the same timeline. Bkfst, breakfast; CGM, continuous glucose monitor.
Each subject was equipped with two Dexcom (San Diego, CA) SEVEN PLUS® continuous glucose monitors 24–72 h prior to each admission and was asked to calibrate per the manufacturer's instructions (self-monitoring of BG every 12 h). Failing sensors were replaced. At the time of admission, the subject's insulin pump was disconnected, and an Omnipod® Insulin Management System (Insulet Corp., Bedford, MA) filled with Humalog® insulin (Eli Lilly, Indianapolis, IN) was initiated for the duration of the admission. The subjects were then trained in the use of the DiAs portable AP platform running on an Android® (Google, Mountainview, CA) smartphone that was communicating with the continuous glucose monitor and with the insulin pump as previously described.15 The CTR and CTR+HR algorithms (see below) were implemented on the DiAs platform. HR data were collected using an HR monitor (model RS800CX; Polar®, Lake Success, NY), and during the CTR+HR admission the CTR system was informed manually when the HR exceeded an empirically determined 125% of resting HR by pressing the exercise button, thereby activating CTR+HR. The button was pressed a second time when the HR returned to below 125% of resting HR, returning the system to regular CTR. During the CTR admission the HR was measured but not used as an input to the system.
Control algorithms
The CTR algorithm has been introduced in a previous publication.11 Based on an internal mathematical model of the subject's glucose–insulin dynamic, derived from the minimal model of glucose kinetics,27 CTR uses Kalman filtering to estimate the subject's metabolic state. The system then predicts glycemic excursions 30–45 min ahead and computes a predicted hypoglycemic and hyperglycemic risks.28 If the predicted hypoglycemic risk increases, CTR attenuates basal insulin delivery; if hyperglycemia is predicted, CTR delivers frequent (up to one per hour) correction boluses13 (in this study, these automatic corrections were deactivated overnight between 11 p.m. and 7 a.m.). Meal insulin computations were based on the subject's insulin pump parameters and his or her estimation of the meal carbohydrate content. CTR+HR is the HR-informed version of CTR that used a different definition of hypoglycemic risk during exercise—when informed by HR change, the risk increases at higher predicted glucose level and faster than our standard CTR. As a result, given an HR signal, the basal attenuation will occur earlier and reduce the insulin dose more. During the CTR+HR admission, the exercise mode was manually triggered when the subject's HR reached 125% of his or her resting HR (measured while the subject was sitting at the beginning of the admission) and turned off when the HR returned below this threshold. The CTR+HR algorithm was designed and tested in silico using computer simulation.29
Outcomes
This was an early feasibility study of the use of HR information in CTR algorithms, not powered to achieve statistical significance. To assess hypoglycemia protection and overall glycemic control, we report the following: the decline in plasma glucose level created by the exercise bout, the Low BG Index (LBGI),30 which quantifies the risk for hypoglycemia; the number of hypoglycemic events (BG <70 mg/dL); the percentage of time spent in the target range of 70–180 mg/dL; and the average glucose level. LBGI and percentage in target were compared using the paired t test, hypoglycemia counts were compared using the paired Wilcoxon rank test, and the glycemic decline during exercise was studied using repeated-measure analysis of variance.
Results
Twelve subjects completed the study. The first two subjects were excluded from the analysis because of protocol violations during the exercise session (the intensity chosen was very high: rating of perceived exertion of 9 out of 10 instead of rating of perceived exertion of 9 out of 20). Results from the 10 remaining subjects are presented below.
