Abstract
Significance: The family of purinergic P2X receptors (P2XRs) is a part of ligand-gated superfamily of channels activated by extracellular adenosine-5′-triphosphate. P2XRs are present in virtually all mammalian tissues as well as in tissues of other vertebrate and nonvertebrate species and mediate a large variety of functions, including fast transmission at central synapses, contraction of smooth muscle cells, platelet aggregation, and macrophage activation to proliferation and cell death. Recent Advances: The recent solving of crystal structure of the zebrafish P2X4.1R is a major advance in the understanding of structural correlates of channel activation and regulation. Combined with growing information obtained in the post-structure era and the reinterpretation of previous work within the context of the tridimensional structure, these data provide a better understanding of how the channel operates at the molecular levels. Critical Issues: This review focuses on the relationship between redox signaling and P2XR function. We also discuss other allosteric modulation of P2XR gating in the physiological/pathophysiological context. This includes the summary of extracellular actions of trace metals, which can be released to the synaptic cleft, pH decrease that happens during ischemia and inflammation, and calcium, an extracellular and intracellular messenger. Future Directions: Our evolving understanding of activation and regulation of P2XRs is helpful in clarifying the mechanism by which these channels trigger and modulate cellular functions. Further research is required to identify the signaling pathways contributing to the regulation of the receptor activity and to develop novel and receptor-specific allosteric modulators, which could be used in vivo with therapeutic potential. Antioxid. Redox Signal. 21, 953–970.
Introduction
The adenosine-5′-triphosphate (ATP)-gated (purinergic) receptors (P2XRs) channels are one of the three families of extracellular ligand-gated ion channels, with each containing three functional domains: an extracellular domain that binds a native agonist, a transmembrane (TM) domain forming an ion channel pore, and an intracellular domain which is critical for regulation and crosstalk with various signaling pathways. P2XRs have two TM domains (TM1 and TM2), with the N- and C-termini facing the cytoplasm and a large extracellular loop (hereafter referred to as the ectodomain), and functional channels are trimers (35). Since 1994, seven mammalian P2X subunits, termed P2X1–P2X7, have been cloned (141). This was followed by the discovery of P2XRs in numerous vertebrates. The first invertebrate P2XR was found in parasitic trematode Schistosoma mansoni (3). These receptors have also been discovered in more primitive life forms such as the unicellular Dictyostelium discoideum and the eukaryote green algae Ostreococcus tauri; for evolutionary relationship of P2XRs, see Kaczmarek-Hajek et al. (87). The cloned human and rat P2X subunits are between 379 and 595 amino acids long and share 35%–54% sequence identity. The primary sequence of P2X subunits shares no significant homology with other ligand-gated ion channels (141).
The ectodomain contains several conserved residues accounting for the formation of three ATP binding sites at the subunit interface and nonconserved residues accounting for the formation of numerous allosteric binding sites. The term “orthosteric sites” is used to describe ATP binding sites on P2XRs, because they are the primary binding sites for the native ligand needed for the conformational changes that allow for the opening of the channels. Consistently, orthosteric agonists other than ATP substitute for ATP in binding and gating and are called full or partial agonists, depending on the efficacy of coupling. Orthosteric antagonists bind the same sites and do not trigger activation of receptors but compete with agonists for binding to these sites; these are also called competitive inhibitors. For their detailed description, see Coddou et al. (35). The “allosteric sites” are topographically distinct from the orthosteric sites that are recognized by ATP. Occupancy of these binding sites alone has little or no ability to trigger P2XR pore opening, but could modulate the channel activity in the presence of ATP. Allosteric perturbation arises not only from the binding of small or large molecules extracellularly, but also from changes in temperature, ionic strength or concentration, and from covalent modification tethering, glycosylation, phosphorylation, and ubiquination, which could take place extracellularly, in the TM region, or intracellularly. Allosteric regulators of P2XRs and the corresponding literature are listed in Table 1.
Table 1.
Allosteric Compounds of P2XRs
| Compound | P2X1R | P2X2R | P2X3R | P2X4R | P2X5R | P2X7R |
|---|---|---|---|---|---|---|
| Zinc | −(183) | +(28, 115)a | +(183)a | +(2, 31, 161, 190)a | +(182)a | −(1, 114, 175) |
| Copper | +(115, 190)a | −(2, 31, 34) | −(1, 114, 175) | |||
| Mercury | +(32, 115) | −(32, 33) | ||||
| Cadmium | −(135) | +(115) | −(134) | +(2, 33)a | ||
| Calcium | NE (54) | −(50, 54) | −(176)b | −(95) | −(182) | −(168, 175) |
| Magnesium | −(50) | −(66) | −(136) | −(1, 175) | ||
| Gadolinium | −(134) | −(135) | −(67) | |||
| Protons | −(164, 183) | +(28, 164) | −(64, 164) | −(164, 196) | −(182) | −(1, 113, 175) |
| Hydrogen peroxide | +(32) | NE (32) | −(32) | |||
| Myxothiazol | +(32) | |||||
| Rotenone | +(32) | |||||
| CO | +(184) | NE (184) | −(184) | |||
| Progesterone | +(47) | |||||
| Toluene | +(187) | −(187) | +(187) | |||
| Ethanol | −(45, 146) | +(44) | −(45, 189) | |||
| Cybracon blue | +(4) | +(125) | ||||
| Ivermectin | NE (160) | NE (95) | NE (95) | +(79, 95, 147, 159) | NE (95) | |
| Alfaxalone | +(36) | |||||
| ALP | +(36) | |||||
| THDOC | +(36) | |||||
| Propofol | +(172) | +(132) | ||||
| Ketamine | +(132) | |||||
| GW791343 | +(123) | |||||
| Polymyxin B | +(55) | |||||
| MRS2219 | +(77) | |||||
| Tetramethylpyrazine | +(63) | |||||
| Clemastine | +(140) | |||||
| Gintonin | +(24) |
Biphasic effects.
Additional positive effect in recovery from desensitization.
+, potentiation; −, inhibition; NE, no effect; P2XRs, purinergic P2X receptors; CO, carbon monoxide; ALP, allopregnanolone (3α,5α)-3-hydroxy-pregnan-20-one; THDOC, tetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one).
The short TM domains of P2XRs provide a pathway for cation influx. P2XRs are nonselective cation channels with high calcium permeability and the depolarizing nature of current, leading to additional influx to calcium in cells expressing voltage-gated calcium channels (141). The TM2 plays a dominant role in receptor activity, such as channel assembly, gating, ion selectivity, and the permeability of monovalent and divalent ions. Residues contributing to the formation of the P2XR pore gate are not conserved, further indicating that the same function could be achieved by different structures (52, 93, 109, 110, 155). Both TM domains contain residues that are important for ivermectin action in P2X4R and effects of alcohols on channel gating, which were recently reviewed (35, 94).
The N-terminus of all P2XRs is relatively short (20–30 amino acids), whereas the length of the C-terminus ranges from 26 amino acids (P2X6R) to about 239 residues (P2X7R). Both the N- and C-termini serve as molecular targets for a series of post-transcriptional and post-translational modifications, including RNA splicing and protein–protein interactions (141). The C-termini exhibit only sequence relatedness for the first 25 amino-acid residues, and the C-terminal of P2X7R contains a unique Y358-E375 sequence that contributes to the transition from the open to dilated state (81, 194). The N- and C-termini also contain conserved phosphorylation sites for cAMP-dependent protein kinase A (PKA) and protein kinase C (PKC) and other important regions that are responsible for the regulation of P2XRs. Finally, the C-termini contain several motifs that are involved in trafficking and stabilization of the receptors in the plasma membrane and specific protein interactions (98, 151, 180), as well as motifs which are responsible for the regulation of P2XR activities by lipids, a topic recently reviewed (35).
ATP is synthetized in mitochondria as a key product of the oxidative phosphorylation, which is energetically driven by the electron transport, and is released extracellularly through still not well-characterized mechanism. The byproducts of the electron transport are the reactive oxygen species (ROS). Both ATP and ROS are generated in mitochondria in a simultaneous and concerted manner, and ROS is also generated by other mechanisms (19). ATP and its metabolites and ROS play roles in both intracellular and extracellular signaling; our article summarizes the current knowledge of signal crosstalk between P2XRs and ROS. We also review binding sites in P2XRs for essential trace metals, divalent cations and protons, as well as the available data on intracellular regulation of these receptors by calcium and protein kinases. Moreover, protein–protein interactions with either P2XRs or other ligand-gated ionic channels are briefly covered. The reviewed data indicate that allosteric modulation mechanisms together represent a significant diversification of channel structure and activity depending on time and place of receptor activation and patho/physiological environment.
Tridimensional Organization and Gating Properties of P2XRs
Early biophysical experiments suggested that three molecules of ATP were required for channels gating (10). Biochemical (137), pharmacological (165), atomic force microscopy (8), and electron microscopy (197) have corroborated the trimeric nature of P2XRs. A recent solution of the crystal structure confirmed trimeric organization of zebrafish P2X4R (zP2X4R). To describe the structure of P2XR, the authors compared it with the shape of a dolphin with head domain, body segment, left and right flippers, and dorsal fin, with its tail submerged within the lipid bilayer. The ectodomain projects 70 Å above the plasma membrane, where body segments of three subunits mutually intertwine, forming three vestibular cavities along the central axis of the channel (91). A comparison of the zP2X4R with acid-sensing ion channel structure revealed similarity in pore architecture and vestibule polarity (67). The crystal structure confirmed the existence of all five disulfide (SS) bonds (29), with the first three bonds located in the head domain, the SS4 is located within the dorsal fin, and the SS5 at the ends of two β-sheets in the low body region. Crystallization of zP2X4.1R also confirmed the hypothesis that the TMs adopt α-helical structures forming an hourglass shape (91). The homology model of rat (rP2X4R) shows that the TM2 domains form a triangular iris, which represent the pore structure of the closed channel; whereas the three TM1 domains are located parallel to the central axis of the channel on the periphery of TM2 (163).
Numerous scanning mutagenesis studies resulted in a similar conclusion, identifying seven residues to have the potential to contribute to the formation of the ATP binding site (P2X4R numbering): K67, K69, F185, N293, R295, and K313 (53, 82, 149, 195, 199). All of these residues are also present in distantly related P2XRs (algae, slime mold amoebae, and choanoflagellates) (167). The zP2X4.1R crystal structure in open state confirmed that the key residues accounting for ATP binding are located about 45 Å from the plasma membrane. The K67, K69, N293, R295, and K313 residues are critical for recognition of α, β, and γ phosphates, and the adenine moiety lies deeper in the binding cavity. These interactions provided a rationale as to why P2XRs are specific for ATP rather than cytidine 5′-triphosphate (CTP), uridine 5′-triphosphate (UTP), uridine 5′-diphosphate (UDP), or guanosine 5′-triphosphate (GTP) (70). Recent electrophysiological and fluorimetric studies have addressed in detail the ATP binding site and movements of ectodomains regions on receptor activation using an ATP-derived thiol-reactive agonist, NCS-ATP; a compound that covalently binds to the receptor orthosteric site. It has been shown that ATP may have distinct binding orientations within the binding site (83, 85, 116).
