Abstract
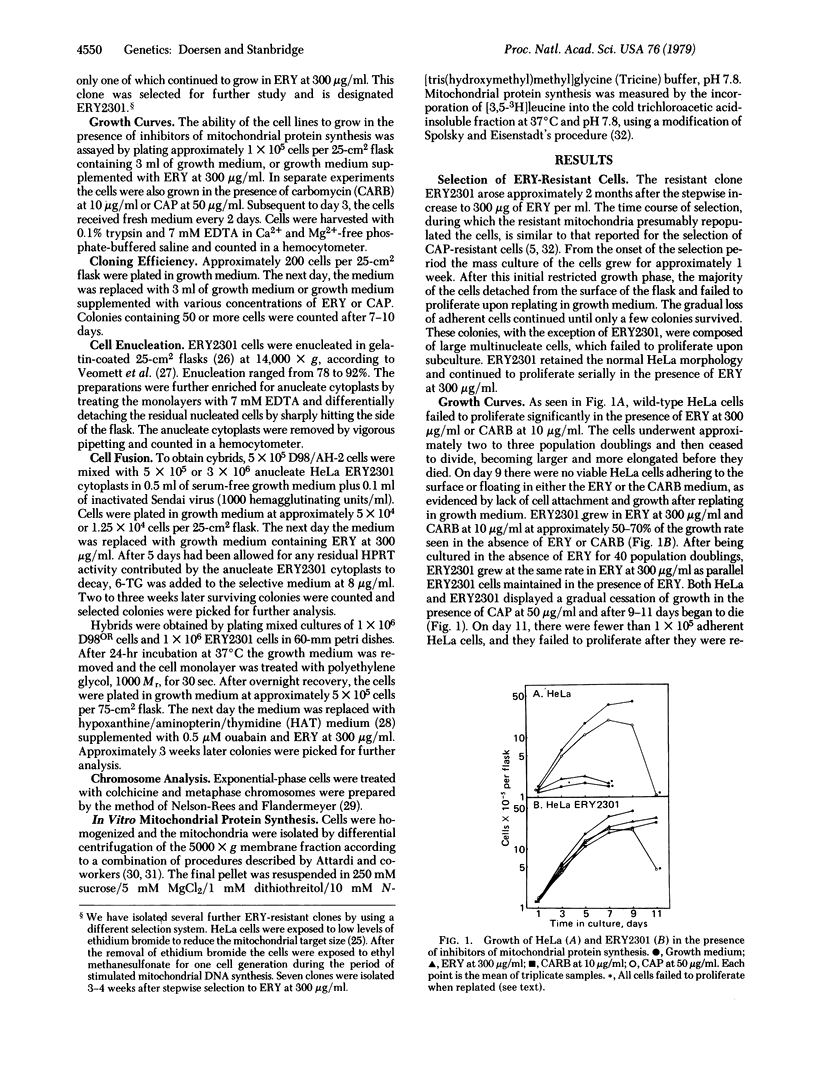
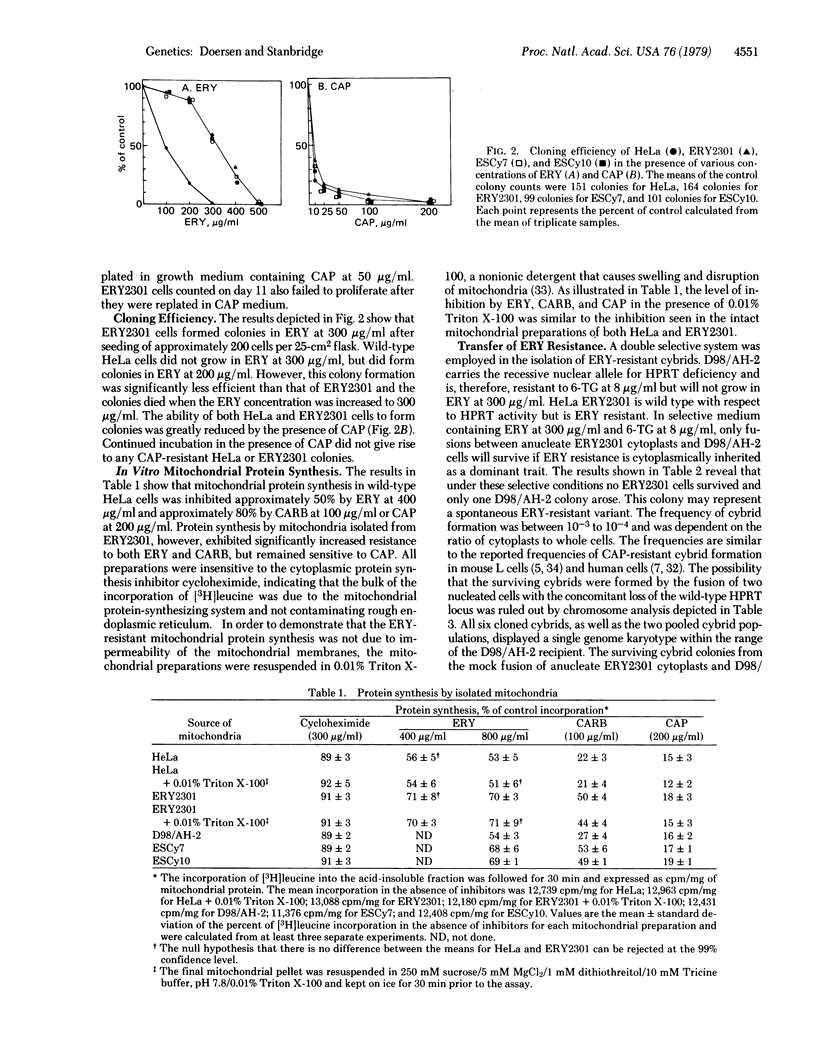
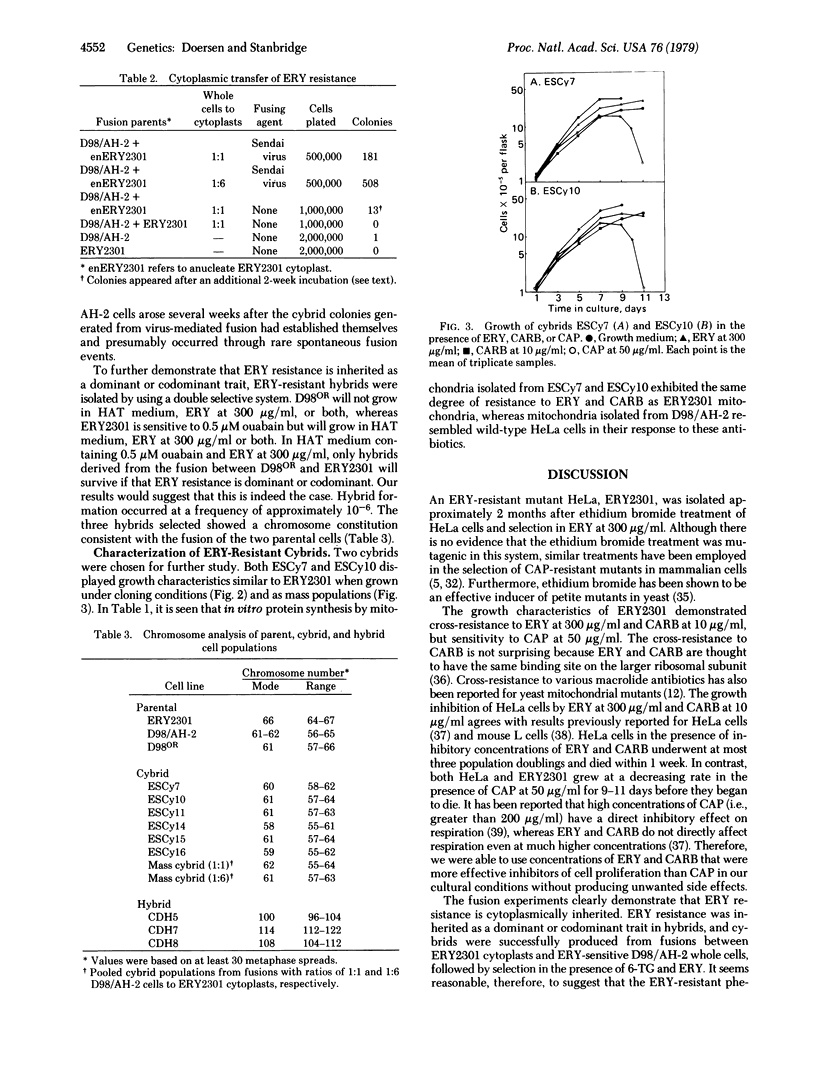
An erythromycin-resistant mutant, ERY2301, was isolated from ethidium bromide-treated HeLa cells in the presence of erythromycin at 300 micrograms/ml. ERY2301 cells were enucleated and the anucleate cytoplasts were fused with D98/AH-2, a hypoxanthine phosphoribosyltransferase-deficient variant of HeLa cells. The resultant cybrids were isolated in a double selective medium containing erythromycin and 6-thioguanine. Cybrid formation occurred at a frequency of 10(-3) to 10(-4). In vitro protein synthesis by intact and Triton X-100 treated mitochondria isolated from ERY2301 was resistant to the macrolide antibiotics erythromycin and carbomycin, but was sensitive to chloramphenicol. These results suggest that the site of erythromycin resistance in ERY2301 may be at the level of mitochondrial protein synthesis and indicate that this trait is cytoplasmically inherited and, therefore, presumably encoded in the mitochondrial genome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi B., Cravioto B., Attardi G. Membrane-bound ribosomes in HeLa cells. I. Their proportion to total cell ribosomes and their association with messenger RNA. J Mol Biol. 1969 Aug 28;44(1):47–70. doi: 10.1016/0022-2836(69)90404-5. [DOI] [PubMed] [Google Scholar]
- Beale G. H., Knowles J. K., Tait A. Mitochondrial genetics in Paramecium. Nature. 1972 Feb 18;235(5338):396–397. doi: 10.1038/235396a0. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Bunn C. L., Eisenstadt J. M. Cybrid formation in mouse L cells: the influence of cytoplast-to-cell ratio. Somatic Cell Genet. 1977 May;3(3):335–341. doi: 10.1007/BF01538751. [DOI] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H., Kellerman G. M., Linnane A. W. XXIV. Effect of mikamycin, carbomycin, spiramycin, erythromycin, and paromomycin on growth and respiration of HeLa cells. Arch Biochem Biophys. 1972 Oct;152(2):869–875. doi: 10.1016/0003-9861(72)90283-4. [DOI] [PubMed] [Google Scholar]
