Abstract
Objective
Cancer patients are advised to quit smoking to reduce treatment complications and future cancer risk. This study's main objective was to evaluate the efficacy of a novel, pre-surgical cessation intervention in newly diagnosed cancer patients scheduled for surgical hospitalization.
Methods
We conducted a parallel-arm, randomized controlled trial comparing the efficacy of our hospital-based, tobacco cessation “best practices” treatment model (BP; cessation counseling and nicotine replacement therapy) with BP enhanced by a behavioral tapering regimen (scheduled reduced smoking; BP+SRS) administered by a handheld computer before hospitalization for surgery. Cessation outcomes were short (hospital admission and three months) and longer-term (6 months) biochemically-verified smoking abstinence. We hypothesized that BP+SRS would be superior to BP alone. One hundred eighty-five smokers were enrolled.
Results
Overall, 7-day-point prevalence, confirmed abstinence rates at six months for BP alone (32%) and BP+SRS (32%) were high; however, no main effect of treatment was observed. Patients who were older and diagnosed with lung cancer were more likely to quit smoking.
Conclusions
Compared to best practices for treating tobacco dependence, a pre-surgical, scheduled reduced smoking intervention did not improve abstinence rates among newly diagnosed cancer patients.
Keywords: Smoking cessation, patients with cancer, scheduled reduced smoking, hospitalized smokers
Continued smoking by tobacco dependent cancer patients is associated with greater risk of recurrence (Fleshner et al., 1999; Kenfield, Stampfer, Chan, & Giovannucci, 2011), reduced survival (Murin & Inciardi, 2001; Parsons, Daley, Begh, & Aveyard, 2010; Waggoner et al., 2006; Warren, Kasza, Reid, Cummings, & Marshall, 2012) and quality of life (Garces et al., 2004; Peppone et al., 2011); and an increased risk of peri-operative complications during treatment(Dresler, Bailey, Roper, Patterson, & Cooper, 1996; Moller, 2006; Theadom & Cropley, 2006; Warner, 2006).
While cancer diagnosis is considered to be a “teachable moment” for smoking cessation (McBride & Ostroff, 2003) and often leads to spontaneous quitting, persistent smoking is a serious and prevalent clinical problem (IOM, 2012; Mayer & Carlson, 2011; Underwood, Townsend, Tai, et al., 2012). About 15.1% of all adult cancer survivors are current smokers (Underwood, Townsend, Stewart, et al., 2012). Persistent smoking among those who smoked prior to diagnosis varies widely by cancer type (Coups & Ostroff, 2005; Cox et al., 2002; Demark-Wahnefried, Aziz, Rowland, & Pinto, 2005; Gritz et al., 2006; Mayer et al., 2007; Ostroff et al., 2000; Park et al., 2012) with continued smoking rates ranging from a low of 20% among lung cancer patients(Cox et al., 2002) to a high of 65% among bladder cancer patients (Ostroff et al., 2000). Thus, cancer-specific health risks and the prevalence of persistent smoking argue for the importance of providing evidence-based treatment of tobacco dependence as a standard of quality care in cancer settings (American Society for Clinical Oncology, 2009; Cox, Africano, Tercyak, & Taylor, 2003; Fleshner et al., 1999; Gritz, Dresler, & Sarna, 2005; National Comprehensive Cancer Network, 2007).
Smoking Cessation Trials with Cancer Patients
Despite the serious risks of persistent smoking, there is little data on how best to promote cessation among cancer patients. Prior randomized controlled cessation trials of pharmacologic and counseling interventions conducted with cancer patients generally have not found statistically significant treatment effects, with 6-month point abstinence rates ranging from 14-30% among those in the intervention conditions (Nayan, Gupta, & Sommer, 2011).
Given the acute and long-term risks and the high rates of persistent smoking, efficacious cessation interventions are needed for cancer patients (de Moor, Elder, & Emmons, 2008). The pre-hospitalization period represents a largely unexplored opportunity for smoking cessation treatment that can capitalize on oncologic providers' advice to quit and leverage a “teachable moment” for smoking cessation among tobacco dependent cancer patients (McBride & Ostroff, 2003). To date, no known trials have focused on preparing cancer patients to quit smoking prior to hospitalization.