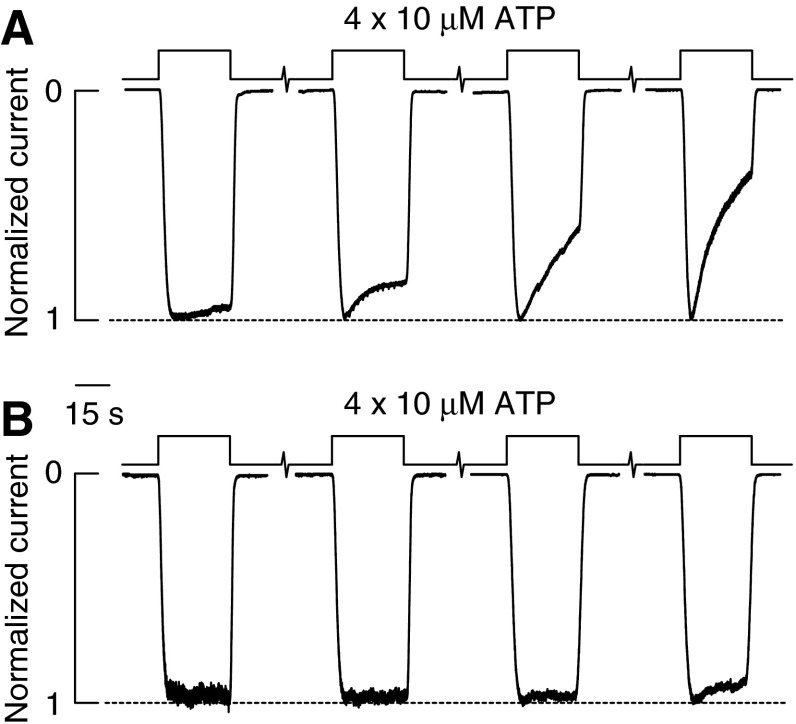
ATP gates rP2XRs in a receptor-specific manner, with the EC50 values ranging from nanomolar to submillimolar concentrations (Fig. 1A). Among receptors, P2X1R and P2X3R have the highest affinity for ATP with an EC50 value in a submicromolar concentration range. ATP is also a full agonist for P2X5R with estimated EC50 values ranging from submicromolar to low micromolar concentration range. P2X2R and P2X4R are also fully activated but less sensitive to ATP, with EC50 values in a low micromolar concentration range. The P2X7Rs is the least sensitive member of the P2XR family to activation by nucleotides, with the EC50 value for ATP in a high micromolar to low millimolar concentration range (35, 94).
FIG. 1.
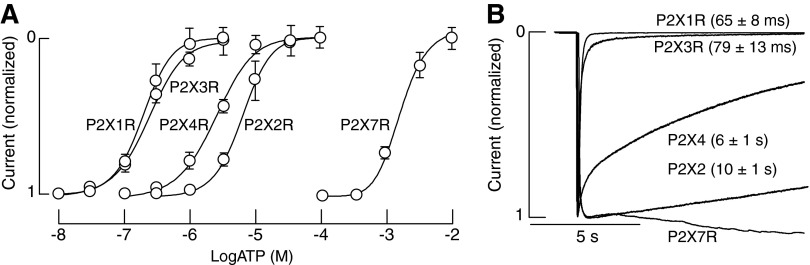
Activation properties of rP2XRs. (A) Concentration–response relationships of five homomeric P2XRs expressed in HEK293 cells. Whole-cell currents were gated with the concentrations of ATP indicated in each case, and currents were normalized against the concentration of ATP that elicited the maximal response. (B) Desensitization profiles of homomeric P2XRs; superimposed recordings showing the different desensitization rates (τdes) derived from monoexponential fitting. Currents were gated with 10 μM (P2X1R and P2X3R), 100 μM (P2X2R and P2X4R), or 3 mM (P2X7R) ATP. P2XRs, purinergic P2×receptors; ATP, adenosine-5′-triphosphate.
The gating of P2XRs usually consists of three phases: a rapid rising phase of inward current induced by the application of agonist (activation phase), a slowly developing decay phase in the presence of an agonist (desensitization phase), and a relatively rapid decay of current after ATP is removed (deactivation phase). The gating kinetics is also receptor specific. Figure 1B illustrates the profile of rP2XR currents in response to supramaximal concentrations of ATP. P2X1R and P2X3R rapidly activate and desensitize, P2X2R and P2X4R slowly desensitize, whereas P2X7R does not show an obvious desensitization but exhibits the secondary current growth (35). Rat P2X5Rs generate low-amplitude nondesensitizing currents, and P2X6R does not express well at the plasma membrane (37). Figure 1 clearly indicates the inverse relationship between the EC50 and desensitization constant values, suggesting that the strength of agonist binding influences receptor desensitization.
Redox Signaling and P2XRs
The redox signaling molecules include both oxygen and nitrogen reactive species, termed ROS and RNS. The oxygen derivate molecules include superoxide anion radical (O2−), hydrogen peroxide (H2O2), and the highly reactive hydroxyl radical (−OH). The nitrogen derivate molecules include nitric oxide (NO), peroxynitrite, nitrogen dioxide, and dinitrogen trioxide (65). In mammalian cells, the physiological generation of ROS occurs in different subcellular compartments: (i) mitochondria (mainly complex I and III); (ii) endoplasmic reticulum (mainly cytochrome P-450 and b5 enzymes); (iii) peroxisomes (mainly fatty acid breakdown); (iv) cytosol (NO synthases, lipoxygenases, prostaglandin endoperoxide (PGH) synthase, and phospholipase A2); and (v) plasma membrane (NAPDH oxidases, lipoxygenase) (19).
During normal metabolism, mitochondria generates ROS as a byproduct of ATP production, but the amount of ROS produced by this mechanism is controversial (19). Phospholipase A2 and lipoxygenase generate H2O2 from the breakdown of fatty acid (105). Xanthine oxidases catabolize the oxidation of xanthine in uric acid, generating two molecules of H2O2 as a byproduct (121). NO synthase catalyzes the conversion of l-arginine in citruline and NO, generating NAD(P)+ and leaking O2− at the expense of NADPH (46). One of the most characterized sources of intracellular ROS is the NADPH oxidase complex; on assembly of these subunits in the plasma membrane, this enzyme via its subunit NOX2 (gp91phox) generates a burst in superoxide anion from the NADPH, which is quickly converted into H2O2 by superoxide dismutases (51). In pathological conditions, such as ischemia, the mitochondria accumulate high concentrations of unpaired electrons (97), which are highly reactive and can combine with the oxygen flowing during reperfusion, producing a burst in superoxide anion generation (97), which is converted in H2O2 by superoxide dismutases. H2O2 can react with Fe2+ or NO and generate −OH or ONOO− (200), which in a form of positive feedback inhibit ATP production and the electron transport chain and increase the production of ROS perpetuating the damage (40).
ROS at high concentrations react with proteins, lipids, carbohydrates, and nucleic acids, causing irreversible functional changes. All of these molecules can induce transient or permanent changes at target proteins through oxidation or reduction of cysteines' sulfhydryls or disulfides. Thiols can be irreversibly oxidized to sulfinic and cysteic acids (65). These irreversible forms of sulfhydryl oxidations are associated with oxidative stress and cell damage. However, during evolution, organisms develop mechanisms to use ROS in physiological processes. At the cellular levels, ROS regulate growth, apoptosis, and intracellular signaling. Several proteins are known to function as effectors for ROS, which are sensitively and reversibly oxidized by these compounds. This includes phosphatases, transcriptional factors, and ion channels. Such ROS-effector proteins commonly have a highly reactive cysteine residue, which reversible oxidation provides transmission of signaling to downstream targets (124).
The relationship between redox signaling and P2XRs has not been well characterized. Early studies documented that the activation of P2X7R increased ROS production in diverse cell types, such as human neutrophils (166), rat microglia (122, 142), rat submandibular glands (57), mouse erythroid cells (178), primary gingival epithelial cells (23), and cultured cortical neurons (138). In concentrations too low to activate P2X7R, ATP also promotes ROS production in mouse myoblasts (157). Extracellular ATP also evokes NO production in type I spiral ganglion neurons through activation of P2XRs (198). A recent report suggests that the P2X4R can indirectly influence ROS production by regulating the P2X7R functions in mouse macrophages (90).
The NADPH oxidase plays a dominant role in P2X7R-induced ROS formation in macrophages and microglia, as estimated in experiments based on the use of pharmacological inhibitors of the NADPH oxidase (72, 122). Experiments with NOX2 knockout mice also support the role of the NADPH oxidase in P2X7R-induced ROS formation in macrophages (127, 139). Moreover, activation of P2X7R in microglia triggers the translocation of NOX2 to the plasma membrane, which is a critical step in the activation of the NADPH oxidase (142). Other studies have proposed that P2X7R-mediated increase in ROS production in human monocytes depends on the MEK/ERK cascade and likely involves the phosphorylation of NOX2 (104). Role of PKC and MAP kinase was also implicated in stimulation of NADPH oxidase and the subsequent generation of ROS in mouse submandibular cells (158). Although the exact mechanism of P2X7R increase in NADPH oxidase activity and ROS production is not known, it seems that the C-terminal domain of the receptor plays a key role because mutant receptors that do not contain specific sequences in this domain are unable to increase ROS production (139). It also appears that P2X7R can trigger mitochondrial ROS production by overloading cells with calcium (178). On the other hand, the action of P2X7R on NO production in THP-1 cells is indirect, by facilitating NO synthase expression (103).
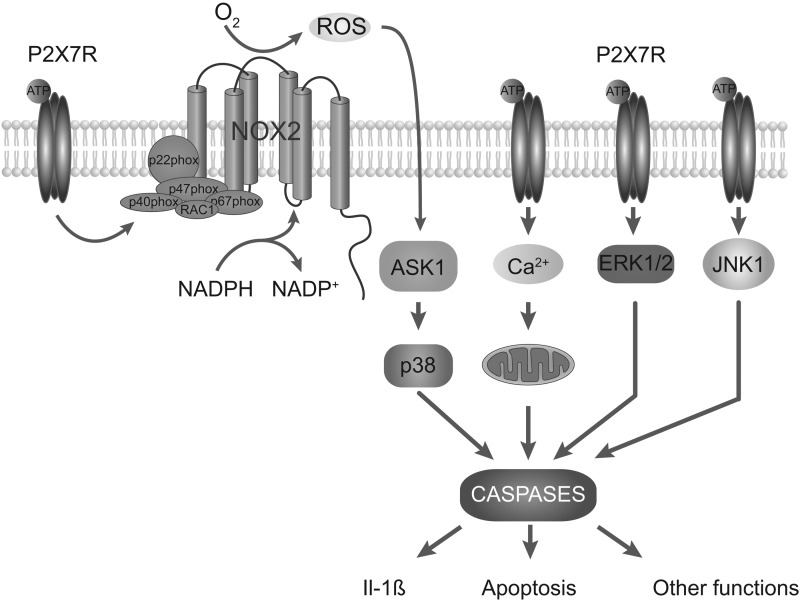
Redox signaling could play a key role in rapid P2X7R induction of interleukin-1β (73), which appears to be coupled to PGE2 release (7). Moreover, it was shown that P2X7R-mediated apoptosis was mediated by ROS production and subsequent activation of the ASK1-p38 MAPK pathway in mice macrophages (139). The P2X7R activation-associated ROS formation is also suggested to account for cell death in murine EOC13 microglia (9). However, in erythroid cells, the P2X7R-induced ROS production is not coupled to apoptosis (178). In cortical neurons, P2X7R-triggered Ca2+ influx and the coupled mitochondrial dysfunction accounted for cell death independently of ROS signaling (138). It has also been suggested that activated P2X7R eliminate Toxoplasma gondii tachyzoites from infected macrophages by ROS (41) and contributes to tissue factor-dependent thrombosis in mice (61). A general scheme of P2X7R-mediated increase and in ROS and ROS-mediated function is shown in Figure 2.
FIG. 2.
Schematic representation of P2X7R-mediated ROS production by NADPH oxidase. Activation of the P2X7R can promote ROS production by NADPH oxidases, a cell membrane complex consisting of several subunits. On assembly of these subunits in the membrane, this enzyme via its NOX2 (gp91phox) catalytic subunit generates a burst of ROS and activation of ASK1-p38 pathway. Activated P2X7Rs trigger NADPH oxidase activity by the translocation of NOX2 to plasma membrane, which is a critical step in the activation of the NADPH oxidase. This represents the major pathway for P2X7R-induced ROS formation. Calcium influx through P2X7R pore and mitochondrial overload could also mediate ROS production. Calcium can also stimulate NO synthase to generate NO, which enhances ROS production by mitochondria. It also appears that P2X7R activates ERK1/2/JNK1 pathways. These pathways trigger activation of various caspases, leading to changes in cellular functions. The best-characterized signaling downstream event of P2X7R activation is mediating the processing and release of IL-1β and IL-18. The P2X7R-induced and caspase-mediated cell apoptosis is also well established. ROS, reactive oxygen species; IL, interleukin.