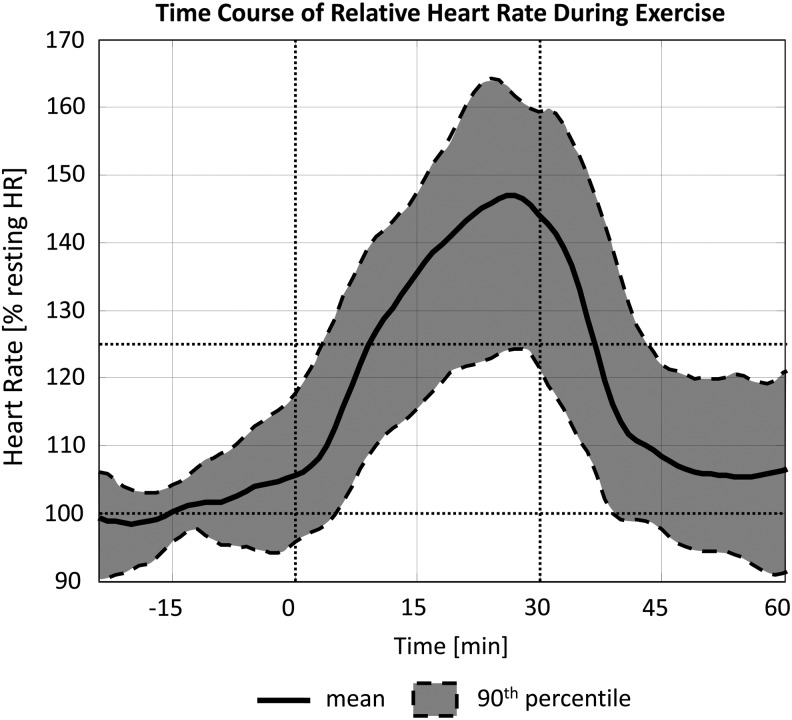
In 19 out of 20 admissions, HR increased consistently during exercise (repeated-measure analysis of variance, P<0.001), crossing the 125% threshold for the first time on average 8:06 min (SD, 7:02) after the onset of exercise (Fig. 2). On average, maximum HR was 155.3±7.3% of the resting value.
FIG. 2.
Heart rate increased consistently during the mild exercise bout and returned to basal values within 15 min afterward. In all bouts, 125% of the basal value was reached within 8 min of onset on average (maximum, 22 min).
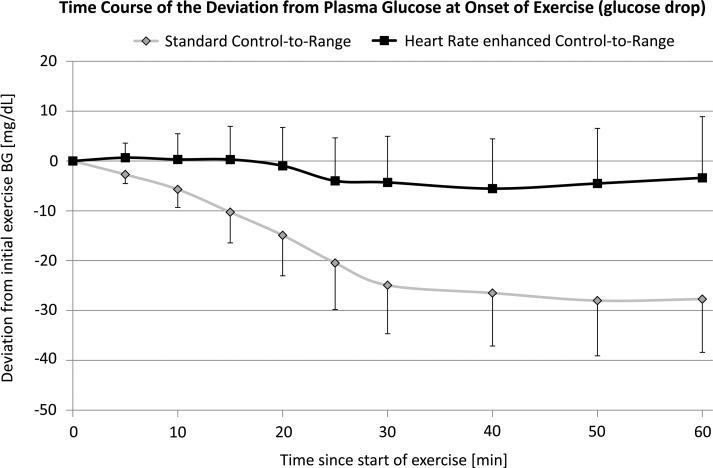
As shown in Figure 3, during the exercise bout the use of HR had a clear impact on the exercise-induced decline in plasma glucose level, with an average maximum BG decline of −29 mg/dL at min 40 with CTR alone and of −5 mg/dL at min 40 with CTR+HR. This difference was significant as indicated by repeated-measure analysis of variance using hypoglycemic treatment as the covariate (P=0.022).
FIG. 3.
On average, the plasma glucose level did not decline during exercise and only moderately afterward when using control-to-range plus heart rate (squares with black line). In contrast, under standard control-to-range the average plasma glucose decline was pronounced throughout the exercise bout and moderately amplified thereafter (diamonds with gray line). Maximum separation was achieved at min 60 after onset of exercise (−3.4 mg/dL vs. −27.7 mg/dL). BG, blood glucose.