Scheduled Reduced Smoking (SRS)
Scheduled reduced smoking (SRS) is a behavioral strategy for preparing to quit in which smokers gradually reduce their daily smoking rate by adhering to predetermined smoking times. Over days or weeks, the inter-cigarette intervals are gradually increased and smoking is delayed until the next scheduled cigarette. As the nonsmoking interval increases, smokers practice behavioral coping with smoking urges (Cinciripini, Wetter, & McClure, 1997). SRS may increase quitting self-efficacy as smokers experience less reward from smoking scheduled cigarettes and successfully manage smoking urges (Catley & Grobe, 2008). To date, two trials conducted with “healthy” smokers have shown that SRS is efficacious (Cinciripini et al., 1994; Cinciripini et al., 1995) with higher abstinence rates and greater coping with smoking urges and quitting self-efficacy for smokers using SRS than those who either quit abruptly, gradually reduced on their own, or smoked on a schedule without reduction (Cinciripini et al., 1995). SRS was chosen as a potential enhancement of the hospital's routine cessation treatment because of the promise of prior work, expectation that SRS would improve self-efficacy, and advantages of using a handheld computer for intervention delivery to reduce patient burden with clinic visits.
The main study goal was to evaluate the efficacy of the SRS intervention combined with best practices (SRS+BP) when compared with BP alone (BP: cessation counseling and pharmacotherapy) on short- and long-term abstinence rates in smokers newly diagnosed with cancer and awaiting hospitalization for surgical treatment. It was hypothesized that patients assigned to the SRS+BP intervention arm would have higher rates of smoking abstinence than those who received BP alone.
Methods
Participants and Procedures
Participants were smokers with newly diagnosed cancer who were scheduled for hospitalization and surgical resection at a comprehensive cancer center in New York City. The study was approved by the Center's institutional review board. Eligible patients were English-speaking adults with a localized solid mass likely to be cancer; awaiting surgical treatment no less than 7 days from study entry1; smoked at least 8 cigarettes per day (cpd) within the past week and had sufficient visual acuity and manual dexterity use a handheld computer. Exclusion criteria included evidence of psychopathology or cognitive impairment severe enough to prevent informed consent or completion of the study. Potential participants were screened via the electronic medical record and recruited from surgical clinics by a trained research assistant (RA). Physicians gave approval to approach their patients.
Randomization/Design
The trial design involved two parallel arms with an equal allocation ratio. Computerized permuted-block randomization was conducted independently by the Centers' Data Management Group. Patients were stratified by baseline daily cigarette consumption (≥ 20 cpd vs < 20 cpd) prior to random assignment. Participants were offered $20 to defray parking expenses incurred.
Intervention Conditions
Best Practices Only
Consistent with best practices (BP) for treating tobacco dependence (USDHHS, 2008), smokers assigned to the BP condition received our hospital's multi-component tobacco cessation treatment. In this setting, smokers are routinely advised to quit smoking by their attending surgeon during their work-up and pre-surgical consultations. All smokers are offered telephonic and bedside cessation counseling on the benefits of cessation for cancer patients, potential barriers to quitting, and behavioral strategies for managing smoking urges, recommendations for use of cessation pharmacotherapy and self-help materials (Ostroff, Burkhalter, O'Brien, Hay, & Dhingra, 2005)2. Individual cessation counseling is provided by designated oncology nurses trained and certified as tobacco treatment specialists (TTS). For this trial, two Tobacco Treatment Specialists (TTS), with at least two years prior clinical experience with cessation treatment, provided the counseling sessions. The TTSs underwent four hours of training using a treatment fidelity checklist. Training was conducted by the PI (JO) and a postdoctoral clinical psychology fellow (LD) using role-play exercises. A demonstration and intervention protocol checklist guided the performance of 16 counseling behaviors based on motivational interviewing for cessation (e.g., dealing with resistance, enhancing self-efficacy, steps to quitting, coping with urges). To minimize protocol drift, group supervision by the PI was conducted weekly.
Participants were offered five individual cessation counseling sessions and nicotine replacement therapy (NRT) at no cost. The first session was prior to surgery and focused on enhancing motivation, preparedness to quit prior to hospitalization, and providing education about cessation pharmacotherapy and adverse effects. The second counseling contact prior to surgery addressed pharmacotherapy use and provided support for patients' quitting efforts. A third counseling session occurred during hospitalization and focused on inpatient management of acute nicotine withdrawal and coping with urges to smoke. Two more counseling sessions were provided during the month after hospital discharge and focused on assessing cessation progress, adherence to pharmacotherapy, reinforcing behavioral strategies for coping with smoking urges, and preventing relapse. All counseling sessions were delivered by telephone with the exception of the third session which was generally delivered in person during the patient's hospital stay. The planned duration of each counseling session was: (Session 1: 30-45 mins; Session 2: 5-10 mins; and Sessions 3, 4 and 5: 15-20 mins). Based on the number and duration of counseling sessions, the BP condition can be classified as consistent with an intensive level (Level 4 out of a Maximum Level 4) of cessation treatment found to be effective for treating hospitalized smokers (Rigotti, Munafo, & Stead, 2008).