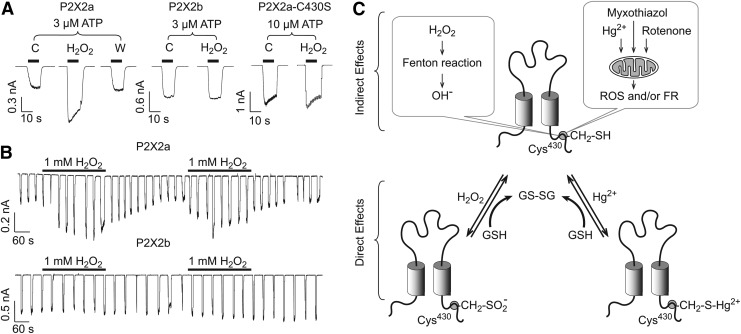
As many other ionic channels (see other articles in this Forum), the activity of P2XRs is affected by changes in the redox balance of cells. Our group reported that H2O2, mercury, and the mitochondrial stress inducers potentiate the activity of rP2X2R. In HEK293 cells, the effect of H2O2 is rapid, reversible, and was prevented with the antioxidant agent N-acetyl-cysteine. Moreover, H2O2 potentiation was only observed in the full-length P2X2aR but not in the shorter P2X2bR. Using site-directed mutagenesis, we further identified a putative intracellular redox sensor, C430. In contrast, the currents mediated by the P2X4R were inhibited by H2O2, suggesting that redox modulation is allosteric and receptor specific (32). These findings indicate that P2XRs can modify their activity depending on the redox state of the cell, as illustrated in Figure 3.
FIG. 3.
The P2X2R activity is regulated by ROS. (A) Representative recordings from HEK293 cells expressing the P2X2aR, P2X2bR, or the P2X2aR-C430S mutant. The potentiation induced by 1 mM H2O2 is observed only in cells expressing the P2X2aR. C, control; W, washout. (B) Effects of H2O2 on 3 μM ATP-evoked currents during repetitive 5 s agonist applications for 10 min in cells expressing P2X2aR (top) or P2X2bR (bottom). (C) Possible mechanisms of P2X2aR modulation by ROS. Cys430 residue could be oxidized indirectly and/or directly by ROS. Top, Indirect effects of H2O2, mercury and the oxidative stress inducers myxothiazol and rotenone could be mediated by ROS and free radicals (FR), including the hydroxyl radical (OH·) produced by mitochondria. H2O2 could also generate OH·through the Fenton reaction. Bottom, ROS could directly oxidize the SH group of Cys430, forming a mercaptide product CH2–S–Hg2+ or a sulfonic acid derivative CH2–SO2−. Endogenous antioxidants such as glutathione (GSH) could partially revert these reactions, resulting in the reduction of the Cys430 residue and the formation of glutathione/glutathione disulfide (GS-SG).
Our results appear to be in conflict with Mason et al. (120), which could reflect experimental differences: (i) we used transient transfected cells, in contrast to stable transfection; (ii) our experiments were done using isolated cells less than 24 h after dissociation, in contrast to nonisolated cells grown in coverslips for 1–5 days; (iii) in contrast to their study, we did not used any pore-forming antifungal in our cell culture to avoid long-term calcium leak or depolarization; (iv) we used divalent and ATP free intracellular buffer, whereas the other group used a different intracellular buffer, which included calcium, ATP, and magnesium, all of which can induce long-term changes in P2X2R activity; (v) our study showed that H2O2, myxothialzol, and rotenone reversible potentiated P2X2R-mediated currents in both HEK 293 cells and Xenopus laevis oocytes; and the Mason et al. (120) study found an initial increase followed by an irreversible decrease in currents after acute application of H2O2, myxothiazol, and rotenone (mitochondrial agents that increase ROS production).
Some reports indicate that ROS and NO can stimulate purinergic signaling in endogenous tissues. For example, in rat vagal lung afferent fibers, H2O2 and hydroxyl radical increase fiber activity and this effect can be prevented with PPADS (154), and ATP-induced NO plays an important role in purinergic signaling in the cochlea (69). When H2O2 was delivered by inhalation to rats, it induced reflex bradypneic responses that are reduced by 50% by PPADS (153). However, it is also possible that ROS have additional effects, increasing ATP release because the bradypneic responses can also be prevented by ATP scavengers (153). Similarly, it has been reported that H2O2 can alter the binding properties of ATP to purinergic receptors cardiac sarcolemmal membranes (129). Recently, an increase of P2X7R-generated current densities in astrocytes treated with rotenone for 48 h (62) has been reported, an effect that could be mediated by ROS. Interestingly, similar effects to that observed with ROS have been described for the gas carbon monoxide; a potentiation of P2X2R-mediated currents; and a modest inhibition or no effect in other subtypes (184). It is known that carbon monoxide can stimulate mitochondrial production of ROS (144), and, therefore, this could explain the similar results between these two studies.
Copper and Zinc as Allosteric Modulators
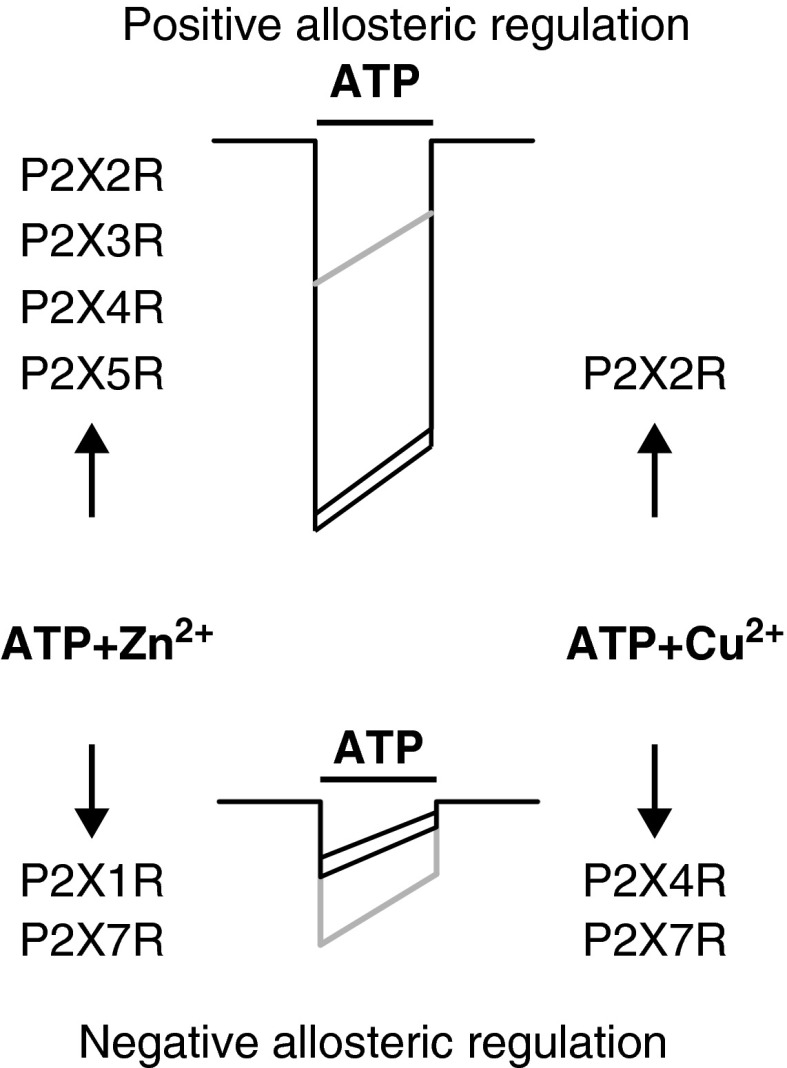
A common aspect of P2XRs is their regulation by essential trace metals, including copper and zinc (Table 1). In general, the nature of trace metal actions, positive or negative, depends on the subtype of receptor and species differences (Fig. 4). In contrast to the ATP-binding sites, the amino acids involved in P2XR trace metal coordination are not conserved and do not appear to be aligned among P2X subunits. This may provide additional rationale for the opposing effects of copper and zinc on receptor function. Experimental observations also support a hypothesis that there are at least two distinct and separate binding sites for trace metals, at least in P2X2R and P2X4R, and that both sites can be occupied by different elements with variable affinities (35).
FIG. 4.
Schematic representation of Zn2+ and Cu2+ allosteric effects. The upper part represents positive allosteric regulation, which results in potentiation of ATP currents in some P2XRs that is exerted by both Zn2+ (left) and Cu2+ (right). The lower part represents negative allosteric regulation, which results in an inhibition of the ATP-currents. Gray traces represent the currents obtained with ATP alone, whereas black traces represent the currents after the joint application of ATP plus Zn2+ or Cu2+.
Both zinc and copper exhibit a biphasic effect on rP2X2R function; a potentiating effect at micromolar concentration ranges; and inhibition at millimolar (35). Zinc also potentiates the ATP responses of native rP2X2Rs and rP2X2/3Rs expressed in neurons from parasympathetic ganglia (118), hypothalamus (177), and dorsal motor nucleus (173). Unlike rat and mouse P2X2Rs that are potentiated, human P2X2R (hP2X2R) is inhibited by zinc (169). Site-directed mutagenesis revealed a critical role of extracellular H120 and H213 in the positive modulation by zinc and copper (28, 115). Histidines 192, 245, and 319 are also partially involved, probably contributing to the stabilization of the allosteric site (115). The allosteric sites for zinc and probably copper are formed by H120 and H213 of adjacent subunits (130), a finding confirmed with the crystallization of P2X4.1R (91). The flexibility of the zinc binding sites of rP2X2R was also tested by shifting two essential histidines 13 residues upstream or downstream from their original position. The ability of zinc to potentiate the mutated channels followed the order H120>H121>H119 and H212>H213>H211 (170). In contrast, it has been difficult to identify the residues that compose the zinc/copper inhibitory site at rP2X2R (28, 59). It appears that high-potency zinc modulation of hP2X2R and low-potency zinc modulation of rP2X2R share a common molecular mechanism (148).
The tridimensional structure of the P2X2R homology model indicates that histidine side chains as coordination ligands of trace metals are spatially close to the ATP binding site, allowing us to propose that these metals modify the ligand binding (35). Figure 5 depicts the histidine moiety (H120 and H213) in the proximity of the ATP-binding site, which differs between the open and closed state of the channel. In the closed channel, the distance between the coordination nitrogens of histidine rings is 2.5-fold longer than in the open channel. Moreover, recent studies have shown that in mutant receptors modified with thiol-reactive fluorophores, zinc is able to induce the opening of the channels even in the absence of ATP (48). Similarly, in P2X2Rs containing the T339S mutation that results in spontaneous openings of the channels, zinc is also able to gate currents in the absence of ATP (84) These studies strongly support the idea that the zinc allosteric site at P2X2R is spatially and mechanistically close to the ATP-binding site.
FIG. 5.