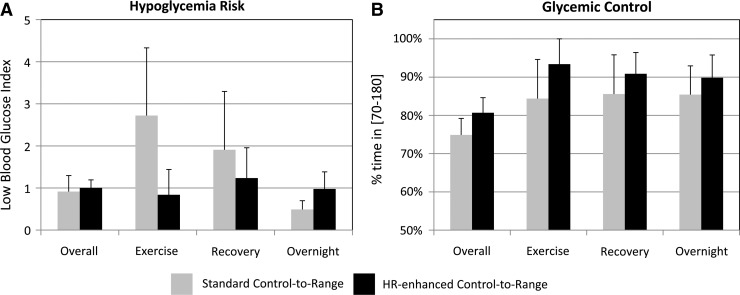
As indicated in Figure 4A, the observed average risk for hypoglycemia during exercise was reduced marginally (no statistical significance) with CTR+HR versus CTR (LBGI, 0.84±0.60 vs. 2.72±1.61 [mean±SE]; P=0.34; effect size=0.49). LBGI remained unchanged overall (1.03±0.20 vs. 0.90±0.37; P=0.66, effect size=0.14) and during recovery (1.81±1.2 vs. 1.83±1.4; P=0.99; effect size<0.1), with a slight increase overnight (0.98±0.41 vs. 0.49±0.21; P=0.35; effect size=0.48). Similarly, when using CTR+HR, hypoglycemic events were less frequent during exercise (none vs. two; P=0.16; effect size=0.45), whereas this effect was less evident during recovery (one vs. two; P=0.56; effect size=0.18) and overnight (none vs. one; P=0.32; effect size=0.32).
FIG. 4.
(A) The risk of hypoglycemia as measured by the Low Blood Glucose Index (mean and SE values reported) was similar overall in both admissions but seems reduced during and in the hours after exercise. (B) This possible reduction did not come at the cost of lesser glycemic control as the time spent in hypoglycemia. In all phases of the admission average percentage in euglycemia seems slightly better using the heart rate (HR)–enhanced system.
As presented in Figure 4B, hypoglycemia risk reduction did not come at the expense of glycemic control. Rather, observed averages of percentage of time in near-normoglycemia (70–180 mg/dL) were consistently (but not significantly) higher during the CTR+HR admission: overall (81±3.9% vs. 75±4.3%; P=0.2; effect size=0.47), during exercise (91±7.3% vs. 85±10.3%; P=0.6; effect size=0.23), during recovery (86±9.3% vs. 84±7.9%; P=0.48; effect size=0.06), and overnight (89±7.1% vs. 84±9.2%; P=0.34; effect size=0.26). These results were confirmed by lower observed average BG on CTR+HR: overall (142±4 mg/dL vs. 150±5 mg/dL; P=0.15; effect size=0.56) and overnight (130±10 mg/dL vs. 141±10 mg/dL; P=0.22; effect size=0.35). Mean BG remained unchanged during exercise (133±10 mg/dL vs. 130±12 mg/dL; P=0.87; effect size=0.07) and during recovery (138±11 mg/dL vs. 135±11 mg/dL; P=0.81; effect size=0.09).
These results held if the rejected two participants were included in the analysis.
Discussion
The goal of this pilot feasibility study was to assess whether HR-activated aggressive basal rate attenuation during closed-loop control could enhance hypoglycemia protection during and after exercise in subjects with T1DM. The results confirm the feasibility of HR-informed AP systems and indicate their potential to decrease hypoglycemic risk during exercise.
Although this first trial had a state-of-the-art randomized crossover design, the study was not powered to reach statistical significance. Nevertheless, the effect size for reduced risk of hypoglycemia during exercise was 0.49, which is typically addressed as a medium effect.31 Thus, future trials enrolling approximately 30 subjects in a similar study design should expect to achieve statistically significant risk reduction using HR-informed control systems.
Despite its small sample size, the study did achieve a statistically significant result showing that the BG rate of decline during exercise was reduced when HR information was supplied to the closed-loop controller. Thus, additional protection against hypoglycemia was indeed provided by an early HR-based warning of physical activity followed by a more conservative insulin injection by the CTR.