- Faye G., Kujawa C., Fukuhara H. Physical and genetic organization of petite and grande yeast mitochondrial DNA. IV. In vivo transcription products of mitochondrial DNA and localization of 23 S ribosomal RNA in petite mutants of saccharomyces cerevisiae. J Mol Biol. 1974 Sep 5;88(1):185–203. doi: 10.1016/0022-2836(74)90304-0. [DOI] [PubMed] [Google Scholar]
- Freeman K. B. Effects of chloramphenicol and its isomers and analogues on the mitochondrial respiratory chain. Can J Biochem. 1970 Apr;48(4):469–478. doi: 10.1139/o70-076. [DOI] [PubMed] [Google Scholar]
- GREVILLE G. D., CHAPPELL J. B. The latent rhodanese of isolated rat-liver mitochondria. Biochim Biophys Acta. 1959 May;33(1):267–269. doi: 10.1016/0006-3002(59)90532-3. [DOI] [PubMed] [Google Scholar]
- Grivell L. A., Netter P., Borst P., Slonimski P. P. Mitochondrial antibiotic resistance in yeast: ribosomal mutants resistant to chloramphenicol, erythromycin and spiramycin. Biochim Biophys Acta. 1973 Jun 23;312(2):358–367. doi: 10.1016/0005-2787(73)90380-8. [DOI] [PubMed] [Google Scholar]
- Grivell L. A., Reijnders L., de Vries H. Altered mitochondrial ribosomes in a cytoplasmic mutant of yeast. FEBS Lett. 1971 Aug 15;16(3):159–163. doi: 10.1016/0014-5793(71)80121-7. [DOI] [PubMed] [Google Scholar]
- Harris M. Cytoplasmic transfer of resistance to antimycin A in Chinese hamster cells. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5604–5608. doi: 10.1073/pnas.75.11.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N. G., Beattie D. S. Protein synthesis on ribosomes isolated from rat liver mitochondria: sensitivity to erythromycin. FEBS Lett. 1973 Oct 1;36(1):102–104. doi: 10.1016/0014-5793(73)80347-3. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lederman M., Attardi G. In vitro protein synthesis in a mitochondrial fraction from HeLa cells: sensitivity to antibiotic and ethidium bromide. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1492–1500. doi: 10.1016/0006-291x(70)90037-9. [DOI] [PubMed] [Google Scholar]
- Lichtor T., Getz G. S. Cytoplasmic inheritance of rutamycin resistance in mouse fibroblasts. Proc Natl Acad Sci U S A. 1978 Jan;75(1):324–328. doi: 10.1073/pnas.75.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Lamb A. J., Christodoulou C., Lukins H. B. The biogenesis of mitochondria, VI. Biochemical basis of the resistance of Saccharomyces cerevisiae toward antibiotics which specifically inhibit mitochondrial protein synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1288–1293. doi: 10.1073/pnas.59.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Nagley P. Mitochondrial genetics in perspective: the derivation of a genetic and physical map of the yeast mitochondrial genome. Plasmid. 1978 Jun;1(3):324–345. doi: 10.1016/0147-619x(78)90049-5. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Wiegand R. G. Mode of action of macrolides. Biochim Biophys Acta. 1968 Apr 22;157(2):404–413. doi: 10.1016/0005-2787(68)90094-4. [DOI] [PubMed] [Google Scholar]
- Mitchell C. H., Attardi G. Cytoplasmic transfer of chloramphenicol resistance in a human cell line. Somatic Cell Genet. 1978 Nov;4(6):737–744. doi: 10.1007/BF01543161. [DOI] [PubMed] [Google Scholar]
- Mitchell C. H., England J. M., Attardi G. Isolation of chloramphenicol-resistant variants from a human cell line. Somatic Cell Genet. 1975 Jul;1(3):215–234. doi: 10.1007/BF01538447. [DOI] [PubMed] [Google Scholar]