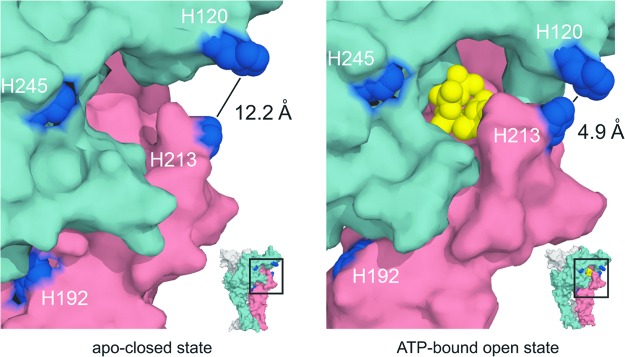
The P2X2R homology model in open and closed state. The model represents the spatial proximity of the ATP-binding site of the crucial Zn2+ and Cu2+-binding histidine residues (blue). The distances between the particular histidine residues (blue) are depicted in a straight line. ATP is depicted in yellow, and particular subunits are indicated in pink and pale green. The particular regions of the molecule are pointed in the miniatures in the inset. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The unique characteristic of P2X4R is its differential modulation by essential trace metals; zinc potentiates receptor activity and copper inhibits it (Fig. 4 and Table 1). Zinc-dependent potentiation is due to an increase in ATP potency, whereas copper decreases the current amplitude without affecting the potency of the agonist. At higher concentrations, zinc inhibits P2X4R function via a reduction in ATP efficacy (2, 183). Among the ectodomain histidines, only the mutation of H140 abolishes the inhibitory action of copper and modifies the pattern of zinc action from a bell-shaped curve, typical of biphasic modulation, to a sigmoid curve, accompanied by an increase in the amplitude of response (34). Subsequent studies revealed that D138 is a second component of the P2X4R inhibitory site, whereas C132 is suggested to account for the positive zinc allosteric effect (31). The homology model of rP2X4R suggested that the H140 residue suggested it is located close to the proposed ATP-binding site which includes N293, R95, and I313 from the same subunit and K67, K69, and F185 from the adjacent subunit (35). Thus, it is reasonable to speculate that D138 and H140 from the same subunit represent the P2X4R-specific region accounting for the inhibitory effects of copper and high concentrations of zinc. The finding that the replacement of C132 abolishes the potentiating effect of zinc also raises the possibility that the SS3 bond is important for zinc potentiation or that this bond is not permanent, but instead breaks and reforms (35). A recent study has indicated additional roles in zinc potentiation for SS1 and SS4; mutations of cysteines that form these bonds resulted in zinc-resistant receptors. Moreover, mutations on cysteines from SS2 resulted in receptors with increased sensitivity to zinc potentiation (108), a similar effect to that observed by our group with the mutation of the inhibitory site (H140, D138). This suggests that the conformational changes induced by mutations in SS1, SS3, or SS4 will disrupt the allosteric site responsible for zinc potentiation, whereas perturbation of SS2 disrupts the inhibitory site for copper and high zinc concentrations. The zinc potentiation is fast and increases with metal preapplication, because it is likely that zinc binds to rP2X4R in the open state in contrast to copper (2), which provides a rationale for why it was easier to predict which residues participate in copper binding based on the crystal structure of the closed receptor (91).
In other P2XRs, there is much less information about trace metal effects and no information about putative allosteric sites. The rP2X7R is inhibited by both copper and zinc, although the latter metal is less potent (1, 114). Copper, but not zinc, also inhibited the mouse P2X7R (128). The mutagenesis studies did not give definitive answers about the binding sites for these metals. One study suggested that the H267A-rP2X7R is a copper-resistant receptor and that H267 and H219 residues account partially for zinc sensitivity (1). The other group has reported a dramatic reduction or almost a complete loss of inhibition by the two metals in cells expressing the H62A and D197A double mutant (114). The function of rP2X1R is inhibited by zinc in a concentration-dependent and competitive manner. In contrast, rP2X3R function is potentiated by zinc, causing a leftward shift in the concentration–response to ATP application, with a maximal effect at 10 μM. However, higher zinc concentrations reduce P2X3R current, suggesting the possible existence of two allosteric sites at this receptor: a high-affinity site with a potentiating effect and a low-affinity site with an inhibitory role (183). The rP2X5R is also modulated in a biphasic fashion by zinc, again suggesting the existence of at least two allosteric sites for this metal (182).
Protons as Allosteric Modulators
Similar to acid-sensing ion channels and the transient receptor potential vanilloid receptor channels, P2XRs can also sense hydrogen ions in the bath medium and respond by modulating the ATP action in a receptor-specific manner (Table 1). In general, P2XRs are inhibited by acidification. The ATP potency for rP2X1R was reduced without altering the maximum current amplitude (164, 183). At rP2X1R, inhibitory effects of extracellular protons and zinc are additive (183). Acidification also reduces the peak amplitude of the rP2X3R current at an EC50 concentration of agonists (164), and it right-shifts the ATP concentration–response curve without altering the peak amplitude of the response (183). Other researchers found an inhibitory effect of acidification that shifts the concentration–response curves to the right and a stimulatory effect which increases the current peak amplitude and activation time constant and accelerates in the recovery from desensitization. The same group also identified the H206 residue as being responsible for these effects (64). It is interesting that fully N-glycosylation of hP2X3Rs is needed to recognize external protons (186). Acidification also decreased the potency of ATP for rP2X4R without a change in the maximum response (71, 164), whereas alkalization enhanced the current amplitude (26) and mutation of H286 removes the extracellular pH sensitivity of this receptor subtype (26, 196). Since this residue is located far from the putative ATP binding site, we may speculate that protons affect gating rather than ATP binding.
Acidification also inhibits the function of P2X5R and P2X7R, but with a pattern of inhibition that differs from other P2XRs; acidification reduced both the potency and efficacy of ATP at rP2X5R (182), whereas it causes a reduction in the peak amplitude of the current without altering the agonist sensitivity at rat and human P2X7R (113). The inhibitory effect of protons on hP2X7R is also visible in single-channel recordings (56). However, changes in the EC50 value of agonists caused by acidification of P2X7R could be masked by the complex biphasic gating properties of these receptors (102, 194) and the sensitization/facilitation of responses during repetitive agonist application (152, 192). It has also been suggested that several residues could contribute to the pH sensitivity of P2X7R, including H130 and D197 (1, 113).
In contrast, for rP2X2R, the potency of all agonists, but not antagonists, is enhanced by acidification 5–10-fold without affecting the maximal agonist response (28, 100, 101, 164). The receptor-specific sensitivity to pH is also obvious in intact cells using single-cell calcium measurements (71). The inhibitory effect is preserved in rP2X2/3R heteromers (164) and is also extremely sensitive to small changes in extracellular pH (about 7.1–7.2) (106, 107); whereas the rP2X1/2R exhibits unique pH sensitivity (20). The pH range is also narrowed for the proton enhancement of the ATP response at the heteromeric rP2X2/6R (99). The basic H319 residue is identified as a putative pH sensor for the rP2X2R (28). This residue is located in a receptor region that has been suggested to operate as a linker region between the ligand-binding domain and the channel pore in other P2XR subunits (150, 195).
The gating of native P2XRs is also altered by changes in pH. Acidic pH potentiates the ATP-gated currents in guinea-pig cochlear outer hair cells (88), mice dorsal root ganglion (DRG) neurons (111), neurons from parasympathetic ganglia (118), and visceral afferent neurons (75). Dictyostelium P2XRs are also modified by acidification, indicating that the pH sensitivity of P2XRs is conserved in evolution (117). These results are consistent with a hypothesis that acidic conditions in the synaptic cleft could modify purinergic transmission, which could be physiologically relevant in certain events, such as pain sensation during inflammation. Finally, ATP-gated receptors can regulate pH-mediated responses, as reflected in the ASIC3 channel sensitization after P2X2R, P2X4R, and P2X5R activation (12).
Extracellular Calcium and Magnesium Affect ATP Gating
There is a large body of information concerning the effects of divalent calcium and magnesium ions on P2XR function. Calcium and magnesium inhibit recombinant channels at millimolar concentrations (141). Native receptors are also sensitive to modulation by calcium (38, 133) and magnesium (126, 171). However, the mechanism of the action of these cations on P2XRs was questioned. The binding of calcium and magnesium to β- and γ-phosphate groups of ATP is a well-established phenomenon (78), but it was unclear as to which form of ATP (ATP4− and/or Mg/Ca-ATP) acts as an orthosteric agonist for this receptor (80). Initially, it was suggested that ATP4− acts as an orthosteric agonist for histamine secretion by mast cells (30). More recently, the effect of ATP on P2X7R currents was studied in the presence and absence of calcium, and the same conclusion was reached (102). In that scenario, effects of divalent cations on P2XR function just reflect change in ratio between ATP4− and Mg/Ca-ATP. Although it has never been supported by direct evidence, this hypothesis has prevailed in the literature during the last two decades.
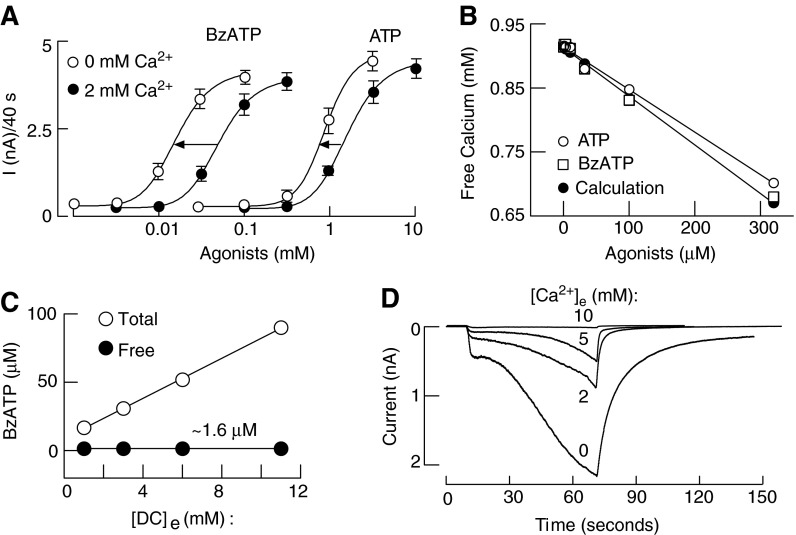
Figure 6A illustrates effects of bath calcium on concentration dependence of BzATP and ATP-induced rP2X7R peak current amplitude reached during 40-s agonist application. For both agonists, there was a leftward shift in agonist concentration of about 0.5 log unit. Figure 6B shows highly comparable concentration-dependent effects of ATP on free calcium concentration obtained by calculations and by measurements for ATP and BzATP, indicating that BzATP also binds calcium. However, these experiments cannot distinguish between the two hypotheses: (i) ATP4− rather than the divalent bound ATP is the native agonist for this receptor subtype or (ii) bath calcium acts as an allosteric inhibitor, and 2 mM calcium causes a rightward shift in the potency of agonists of about 0.5 log unit independent of the concentration of free acid form of agonists. One way to overcome this limitation is to have variable calcium concentrations and identical-free agonist concentrations (Fig. 6C). Under such experimental conditions, the relationship between total agonist concentration and the pattern of current response was lost; practically, the lowest total BzATP concentration was most effective, and the highest concentration was the least effective. Furthermore, although the free acid from of BzATP was identical in all four media, we still observed the full concentration-dependent inhibitory effect of calcium on the peak amplitude of current response (Fig. 6D). This clearly indicates the allosteric nature of action of this cation (193).
FIG. 6.
Calcium antagonism on the P2X7R is allosteric. (A) Concentration–response curves to ATP and BzATP in P2X7R-expressing HEK293 cells. The experiments were performed in the absence (open circles) or in the presence (closed circles) of 2 mM extracellular Ca2+. (B) Concentration-dependent effects of ATP and BzATP on free Ca2+ concentration measured with a calcium ion selective electrode. The calculated free Ca2+ concentrations were obtained using the web Maxc program. (C) A comparable free BzATP concentration of 1.6 μM was achieved with variable total BzATP and variable Ca2+ concentrations. (D) Current amplitudes of the different media shown in (C), containing identical free BzATP concentrations (1.6 μM) but increasing Ca2+ concentrations, demonstrating that Ca2+ itself and not its effects on free BzATP are responsible for current inhibition.
Others reported that extracellular calcium inhibits both the rP2X7R cationic current and dye uptake. The inhibitory effect of this cation on receptor function was mimicked by other divalent cations. For each of these metals, the IC50 values for current and dye uptake were similar: Cu2+>Cd2+∼Zn2+>Ni2+>> Mg2+∼Co2+>Mn2+>Ca2+=Ba2+>> Sr2+. Furthermore, this inhibition was voltage independent, a finding inconsistent with the hypothesis that divalent cations cause functional inhibition by blocking the channel pore (168). Furthermore, the major inhibitory effect of divalent cations was observed in relatively low concentrations, a pattern typical for allosteric modulation (175).