It is important to note that this additional hypoglycemia protection was not associated with an elevated average glucose level or decreased time in target range; rather, the average glucose level may have improved (decreased) with the use of HR (effect size of 0.56). We could speculate that this decrease may be associated with a lower counterregulatory response during exercise, which would be consistent with fewer hypoglycemic events and lower rate of BG decline. However, future studies would be needed to test such speculation.
In terms of technology, the HR signal was not sent automatically to the DiAs platform—the 125% resting HR trigger was manual—therefore we could not observe the full effect of possible false alarms that could occur with a fully automated system observing the subject continually. Retrospective analysis of the HR signal over the entire admission showed 31 potential false alarms over all the subjects, with 21 of these false alarms triggered in the same subject and 66% with a duration of less than 10 min (maximum of 30 min). A retrospective run of the CTR+HR showed these occasional HR spikes would have resulted in different insulin injections in only one subject and would have corresponded to a small amount—less than 0.35 U of insulin added to the 61.5 U total daily dose for this subject.
Although these first results are encouraging, care should be taken in selecting an exercise signal to enhance AP systems. The data presented here included only subjects without significant cardiovascular complications, and the use of medications known to affect HR (e.g., β-blockers) was excluded. Thus, the feasibility of such systems for the general T1DM population is yet to be demonstrated. Systems such as accelerometers could replace the HR signal with appropriate algorithmic treatment, but accelerometer signals are notoriously hard to interpret by automated algorithms and often require redundancy of the sensors and the sensor position to accurately differentiate between physical activity and normal movements. A strategy combining these different data sources is likely to be most efficient.
Finally, the results presented are only representative of glycemic drops engendered by mild exercise. Confirmation at different levels and duration of exercise will be needed, as well as studying different types of physical activity.
In conclusion, HR has been used for the first time to inform an AP system, slowing the rate of glycemic decline associated with exercise and possibly indicating a reduced risk and improved protection against hypoglycemia during and after exercise.
Acknowledgments
Funding was received from grant R21DK085641 from the National Institute of Diabetes and Digestive Disorders and Kidney, National Institutes of Health. Material support was received from Insulet Corp. (Bedford, MA), Dexcom Inc. (San Diego, CA), and the University of California, Santa Barbara.
Author Disclosure Statement
M.D.B., C.H.K., and B.P.K. hold patents or patent applications related to the study technology. B.P.K. has served on advisory boards for Animas and Sanofi-Aventis and received research grant/material support from Abbott, Animas, Becton-Dickinson, Dexcom, Insulet, LifeScan, Tandem, and Sanofi-Aventis. M.D.B. has received research support from Becton-Dickinson, Dexcom, Insulet, Sanofi, and Tandem Diabetes Care. S.M.A. has received research support from Medtronic and Animas. S.A.B. has received research support for Dexcom. C.H.K., L.K., and K.A.T. declare no competing financial interests exist.
M.D.B. designed the study, supervised the algorithm development, served as senior study engineer, and analyzed the data. S.A.B. served as a study physician. C.H.K. participated in algorithm development and served as junior study engineer. L.K. served as study research coordinator and supervised Clinical Research Center admissions. K.A.T. participated in results analysis and writing the manuscript. S.M.A. served as a study physician and participated in study design. B.P.K. supervised study design and algorithm development and edited the manuscript.