- Nagley P., Molloy P. L., Lukins H. B., Linnane A. W. Studies on mitochondrial gene purification using petite mutants of yeast: characterization of mutants enriched in ribosomal RNA cistrons. Biochem Biophys Res Commun. 1974 Mar 15;57(1):232–239. doi: 10.1016/s0006-291x(74)80381-5. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Flandermeyer R. R. Inter- and intraspecies contamination of human breast tumor cell lines HBC and BrCa5 and other cell cultures. Science. 1977 Mar 25;195(4284):1343–1344. doi: 10.1126/science.557237. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Slonimski P. P., Perrodin G., Croft J. H. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal "petites". Biochem Biophys Res Commun. 1968 Feb 15;30(3):232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Jordan J. M., Vinograd J. In vivo effects of intercalating drugs on the superhelix density of mitochondrial DNA isolated from human and mouse cells in culture. J Mol Biol. 1971 Jul 28;59(2):255–272. doi: 10.1016/0022-2836(71)90050-7. [DOI] [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- Towers N. R., Dixon H., Kellerman G. M., Linnane A. W. Biogenesis of mitochondria. 22. The sensitivity of rat liver mitochondria to antibiotics; a phylogenetic difference between a mammalian system and yeast. Arch Biochem Biophys. 1972 Aug;151(2):361–369. doi: 10.1016/0003-9861(72)90510-3. [DOI] [PubMed] [Google Scholar]
- Veomett G., Shay J., Hough P. V., Prescott D. M. Large-scale enucleation of mammalian cells. Methods Cell Biol. 1976;13:1–6. doi: 10.1016/s0091-679x(08)61794-x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman A., Attardi G. Cytoplasmically inherited mutations of a human cell line resulting in deficient mitochondrial protein synthesis. Somatic Cell Genet. 1979 Mar;5(2):241–262. doi: 10.1007/BF01539164. [DOI] [PubMed] [Google Scholar]
- Wiseman A., Attardi G. Reversible tenfod reduction in mitochondria DNA content of human cells treated with ethidium bromide. Mol Gen Genet. 1978 Nov 16;167(1):51–63. doi: 10.1007/BF00270321. [DOI] [PubMed] [Google Scholar]