The rP2X7R-H130A mutant is resistant to magnesium inhibition, reinforcing the hypothesis that this metal acts through an allosteric mechanism (1), but not to calcium inhibition (193), further suggesting separate binding sites for these metals. A distinct role of calcium and magnesium in the modulation of native P2X3Rs from cultured rat DRG neurons was also reported. Calcium positively modulated these channels, while magnesium had an inhibitory effect (66). Single-channel recordings of P2X4R revealed that magnesium reversibly decreases the amplitude of ATP-evoked single channel currents in a concentration-dependent manner that is independent of the membrane potential. They also found that magnesium shortens the mean open time without affecting the mean closed time, suggesting that magnesium inhibits the function of human P2X4R by means of an open-channel block via a binding site located at the exterior surface of the pore (136).
Calcium also exhibits a strong inhibitory effect on rP2X2Rs, with a half-maximal block of the current at about 5 mM, but not on hP2X1Rs (54). However, rP2X3R was more than 10-fold less sensitive to blockade by bath calcium, whereas heteromeric rP2X2/3Rs were more sensitive. Thus, the rank of order for inhibition by calcium at these receptors is P2X2R>P2X2/3R>P2X3R>P2X1R (176). Single-channel recordings also confirmed the inhibitory effects of calcium and magnesium on recombinant rP2X2R. These effects manifest as a fast block, visible as a reduction in amplitude of the unitary currents (49).
Calcium has additional effects on P2XR gating. It increases the rates of rP2X2R desensitization, an action that is mimicked by magnesium, barium, and manganese, although the calcium-induced desensitization exhibited a higher affinity and co-operativity (50). Our recent experiments revealed very complex effects of bath calcium on P2X2R gating. During sustained agonist application, receptors continued to desensitize in calcium-independent and calcium-dependent modes. Calcium-independent desensitization was more pronounced in P2X2bR and calcium-dependent desensitization in P2X2aR. In whole-cell recordings, we also observed use-dependent facilitation of desensitization of both receptors (92). Figure 7 illustrates development of use-dependent desensitization of P2X2R in cells bathed in Ca2+-containing medium (Fig. 7A) but not in cells bathed in Ca2+-deficient medium (Fig. 7B). A stimulatory role of calcium in P2X3R recovery from desensitization was also reported (39). These effects of calcium are not necessarily a manifestation of the extracellular allosteric action of this cation, but they probably reflect its intracellular actions (for details, see next).
FIG. 7.
Calcium use-dependent desensitization on the P2X2R. (A) Recordings from the same HEK293 cell expressing the P2X2aR and stimulated with 10 μM ATP for 30 s and washout periods of 3 min in the continuous presence of 2 mM extracellular Ca2+. Note the increase in receptor desensitization after four ATP stimulations. (B) The same protocols as in (A), but using an extracellular media without Ca2+; no use-dependent desensitization is observed under these conditions.
Roles of Intracellular Calcium and Protein Kinases in P2XR Activity
All P2XRs conduct calcium directly through their pores and indirectly by depolarizing cell membrane and activating voltage-gated calcium channels. Intracellularly, calcium binds to various signaling proteins, such as calcineurin or calmodulin (CaM)-kinases, activating its phosphatase or kinase activity. Moreover, CaM alone also modulates channel activity, as has been described for the P2X7R. In cells expressing this receptor, CaM facilitates both current and membrane blebbing. The proposed CaM-binding site is located in the C-terminal sequence I541–S552 (152). Later, it was shown that the hP2X7R is resistant to CaM modulation because it lacks three critical residues of the CaM allosteric site; substitution of these residues with the ones present in the rat receptor resulted in CaM-sensitive receptor (151). In DRG neurons, CaM-dependent protein kinase II potentiates purinergic responses, probably by promoting trafficking of P2XRs (191). In addition, the neuronal calcium sensor VILIP1 was shown to regulate some aspects of P2X2R gating, such as affinity and efficacy of ATP (22).
Calcium, directly or indirectly, can also regulate the activity of PKA, some PKC isoforms, as well as (NO) oxide synthase, leading to generation of NO, stimulation of soluble guanylyl cyclase, and activation of cGMP-dependent protein kinase. PKA has been shown to regulate the rP2X2R; both 8-Br-cAMP and the PKA catalytic subunit inhibit the current amplitudes in cells expressing this receptor and mutation of the C-terminal residue S431 abolishes this effect (25). PKA also potentiates the P2X4R; it has been suggested that this effect is indirect and requires an accessory protein which interacts with the YXXGL endocytosis motif (18). The potentiation of P2X3R currents in DRG neurons mediated by prostaglandin E2 is also PKA dependent (179), as well as the glucocorticoid-induced inhibition of P2XR-mediated currents in HT4 neuroblastoma cells (68). A critical role of the cAMP sensor Epac in the sensitization of native P2X3R has also been reported (179).
Experiments with the PKC activator PMA had shown that this kinase could specifically potentiate the currents mediated by P2X1R and P2X3R currents (17, 143, 174), but not by other subtypes (17). P2X1R-mediated currents are potentiated by serotonin-2A receptor activation, an effect that is probably mediated by PKC (5). Other groups have suggested that PKCγ potentiates P2X7R function in the type-2 astrocyte cell line (76), whereas PMA inhibits P2XR-mediated currents in isolated DRG neurons from adult rats (11).
The N-terminal of all P2X subunits contains a putative binding motif (TXR/K) for PKC phosphorylation. Mutation of the conserved threonine residue results in dramatic changes in receptor activity, altering the current amplitude (P2X1R, P2X2R, and P2X3R) and desensitization profile (P2X2R) (13, 58, 112). However, biochemical experiments failed to show a direct phosphorylation at this conserved threonine residue (17, 58, 174), suggesting that the structural changes induced by mutagenesis and no phosphorylation accounts for the changes observed in receptor function. Similarly, the permeation properties of the P2X7R pore change by replacing T15, and this effect is not related to PKC phosphorylation of this residue (194). An alternative target for ecto-PKC phosphorylation has been proposed for the hP2X3R; point mutations of putative extracellular PKC phosphorylation sites abolished (T134A, S178A) the potentiation of currents observed with UTP (162, 185). It is also possible that the regulation of P2XRs by phosphorylation is indirect. A recent report has shown that P2X3R currents are potentiated by pro-inflammatory agents which activate protease-activated receptor-2, and this effect can be prevented by inhibition of PKA or PKC in DRG neurons (180). Parkin, an ubiquitin E3 ligase, can also regulate the function of P2XRs in PC12 cells by indirectly modulating the activity of PKA and other kinases (156).
Other protein kinases also affect the P2XR function. P2X3R-mediated currents are decreased by both the C-terminal Src kinase and the cyclin-dependent kinase-5 (Cdk5). For the C-terminal Src kinase-dependent regulation, the C-terminal T393 residue has been proposed as a target for phosphorylation, whereas the Cdk5 regulation could be mediated by an accessory protein (42, 131). In mouse trigeminal sensory neurons, phosphorylation of the P2X3R is controlled by nerve growth factors (43).
Regulation of P2XR Activity by Interaction with Other Proteins
There is growing evidence that P2XRs can associate with other proteins and these interactions can modify the gating properties of channels. In addition to the previously mentioned VILIP1 and CaM which regulate the activity of the P2X2R and the P2X7R, it has also been proposed that the beta-amyloid precursor protein-binding proteins Fe65 interacts with the P2X2R, resulting in changes in the permeability properties of this receptor (119). More interestingly, it has been also shown that P2XRs can interact with other ligand-gated ionic channels, as the observed cross-inhibition (the magnitude of the current gated by simultaneous activation is smaller than the sum predicted by activating each one separately) between P2X2Rs and nicotinic AChRs that is observed in both heterologous expressed and endogenous receptors (96). Moreover, cross-inhibition has been observed between RHO1 GABA receptors and both the P2X2R (15) and the P2X4R (188). Similar interactions have been found between these P2XRs and GABAA receptors (16, 86). Another study also points to a mutual cross-inhibition between P2X2 and 5-HT3 receptors in heterologous and native systems (14). Finally, the level of receptor expression also influences the gating properties of the P2X2R, such as ATP affinity (27), ionic permeability, and rectification properties (60), suggesting that receptor-receptor interactions and cooperative mechanisms play a role in P2XR-mediated responses.
Physiological and Pathological Implications of P2XR Allosteric Regulation
After 40 years from the proposal of purinergic signaling by Burnstock (21), there is no doubt that P2XRs are allosterically regulated by a myriad of endogenous and exogenous compounds. The pharmacological relevance of such modulation is obvious. Allosteric modulation of P2XRs may also be of physiological relevance. For example, both zinc and copper are released during synaptic transmission (6, 89) and they can reach micromolar concentrations in the synaptic cleft where they could exert their allosteric actions. In this context, a remarkable example is the zinc potentiation of glycinergic transmission. A knock-in mouse that expresses a zinc-insensitive GlyR instead of the wild type resulted in a hyperekplexia phenotype (74). This mutated receptor had similar expression and agonist affinity compared with the wild type, and the only difference was its insensitivity to zinc, unmasking that endogenous allosteric regulation by this metal is crucial for normal glycinergic transmission. Similar approaches with P2XRs transgenic mice could unmask the role of allosteric modulation in this family of receptors.
On the other hand, some pathological conditions could result in an excessive accumulation of divalent metals, impairing normal synaptic transmission; that is, the case of Wilson's disease in which a nonfunctional P-type copper transporter ATPase ATP7B results in copper accumulation that can lead to neurological disorders, within other symptoms (181) or during the immune response, where the copper levels can drastically change (145). At least a part of these effects may result from constant allosteric modulation of ionic channels, including P2XRs. In this context, the identification of binding sites and mechanisms of action could have relevance in the design of drugs and therapeutic strategies to treat these and other pathologies.
Similarly, allosteric modulation by protons and ROS could be of physiological relevance; as well as in divalent metals, the levels of ROS and pH may not only dramatically change during pathological events, but they can also slightly change during normal physiological processes. In this scenario, P2XRs are polymodal sensors that can change their gating properties not only after binding extracellular ATP, but also after changes in its extracellular and intracellular milieu. Again, knowing and understanding allosteric modulation could provide a valuable tool to treat diverse pathologies, such us pain-related diseases and states. In this regard, the availability of both closed and open crystal of the zP2X4.1R constitutes a significant advantage to achieve this goal.
Conclusions and Future Perspectives
P2XRs have numerous regulatory sites in both the extra- and intracellular domains, which provide the physiological and pharmacological frames for the modulation of the activity of these channels by a complex set of synapse- and/or paracrine-derived native agents, intracellular metabolites, and drugs. In contrast to the ATP-binding sites, the nonconserved residues appear to play a critical role in allosteric modulation of these channels by trace metals, divalent cations, and protons, which explain the receptor specificity of the actions of these molecules. P2XRs are also modulated by second messengers and membrane lipids acting intracellularly. The gating of P2XRs can also be regulated by redox signaling, and the formation and production of ROS can be facilitated by activation of P2XRs, constituting a mutual feedback that is of physiological relevance. Thus, as with other ligand-gated receptor channels, the P2XR affinity for agonists and/or the changes in their maximal responses is not only determined by the orthosteric ligand concentration, but also depends on the availability of allosteric regulators in the receptor milieu. These findings provide a basis for further studies addressing the dependence of the activity of a particular receptor on the metabolic state of cells, tissues, and organs.
In general, allosteric modulators constitute a valuable alternative for future drug design, especially for receptor-specific drugs. The current information regarding the residues involved in orthosteric and allosteric modulation combined with the channel structure provided by crystallization will also lead to a better understanding of the structure–activity relationships that govern the gating of P2XRs. Specifically, the close topological location of the trace metal coordination amino acid residues to the orthosteric ligand sites provides important clues for further structural modeling and investigation into the actions of metals in the receptor ectodomain. Since trace metals can be released to the synaptic cleft and exert effects on P2XR signaling, further studies are needed to clarify the physiological conditions for this action in vivo. Changes in cellular pH caused by ischemia could affect P2X3R activity places allosteric modulation by protons in a more physiological/pathophysiological context. Future experiments should also be directed in order to identify mechanisms and allosteric sites for ROS at P2XRs and to elucidate the signaling pathways that are responsible for P2XR-mediated ROS formation. In this regard, it is also necessary to test whether other P2XRs are regulated by or regulate the redox balance of cells.