References
- 1.Cryer PE: Hypoglycaemia: The limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 2002;45:937–948 [DOI] [PubMed] [Google Scholar]
- 2.Cryer PE: Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 2005;54:3592–3601 [DOI] [PubMed] [Google Scholar]
- 3.van Bon AC, Verbitskiy E, von Basum G, Hoekstra JBL, DeVries JH: Exercise in closed-loop control: a major hurdle. J Diabetes Sci Technol 2011;5:1337–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon SK, Ferreira LD, Ratnam N, Davey RJ, Youngs LM, Davis EA, Fournier PA, Jones TW: Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab 2007;92:963–968 [DOI] [PubMed] [Google Scholar]
- 5.Goodyear LJ, Kahn BB: Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 1998;49:235–261 [DOI] [PubMed] [Google Scholar]
- 6.Dohm GL: Invited review: regulation of skeletal muscle GLUT-4 expression by exercise. J Appl Physiol 2002;93:782–787 [DOI] [PubMed] [Google Scholar]
- 7.Younk LM, Mikeladze M, Tate D, Davis SN: Exercise-related hypoglycemia in diabetes mellitus. Expert Rev Endocrinol Metab 2011;6:93–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toni S, Reali MF, Barni F, Lenzi L, Festini F: Managing insulin therapy during exercise in type 1 diabetes mellitus. Acta Biomed 2006;77(Suppl 1):34–40 [PubMed] [Google Scholar]
- 9.Kumareswaran K, Elleri D, Allen JM, Caldwell K, Nodale M, Wilinska ME, Amiel SA, Hovorka R, Murphy HR: Accuracy of continuous glucose monitoring during exercise in type 1 diabetes pregnancy. Diabetes Technol Ther 2013;15:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddell MC, Milliken J: Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 2011;13:819–825 [DOI] [PubMed] [Google Scholar]
- 11.Cryer PE: The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iscoe KE, Campbell JE, Jamnik V, Perkins BA, Riddell MC: Efficacy of continuous real-time blood glucose monitoring during and after prolonged high-intensity cycling exercise: spinning with a continuous glucose monitoring system. Diabetes Technol Ther 2006;8:627–635 [DOI] [PubMed] [Google Scholar]
- 13.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Toffanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ, 3rd, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B, International Artificial Pancreas Study Group: Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherr JL, Cengiz E, Palerm CC, Clark B, Kurtz N, Roy A, Carria L, Cantwell M, Tamborlane WV, Weinzimer SA: Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care 2013;36:2909–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Farret A, Pelletier M, Place J, Bruttomesso D, Del Favero S, Visentin R, Filippi A, Scotton R, Avogaro A, Doyle FJ, III: Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancrea. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, Kollman C, Nodale M, Murphy HR, Dunger DB, Amiel SA, Heller SR, Wilinska ME, Evans ML: Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB: Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 18.Cobelli C, Renard E, Kovatchev BP, Keith-Hynes P, Ben Brahim N, Place J, Del Favero S, Breton M, Farret A, Bruttomesso D, Dassau E, Zisser H, Doyle FJ, 3rd, Patek SD, Avogaro A: Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renard EM, Farret A, Place J, Cobelli C, Kovatchev BP, Breton MD: Closed-loop insulin delivery using subcutaneous infusion and glucose sensing, and equipped with a dedicated safety supervision algorithm, improves safety of glucose control in type 1 diabetes [abstract]. Diabetologia 2010;53(Suppl 1):OP08 [Google Scholar]
- 20.Cobelli C, Renard E, Kovatchev B: Artificial pancreas: past, present, future. Diabetes 2011;60:2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elleri D, Allen JM, Kumareswaran K, Leelarathna L, Nodale M, Caldwell K, Cheng P, Kollman C, Haidar A, Murphy HR, Wilinska M, Acerini CL, Dunger DB, Hovorka R: Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care 2013;36:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER: A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breton MD: Physical activity—the major unaccounted impediment to closed loop control. J Diabetes Sci Technol 2008;2:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A: Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther 2013;15:386–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turksoy K, Quinn L, Littlejohn E, Cinar A: Multivariable adaptive identification and control for artificial pancreas systems. IEEE Trans Biomed Eng 2014;61:883–891 [DOI] [PubMed] [Google Scholar]
- 26.Borg G, Noble BJ: Perceived exertion In: Wilmore J, ed. Exercise and Sport Science Reviews. New York: Academic Press, 1974:131–154 [PubMed] [Google Scholar]
- 27.Bergman RN, Ider YZ, Bowden CR, Cobelli C: Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 28.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W: Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care 1997;20:1655–1658 [DOI] [PubMed] [Google Scholar]
- 29.Kovatchev BP, Breton M, Man CD, Cobelli C: In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol 2009;3:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W: Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 32.Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates, 1988 [Google Scholar]