Abbreviations Used
- ALP
allopregnanolone (3α,5α)-3-hydroxy-pregnan-20-one
- ATP
adenosine-5′-phosphate
- CaM
calmodulin
- Cdk5
cyclin-dependent kinase-5
- CTP
cytidine 5′-triphosphate
- DRG
dorsal root ganglion
- H2O2
hydrogen peroxide
- IL
interleukin
- NO
nitric oxide
- PGH
prostaglandin endoperoxide synthase
- PKA
protein kinase A
- PKC
protein kinase C
- P2XRs
purinergic P2X receptors
- ROS
reactive oxygen species
- SS
disulfide
- THDOC
tetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one)
- TM
transmembrane
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-triphosphate
- zP2X4R
zebrafish P2X4R
Acknowledgments
This research was funded by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH (S.S., E.L.-S., and C.C.) and the FONDECYT Initiation Grant 11121302 (C.C.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1.Acuna-Castillo C, Coddou C, Bull P, Brito J, and Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem 101: 17–26, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Acuna-Castillo C, Morales B, and Huidobro-Toro JP. Zinc and copper modulate differentially the P2X4 receptor. J Neurochem 74: 1529–1537, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Agboh KC, Webb TE, Evans RJ, and Ennion SJ. Functional characterization of a P2×receptor from Schistosoma mansoni. J Biol Chem 279: 41650–41657, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Alexander K, Niforatos W, Bianchi B, Burgard EC, Lynch KJ, Kowaluk EA, Jarvis MF, and van Biesen T. Allosteric modulation and accelerated resensitization of human P2X(3) receptors by cibacron blue. J Pharmacol Exp Ther 291: 1135–1142, 1999 [PubMed] [Google Scholar]
- 5.Ase AR, Raouf R, Belanger D, Hamel E, and Seguela P. Potentiation of P2X1 ATP-gated currents by 5-hydroxytryptamine 2A receptors involves diacylglycerol-dependent kinases and intracellular calcium. J Pharmacol Exp Ther 315: 144–154, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Assaf SY. and Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature 308: 734–736, 1984 [DOI] [PubMed] [Google Scholar]
- 7.Barbera-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, and Pelegrin P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1beta release. FASEB J 26: 2951–2962, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, and Edwardson JM. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem 280: 10759–10765, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bartlett R, Yerbury JJ, and Sluyter R. P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm 2013: 18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bean BP, Williams CA, and Ceelen PW. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci 10: 11–19, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bie BH, Zhang YH, and Zhao ZQ. Inhibition of P2×receptor-mediated inward current by protein kinase C in small-diameter dorsal root ganglion neurons of adult rats. Neurosci Bull 25: 179–186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, and McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68: 739–749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boue-Grabot E, Archambault V, and Seguela P. A protein kinase C site highly conserved in P2×subunits controls the desensitization kinetics of P2X(2) ATP-gated channels. J Biol Chem 275: 10190–10195, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Boue-Grabot E, Barajas-Lopez C, Chakfe Y, Blais D, Belanger D, Emerit MB, and Seguela P. Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. J Neurosci 23: 1246–1253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boue-Grabot E, Emerit MB, Toulme E, Seguela P, and Garret M. Cross-talk and co-trafficking between rho1/GABA receptors and ATP-gated channels. J Biol Chem 279: 6967–6975, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Boue-Grabot E, Toulme E, Emerit MB, and Garret M. Subunit-specific coupling between gamma-aminobutyric acid type A and P2X2 receptor channels. J Biol Chem 279: 52517–52525, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Brown DA. and Yule DI. Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim Biophys Acta 1773: 166–175, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DA. and Yule DI. Protein kinase A regulation of P2X(4) receptors: requirement for a specific motif in the C-terminus. Biochim Biophys Acta 1803: 275–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GC. and Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 12: 1–4, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Brown SG, Townsend-Nicholson A, Jacobson KA, Burnstock G, and King BF. Heteromultimeric P2X(1/2) receptors show a novel sensitivity to extracellular pH. J Pharmacol Exp Ther 300: 673–680, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnstock G. Purinergic nerves. Pharmacol Rev 24: 509–581, 1972 [PubMed] [Google Scholar]
- 22.Chaumont S, Compan V, Toulme E, Richler E, Housley GD, Rassendren F, and Khakh BS. Regulation of P2X2 receptors by the neuronal calcium sensor VILIP1. Sci Signal 1: ra8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi CH, Spooner R, DeGuzman J, Koutouzis T, Ojcius DM, and Yilmaz O. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell Microbiol 15: 961–976, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, Kim HJ, Kim BR, Shin TJ, Hwang SH, Lee BH, Lee SM, Rhim H, and Nah SY. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, potentiates ATP-gated P2X(1) receptor channel currents. Mol Cells 35: 142–150, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow YW. and Wang HL. Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J Neurochem 70: 2606–2612, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Clarke CE, Benham CD, Bridges A, George AR, and Meadows HJ. Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol 523(Pt 3): 697–703, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clyne JD, Brown TC, and Hume RI. Expression level dependent changes in the properties of P2X2 receptors. Neuropharmacology 44: 403–412, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Clyne JD, LaPointe LD, and Hume RI. The role of histidine residues in modulation of the rat P2X(2) purinoceptor by zinc and pH. J Physiol 539: 347–359, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clyne JD, Wang LF, and Hume RI. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci 22: 3873–3880, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockcroft S, and Gomperts BD. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol 296: 229–243, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coddou C, Acuna-Castillo C, Bull P, and Huidobro-Toro JP. Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J Biol Chem 282: 36879–36886, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Coddou C, Codocedo JF, Li S, Lillo JG, Acuna-Castillo C, Bull P, Stojilkovic SS, and Huidobro-Toro JP. Reactive oxygen species potentiate the P2X2 receptor activity through intracellular Cys430. J Neurosci 29: 12284–12291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coddou C, Lorca RA, Acuna-Castillo C, Grauso M, Rassendren F, and Huidobro-Toro JP. Heavy metals modulate the activity of the purinergic P2X4 receptor. Toxicol Appl Pharmacol 202: 121–131, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Coddou C, Morales B, Gonzalez J, Grauso M, Gordillo F, Bull P, Rassendren F, and Huidobro-Toro JP. Histidine 140 plays a key role in the inhibitory modulation of the P2X4 nucleotide receptor by copper but not zinc. J Biol Chem 278: 36777–36785, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, and Stojilkovic SS. Activation and regulation of purinergic P2×receptor channels. Pharmacol Rev 63: 641–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Codocedo JF, Rodriguez FE, and Huidobro-Toro JP. Neurosteroids differentially modulate P2×ATP-gated channels through non-genomic interactions. J Neurochem 110: 734–744, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, and Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16: 2495–2507, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook SP. and McCleskey EW. Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2×receptors in nociceptors. Neuropharmacology 36: 1303–1308, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Cook SP, Rodland KD, and McCleskey EW. A memory for extracellular Ca2+ by speeding recovery of P2×receptors from desensitization. J Neurosci 18: 9238–9244, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper CE. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem Sci 27: 33–39, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Correa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, and Coutinho-Silva R. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect 12: 497–504, 2010 [DOI] [PubMed] [Google Scholar]
- 42.D'Arco M, Giniatullin R, Leone V, Carloni P, Birsa N, Nair A, Nistri A, and Fabbretti E. The C-terminal Src inhibitory kinase (Csk)-mediated tyrosine phosphorylation is a novel molecular mechanism to limit P2X3 receptor function in mouse sensory neurons. J Biol Chem 284: 21393–21401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, and Fabbretti E. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci 27: 8190–8201, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, and Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology 49: 243–253, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Davies DL, Machu TK, Guo Y, and Alkana RL. Ethanol sensitivity in ATP-gated P2×receptors is subunit dependent. Alcohol Clin Exp Res 26: 773–778, 2002 [PubMed] [Google Scholar]
- 46.Dawson TM, Dawson VL, and Snyder SH. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol 32: 297–311, 1992 [DOI] [PubMed] [Google Scholar]
- 47.De Roo M, Boue-Grabot E, and Schlichter R. Selective potentiation of homomeric P2X2 ionotropic ATP receptors by a fast non-genomic action of progesterone. Neuropharmacology 58: 569–577, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Dellal SS. and Hume RI. Covalent modification of mutant rat P2X2 receptors with a thiol-reactive fluorophore allows channel activation by zinc or acidic pH without ATP. PLoS One 7: e47147, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding S. and Sachs F. Ion permeation and block of P2X(2) purinoceptors: single channel recordings. J Membr Biol 172: 215–223, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Ding S. and Sachs F. Inactivation of P2X2 purinoceptors by divalent cations. J Physiol 522(Pt 2): 199–214, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Egan TM, Haines WR, and Voigt MM. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J Neurosci 18: 2350–2359, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ennion S, Hagan S, and Evans RJ. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J Biol Chem 275: 35656, 2000 [PubMed] [Google Scholar]
- 54.Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, and North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2×receptors) expressed in mammalian cells. J Physiol 497(Pt 2): 413–422, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, and Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Flittiger B, Klapperstuck M, Schmalzing G, and Markwardt F. Effects of protons on macroscopic and single-channel currents mediated by the human P2X7 receptor. Biochim Biophys Acta 1798: 947–957, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Fontanils U, Seil M, Pochet S, El Ouaaliti M, Garcia-Marcos M, Dehaye JP, and Marino A. Stimulation by P2X(7) receptors of calcium-dependent production of reactive oxygen species (ROS) in rat submandibular glands. Biochim Biophys Acta 1800: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Franklin C, Braam U, Eisele T, Schmalzing G, and Hausmann R. Lack of evidence for direct phosphorylation of recombinantly expressed P2X(2) and P2×(3) receptors by protein kinase C. Purinergic Signal 3: 377–388, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friday SC. and Hume RI. Contribution of extracellular negatively charged residues to ATP action and zinc modulation of rat P2X2 receptors. J Neurochem 105: 1264–1275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujiwara Y. and Kubo Y. Density-dependent changes of the pore properties of the P2X2 receptor channel. J Physiol 558: 31–43, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, and Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest 121: 2932–2944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao XF, Wang W, Yu Q, Burnstock G, Xiang ZH, and He C. Astroglial P2X7 receptor current density increased following long-term exposure to rotenone. Purinergic Signal 7: 65–72, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Y, Xu C, Yu K, Li G, Wan F, Liu S, Lin J, Liu H, Zhang J, Li X, and Liang S. Effect of tetramethylpyrazine on DRG neuron P2X3 receptor involved in transmitting pain after burn. Burns 36: 127–134, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Gerevich Z, Zadori ZS, Koles L, Kopp L, Milius D, Wirkner K, Gyires K, and Illes P. Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J Biol Chem 282: 33949–33957, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Ghezzi P. and Bonetto V. Redox proteomics: identification of oxidatively modified proteins. Proteomics 3: 1145–1153, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Giniatullin R, Sokolova E, and Nistri A. Modulation of P2X3 receptors by Mg2+ on rat DRG neurons in culture. Neuropharmacology 44: 132–140, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Gonzales EB, Kawate T, and Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2×receptors. Nature 460: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han JZ, Lin W, and Chen YZ. Inhibition of ATP-induced calcium influx in HT4 cells by glucocorticoids: involvement of protein kinase A. Acta Pharmacol Sin 26: 199–204, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Harada N. Role of nitric oxide on purinergic signalling in the cochlea. Purinergic Signal 6: 211–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hattori M. and Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2×receptors. Nature 485: 207–212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He ML, Zemkova H, Koshimizu TA, Tomic M, and Stojilkovic SS. Intracellular calcium measurements as a method in studies on activity of purinergic P2×receptor channels. Am J Physiol Cell Physiol 285: C467–C479, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Hewinson J. and Mackenzie AB. P2X(7) receptor-mediated reactive oxygen and nitrogen species formation: from receptor to generators. Biochem Soc Trans 35: 1168–1170, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Hewinson J, Moore SF, Glover C, Watts AG, and MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1 beta processing in human monocytes. J Immunol 180: 8410–8420, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Hirzel K, Muller U, Latal AT, Hulsmann S, Grudzinska J, Seeliger MW, Betz H, and Laube B. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn(2+) as an essential endogenous modulator of glycinergic neurotransmission. Neuron 52: 679–690, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 201: 63–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hung AC, Chu YJ, Lin YH, Weng JY, Chen HB, Au YC, and Sun SH. Roles of protein kinase C in regulation of P2X7 receptor-mediated calcium signalling of cultured type-2 astrocyte cell line, RBA-2. Cell Signal 17: 1384–1396, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Jacobson KA, Kim YC, Wildman SS, Mohanram A, Harden TK, Boyer JL, King BF, and Burnstock G. A pyridoxine cyclic phosphate and its 6-azoaryl derivative selectively potentiate and antagonize activation of P2X1 receptors. J Med Chem 41: 2201–2206, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jahngen JH. and Rossomando EF. Resolution of an ATP-metal chelate from metal-free ATP by reverse-phase high-performance liquid chromatography. Anal Biochem 130: 406–415, 1983 [DOI] [PubMed] [Google Scholar]
- 79.Jelinkova I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, and Zemkova H. Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun 349: 619–625, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Jiang LH. Inhibition of P2X(7) receptors by divalent cations: old action and new insight. Eur Biophys J 38: 339–346, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, and North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. Am J Physiol Cell Physiol 289: C1295–C1302, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Jiang LH, Rassendren F, Surprenant A, and North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2×receptor. J Biol Chem 275: 34190–34196, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Jiang R, Lemoine D, Martz A, Taly A, Gonin S, Prado de Carvalho L, Specht A, and Grutter T. Agonist trapped in ATP-binding sites of the P2X2 receptor. Proc Natl Acad Sci USA 108: 9066–9071, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang R, Taly A, Lemoine D, Martz A, Cunrath O, and Grutter T. Tightening of the ATP-binding sites induces the opening of P2×receptor channels. EMBO J 31: 2134–2143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang R, Taly A, Lemoine D, Martz A, Specht A, and Grutter T. Intermediate closed channel state(s) precede(s) activation in the ATP-gated P2X2 receptor. Channels (Austin) 6: 398–402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jo YH, Donier E, Martinez A, Garret M, Toulme E, and Boue-Grabot E. Cross-talk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. J Biol Chem 286: 19993–20004, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, and Nicke A. Molecular and functional properties of P2×receptors—recent progress and persisting challenges. Purinergic Signal 8: 375–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanjhan R, Raybould NP, Jagger DJ, Greenwood D, and Housley GD. Allosteric modulation of native cochlear P2×receptors: insights from comparison with recombinant P2X2 receptors. Audiol Neurootol 8: 115–128, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Kardos J, Kovacs I, Hajos F, Kalman M, and Simonyi M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci Lett 103: 139–144, 1989 [DOI] [PubMed] [Google Scholar]
- 90.Kawano A, Tsukimoto M, Mori D, Noguchi T, Harada H, Takenouchi T, Kitani H, and Kojima S. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun 420: 102–107, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Kawate T, Michel JC, Birdsong WT, and Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460: 592–598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khadra A, Yan Z, Coddou C, Tomic M, Sherman A, and Stojilkovic SS. Gating properties of the P2X2a and P2X2b receptor channels: experiments and mathematical modeling. J Gen Physiol 139: 333–348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khakh BS. and Egan TM. Contribution of transmembrane regions to ATP-gated P2X2 channel permeability dynamics. J Biol Chem 280: 6118–6129, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Khakh BS. and North RA. Neuromodulation by extracellular ATP and P2×receptors in the CNS. Neuron 76: 51–69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, and Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci 19: 7289–7299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khakh BS, Zhou X, Sydes J, Galligan JJ, and Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature 406: 405–410, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Khodorov B, Pinelis V, Vergun O, Storozhevykh T, and Vinskaya N. Mitochondrial deenergization underlies neuronal calcium overload following a prolonged glutamate challenge. FEBS Lett 397: 230–234, 1996 [DOI] [PubMed] [Google Scholar]
- 98.Kim M, Jiang LH, Wilson HL, North RA, and Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 20: 6347–6358, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.King BF, Townsend-Nicholson A, Wildman SS, Thomas T, Spyer KM, and Burnstock G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J Neurosci 20: 4871–4877, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.King BF, Wildman SS, Ziganshina LE, Pintor J, and Burnstock G. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br J Pharmacol 121: 1445–1453, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.King BF, Ziganshina LE, Pintor J, and Burnstock G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br J Pharmacol 117: 1371–1373, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klapperstuck M, Buttner C, Schmalzing G, and Markwardt F. Functional evidence of distinct ATP activation sites at the human P2X(7) receptor. J Physiol 534: 25–35, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee WG, Lee SD, Cho JH, Jung Y, Kim JH, Hien TT, Kang KW, Ko H, and Kim YC. Structure-activity relationships and optimization of 3,5-dichloropyridine derivatives as novel P2X(7) receptor antagonists. J Med Chem 55: 3687–3698, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Lenertz LY, Gavala ML, Hill LM, and Bertics PJ. Cell signaling via the P2X(7) nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal 5: 175–187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lewis RA, Austen KF, and Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med 323: 645–655, 1990 [DOI] [PubMed] [Google Scholar]
- 106.Li C, Peoples RW, and Weight FF. Acid pH augments excitatory action of ATP on a dissociated mammalian sensory neuron. Neuroreport 7: 2151–2154, 1996 [DOI] [PubMed] [Google Scholar]
- 107.Li C, Peoples RW, and Weight FF. Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. J Neurophysiol 76: 3048–3058, 1996 [DOI] [PubMed] [Google Scholar]
- 108.Li CY, Xiong KM, Wu YX, Liu YW, Chen L, Stewart RR, Peoples RW, and Yi CL. Conserved extracellular cysteines differentially regulate the potentiation produced by Zn2+ in rat P2X4 receptors. Eur J Pharmacol 707: 11–16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li M, Chang TH, Silberberg SD, and Swartz KJ. Gating the pore of P2×receptor channels. Nat Neurosci 11: 883–887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Z, Migita K, Samways DS, Voigt MM, and Egan TM. Gain and loss of channel function by alanine substitutions in the transmembrane segments of the rat ATP-gated P2X2 receptor. J Neurosci 24: 7378–7386, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, and Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2×, and TRPV1. J Neurophysiol 100: 1184–1201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu GJ, Brockhausen J, and Bennett MR. P2X1 receptor currents after disruption of the PKC site and its surroundings by dominant negative mutations in HEK293 cells. Auton Neurosci 108: 12–16, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Liu X, Ma W, Surprenant A, and Jiang LH. Identification of the amino acid residues in the extracellular domain of rat P2X(7) receptor involved in functional inhibition by acidic pH. Br J Pharmacol 156: 135–142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu X, Surprenant A, Mao HJ, Roger S, Xia R, Bradley H, and Jiang LH. Identification of key residues coordinating functional inhibition of P2X7 receptors by zinc and copper. Mol Pharmacol 73: 252–259, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Lorca RA, Coddou C, Gazitua MC, Bull P, Arredondo C, and Huidobro-Toro JP. Extracellular histidine residues identify common structural determinants in the copper/zinc P2X2 receptor modulation. J Neurochem 95: 499–512, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Lorinczi E, Bhargava Y, Marino SF, Taly A, Kaczmarek-Hajek K, Barrantes-Freer A, Dutertre S, Grutter T, Rettinger J, and Nicke A. Involvement of the cysteine-rich head domain in activation and desensitization of the P2X1 receptor. Proc Natl Acad Sci USA 109: 11396–11401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ludlow MJ, Traynor D, Fisher PR, and Ennion SJ. Purinergic-mediated Ca2+ influx in Dictyostelium discoideum. Cell Calcium 44: 567–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma B, Ruan HZ, Burnstock G, and Dunn PM. Differential expression of P2×receptors on neurons from different parasympathetic ganglia. Neuropharmacology 48: 766–777, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Masin M, Kerschensteiner D, Dumke K, Rubio ME, and Soto F. Fe65 interacts with P2X2 subunits at excitatory synapses and modulates receptor function. J Biol Chem 281: 4100–4108, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Mason HS, Bourke S, and Kemp PJ. Selective modulation of ligand-gated P2×purinoceptor channels by acute hypoxia is mediated by reactive oxygen species. Mol Pharmacol 66: 1525–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 121.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163, 1985 [DOI] [PubMed] [Google Scholar]
- 122.Mead EL, Mosley A, Eaton S, Dobson L, Heales SJ, and Pocock JM. Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial-neuronal interactions. J Neurochem 121: 287–301, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Michel AD, Chambers LJ, and Walter DS. Negative and positive allosteric modulators of the P2X(7) receptor. Br J Pharmacol 153: 737–750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miki H. and Funato Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J Biochem 151: 255–261, 2012 [DOI] [PubMed] [Google Scholar]
- 125.Miller KJ, Michel AD, Chessell IP, and Humphrey PP. Cibacron blue allosterically modulates the rat P2X4 receptor. Neuropharmacology 37: 1579–1586, 1998 [DOI] [PubMed] [Google Scholar]
- 126.Miyoshi H, Yamaoka K, Urabe S, Kodama M, and Kudo Y. Functional expression of purinergic P2X7 receptors in pregnant rat myometrium. Am J Physiol Regul Integr Comp Physiol 298: R1117–R1124, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Moore SF. and MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol 183: 3302–3308, 2009 [DOI] [PubMed] [Google Scholar]
- 128.Moore SF. and Mackenzie AB. Species and agonist dependent zinc modulation of endogenous and recombinant ATP-gated P2X7 receptors. Biochem Pharmacol 76: 1740–1747, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Musat S. and Dhalla NS. Alteration in cardiac sarcolemmal ATP receptors by oxyradicals. Ann N Y Acad Sci 793: 1–12, 1996 [DOI] [PubMed] [Google Scholar]
- 130.Nagaya N, Tittle RK, Saar N, Dellal SS, and Hume RI. An intersubunit zinc binding site in rat P2X2 receptors. J Biol Chem 280: 25982–25993, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nair A, Simonetti M, Fabbretti E, and Nistri A. The Cdk5 kinase downregulates ATP-gated ionotropic P2X3 receptor function via serine phosphorylation. Cell Mol Neurobiol 30: 505–509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakanishi M, Mori T, Nishikawa K, Sawada M, Kuno M, and Asada A. The effects of general anesthetics on P2X7 and P2Y receptors in a rat microglial cell line. Anesth Analg 104: 1136–1144, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Nakazawa K. and Hess P. Block by calcium of ATP-activated channels in pheochromocytoma cells. J Gen Physiol 101: 377–392, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakazawa K. and Ohno Y. Effects of neuroamines and divalent cations on cloned and mutated ATP-gated channels. Eur J Pharmacol 325: 101–108, 1997 [DOI] [PubMed] [Google Scholar]
- 135.Nakazawa K, Liu M, Inoue K, and Ohno Y. pH dependence of facilitation by neurotransmitters and divalent cations of P2X2 purinoceptor/channels. Eur J Pharmacol 337: 309–314, 1997 [DOI] [PubMed] [Google Scholar]
- 136.Negulyaev YA. and Markwardt F. Block by extracellular Mg2+ of single human purinergic P2X4 receptor channels expressed in human embryonic kidney cells. Neurosci Lett 279: 165–168, 2000 [DOI] [PubMed] [Google Scholar]
- 137.Nicke A, Baumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, and Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17: 3016–3028, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nishida K, Nakatani T, Ohishi A, Okuda H, Higashi Y, Matsuo T, Fujimoto S, and Nagasawa K. Mitochondrial dysfunction is involved in P2X7 receptor-mediated neuronal cell death. J Neurochem 122: 1118–1128, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, and Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem 283: 7657–7665, 2008 [DOI] [PubMed] [Google Scholar]
- 140.Norenberg W, Hempel C, Urban N, Sobottka H, Illes P, and Schaefer M. Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations. J Biol Chem 286: 11067–11081, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.North RA. Molecular physiology of P2×receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, and Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem 278: 13309–13317, 2003 [DOI] [PubMed] [Google Scholar]
- 143.Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, and Grunder S. Inflammatory mediators potentiate ATP-gated channels through the P2X(3) subunit. J Biol Chem 276: 21077–21082, 2001 [DOI] [PubMed] [Google Scholar]
- 144.Peers C, Dallas M, and Scragg JL. Ion channels as effectors in carbon mnonoxide signaling. Commun Integr Biol 2: 241–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Percival SS. Copper and immunity. Am J Clin Nutr 67: 1064S–1068S, 1998 [DOI] [PubMed] [Google Scholar]
- 146.Popova M, Asatryan L, Ostrovskaya O, Wyatt LR, Li K, Alkana RL, and Davies DL. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem 112: 307–317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Priel A. and Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol 123: 281–293, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Punthambaker S, Blum JA, and Hume RI. High potency zinc modulation of human P2X2 receptors and low potency zinc modulation of rat P2X2 receptors share a common molecular mechanism. J Biol Chem 287: 22099–22111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roberts JA. and Evans RJ. Contribution of conserved polar glutamine, asparagine and threonine residues and glycosylation to agonist action at human P2X1 receptors for ATP. J Neurochem 96: 843–852, 2006 [DOI] [PubMed] [Google Scholar]
- 150.Roberts JA. and Evans RJ. Cysteine substitution mutants give structural insight and identify ATP binding and activation sites at P2×receptors. J Neurosci 27: 4072–4082, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roger S, Gillet L, Baroja-Mazo A, Surprenant A, and Pelegrin P. C-terminal calmodulin-binding motif differentially controls human and rat P2X7 receptor current facilitation. J Biol Chem 285: 17514–17524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Roger S, Pelegrin P, and Surprenant A. Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28: 6393–6401, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ruan T, Lin YS, Lin KS, and Kou YR. Mediator mechanisms involved in TRPV1 and P2×receptor-mediated, ROS-evoked bradypneic reflex in anesthetized rats. J Appl Physiol 101: 644–654, 2006 [DOI] [PubMed] [Google Scholar]
- 154.Ruan T, Lin YS, Lin KS, and Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol 565: 563–578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Samways DS. and Egan TM. Acidic amino acids impart enhanced Ca2+ permeability and flux in two members of the ATP-gated P2×receptor family. J Gen Physiol 129: 245–256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sato A, Arimura Y, Manago Y, Nishikawa K, Aoki K, Wada E, Suzuki Y, Osaka H, Setsuie R, Sakurai M, Amano T, Aoki S, Wada K, and Noda M. Parkin potentiates ATP-induced currents due to activation of P2×receptors in PC12 cells. J Cell Physiol 209: 172–182, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Sciancalepore M, Luin E, Parato G, Ren E, Giniatullin R, Fabbretti E, and Lorenzon P. Reactive oxygen species contribute to the promotion of the ATP-mediated proliferation of mouse skeletal myoblasts. Free Radic Biol Med 53: 1392–1398, 2012 [DOI] [PubMed] [Google Scholar]
- 158.Seil M, Fontanils U, Etxebarria IG, Pochet S, Garcia-Marcos M, Marino A, and Dehaye JP. Pharmacological evidence for the stimulation of NADPH oxidase by P2X(7) receptors in mouse submandibular glands. Purinergic Signal 4: 347–355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Silberberg SD, Li M, and Swartz KJ. Ivermectin Interaction with transmembrane helices reveals widespread rearrangements during opening of P2×receptor channels. Neuron 54: 263–274, 2007 [DOI] [PubMed] [Google Scholar]
- 160.Sim JA, Park CK, Oh SB, Evans RJ, and North RA. P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol 152: 1283–1290, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, and Stuhmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci USA 93: 3684–3688, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stanchev D, Flehmig G, Gerevich Z, Norenberg W, Dihazi H, Furst S, Eschrich K, Illes P, and Wirkner K. Decrease of current responses at human recombinant P2X3 receptors after substitution by Asp of Ser/Thr residues in protein kinase C phosphorylation sites of their ecto-domains. Neurosci Lett 393: 78–83, 2006 [DOI] [PubMed] [Google Scholar]
- 163.Stojilkovic SS, Yan Z, Obsil T, and Zemkova H. Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells. Cell Mol Neurobiol 30: 1251–1258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stoop R, Surprenant A, and North RA. Different sensitivities to pH of ATP-induced currents at four cloned P2×receptors. J Neurophysiol 78: 1837–1840, 1997 [DOI] [PubMed] [Google Scholar]
- 165.Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, and North RA. Contribution of individual subunits to the multimeric P2X(2) receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol 56: 973–981, 1999 [DOI] [PubMed] [Google Scholar]
- 166.Suh BC, Kim JS, Namgung U, Ha H, and Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol 166: 6754–6763, 2001 [DOI] [PubMed] [Google Scholar]
- 167.Surprenant A. and North RA. Signaling at purinergic P2×receptors. Annu Rev Physiol 71: 333–359, 2009 [DOI] [PubMed] [Google Scholar]
- 168.Surprenant A, Rassendren F, Kawashima E, North RA, and Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2×receptor (P2X7). Science 272: 735–738, 1996 [DOI] [PubMed] [Google Scholar]
- 169.Tittle RK. and Hume RI. Opposite effects of zinc on human and rat P2X2 receptors. J Neurosci 28: 11131–11140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tittle RK, Power JM, and Hume RI. A histidine scan to probe the flexibility of the rat P2X2 receptor zinc-binding site. J Biol Chem 282: 19526–19533, 2007 [DOI] [PubMed] [Google Scholar]
- 171.Tomic M, Jobin RM, Vergara LA, and Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem 271: 21200–21208, 1996 [DOI] [PubMed] [Google Scholar]
- 172.Tomioka A, Ueno S, Kohama K, Goto F, and Inoue K. Propofol potentiates ATP-activated currents of recombinant P2X(4) receptor channels expressed in human embryonic kidney 293 cells. Neurosci Lett 284: 167–170, 2000 [DOI] [PubMed] [Google Scholar]
- 173.Ueno T, Ueno S, Kakazu Y, Akaike N, and Nabekura J. Bidirectional modulation of P2×receptor-mediated response by divalent cations in rat dorsal motor nucleus of the vagus neurons. J Neurochem 78: 1009–1018, 2001 [DOI] [PubMed] [Google Scholar]
- 174.Vial C, Tobin AB, and Evans RJ. G-protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem J 382: 101–110, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Virginio C, Church D, North RA, and Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 36: 1285–1294, 1997 [DOI] [PubMed] [Google Scholar]
- 176.Virginio C, North RA, and Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2×receptors in rat nodose neurones. J Physiol 510(Pt 1): 27–35, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vorobjev VS, Sharonova IN, Sergeeva OA, and Haas HL. Modulation of ATP-induced currents by zinc in acutely isolated hypothalamic neurons of the rat. Br J Pharmacol 139: 919–926, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang B. and Sluyter R. P2X7 receptor activation induces reactive oxygen species formation in erythroid cells. Purinergic Signal 9: 101–112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang C, Gu Y, Li GW, and Huang LY. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol 584: 191–203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang S, Dai Y, Kobayashi K, Zhu W, Kogure Y, Yamanaka H, Wan Y, Zhang W, and Noguchi K. Potentiation of the P2X3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain. Eur J Neurosci 36: 2293–2301, 2012 [DOI] [PubMed] [Google Scholar]
- 181.Weiss KH. and Stremmel W. Evolving perspectives in Wilson disease: diagnosis, treatment and monitoring. Curr Gastroenterol Rep 14: 1–7, 2012 [DOI] [PubMed] [Google Scholar]
- 182.Wildman SS, Brown SG, Rahman M, Noel CA, Churchill L, Burnstock G, Unwin RJ, and King BF. Sensitization by extracellular Ca(2+) of rat P2X(5) receptor and its pharmacological properties compared with rat P2X(1). Mol Pharmacol 62: 957–966, 2002 [DOI] [PubMed] [Google Scholar]
- 183.Wildman SS, King BF, and Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br J Pharmacol 128: 486–492, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wilkinson WJ, Gadeberg HC, Harrison AW, Allen ND, Riccardi D, and Kemp PJ. Carbon monoxide is a rapid modulator of recombinant and native P2X(2) ligand-gated ion channels. Br J Pharmacol 158: 862–871, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wirkner K, Stanchev D, Koles L, Klebingat M, Dihazi H, Flehmig G, Vial C, Evans RJ, Furst S, Mager PP, Eschrich K, and Illes P. Regulation of human recombinant P2X3 receptors by ecto-protein kinase C. J Neurosci 25: 7734–7742, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wirkner K, Stanchev D, Milius D, Hartmann L, Kato E, Zadori ZS, Mager PP, Rubini P, Norenberg W, and Illes P. Regulation of the pH sensitivity of human P2×receptors by N-linked glycosylation. J Neurochem 107: 1216–1224, 2008 [DOI] [PubMed] [Google Scholar]
- 187.Woodward JJ, Nowak M, and Davies DL. Effects of the abused solvent toluene on recombinant P2×receptors expressed in HEK293 cells. Brain Res Mol Brain Res 125: 86–95, 2004 [DOI] [PubMed] [Google Scholar]
- 188.Xia R, Mei ZZ, Milligan C, and Jiang LH. Inhibitory interaction between P2X4 and GABA(C) rho1 receptors. Biochem Biophys Res Commun 375: 38–43, 2008 [DOI] [PubMed] [Google Scholar]
- 189.Xiong K, Li C, and Weight FF. Inhibition by ethanol of rat P2X(4) receptors expressed in Xenopus oocytes. Br J Pharmacol 130: 1394–1398, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, and Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol 81: 2088–2094, 1999 [DOI] [PubMed] [Google Scholar]
- 191.Xu GY. and Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2×receptors. Proc Natl Acad Sci USA 101: 11868–11873, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan Z, Khadra A, Li S, Tomic M, Sherman A, and Stojilkovic SS. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 30: 14213–14224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yan Z, Khadra A, Sherman A, and Stojilkovic SS. Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol 138: 437–452, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yan Z, Li S, Liang Z, Tomic M, and Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol 132: 563–573, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yan Z, Liang Z, Obsil T, and Stojilkovic SS. Participation of the Lys313-Ile333 sequence of the purinergic P2X4 receptor in agonist binding and transduction of signals to the channel gate. J Biol Chem 281: 32649–32659, 2006 [DOI] [PubMed] [Google Scholar]
- 196.Yan Z, Liang Z, Tomic M, Obsil T, and Stojilkovic SS. Molecular determinants of the agonist binding domain of a P2×receptor channel. Mol Pharmacol 67: 1078–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 197.Young MT, Fisher JA, Fountain SJ, Ford RC, North RA, and Khakh BS. Molecular shape, architecture, and size of P2X4 receptors determined using fluorescence resonance energy transfer and electron microscopy. J Biol Chem 283: 26241–26251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yukawa H, Shen J, Harada N, Cho-Tamaoka H, and Yamashita T. Acute effects of glucocorticoids on ATP-induced Ca2+ mobilization and nitric oxide production in cochlear spiral ganglion neurons. Neuroscience 130: 485–496, 2005 [DOI] [PubMed] [Google Scholar]
- 199.Zemkova H, Yan Z, Liang Z, Jelinkova I, Tomic M, and Stojilkovic SS. Role of aromatic and charged ectodomain residues in the P2X(4) receptor functions. J Neurochem 102: 1139–1150, 2007 [DOI] [PubMed] [Google Scholar]
- 200.Zhang DX. and Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007 [DOI] [PubMed] [Google Scholar]