The primary aim was to test the effectiveness of a novel behavioral intervention and NRT use was recommended but not required for study participation. To be most representative of actual clinical care, cessation pharmacotherapy recommendations were tailored to the specific needs and preferences of patients. The study provided free NRT to all participants, and evaluated equivalence in NRT use between the treatment groups.
Best Practices + Scheduled Reduced Smoking (BP+SRS)
The BP+SRS treatment condition included all of the best practices described above plus a handheld computer pre-programmed to administer the SRS pre-surgical gradual tapering regimen (Cinciripini et al., 1994; 1995). Participants randomized to BP+SRS were trained by the RA to use the handheld computer, or “QuitPal”. Training sessions concluded with an assessment of QuitPal mastery, and each participant received a helpline number and instructional manual. In addition, the RA contacted each participant within 3 days after enrollment to answer any questions about QuitPal use. Each patient's individualized SRS schedule was tailored to three parameters: a) typical waking and bedtimes; b) daily average smoking rate; and c) number of days from enrollment until hospitalization, with a quit date planned at least 24 hours prior to an inpatient admission. Patients were instructed to smoke within 10 minutes of the auditory prompt and all entries and events were time-stamped. Patients were instructed to use the QuitPal daily prior to their hospital admission and return it upon admission.
Measures
Patients' medical charts were reviewed to collect data on disease and treatment characteristics. Demographic and tobacco use characteristics were also assessed. Nicotine dependence was assessed at baseline by the Fagerstrom Test for Nicotine Dependence (Fagerstrom, Heatherton, & Kozlowski, 1990; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), a 6-item scale, with scores > 6 indicating heavy nicotine dependence. The Commitment to Abstinence Scale (Hall, Havassy, & Wasserman, 1990) measured baseline commitment to one of six smoking abstinence goals ranging from 1 (“I have no goal/intentions to quit”) to 6 (“My goal is total abstinence”). Ten items from the Confidence Questionnaire Form (Baer & Lichtenstein, 1988) assessed baseline self-efficacy appraisals for coping with smoking urges in tempting situations. Patients rated the likelihood of being able to resist smoking in each context on a 0-100% scale, with higher scores indicating greater quitting self-efficacy.
Smoking Cessation Outcomes
The primary study outcome was 7-day point prevalence abstinence assessed at six months post-hospitalization and verified by cotinine assays using saliva samples as recommended for cessation clinical trials (Hughes et al., 2003; Velicer, Prochaska, Rossi, & Snow, 1992). Secondary abstinence outcomes were assessed at hospital admission (24-hour point-prevalence abstinence) and verified by carbon monoxide (CO) breath test, and at three months post-hospitalization (7-day point abstinence) and verified by salivary cotinine assays. To minimize misreporting, patients were informed that their breath (or saliva) would be tested for recent tobacco smoke exposure before smoking status was self-reported. Patients with CO levels < 10 ppm were classified as point abstinent (SRNT Subcommittee on biochemical verification, 2002). Patients with salivary cotinine levels < 15ng/ml were classified as abstinent (SRNT Subcommittee on biochemical verification, 2002). ITT principal was applied to biochemical verification of abstinence so that a participant is coded as smoking unless biochemically verified. Thus, missing abstinence data and incidences of misreporting were coded as smoking. Additionally, the self-reported number of cigarettes smoked per day (cpd) and an overall assessment of recent changes in their smoking rate (reduced, about the same, increased) were measured at hospital admission and at the three- and six-month (post-hospital admission) follow-up assessments.
Treatment Implementation Fidelity, Usage, and Program Evaluation
To confirm that cessation treatment conditions were delivered as intended (Bellg et al., 2004), four treatment components were assessed: advice to quit, offering of NRT, behavioral counseling delivery, and training for use of the handheld computer (BP+SRS participants only). Patient-reported receipt of advice to quit from the surgical oncologist was collected at baseline. Counseling and medical records were audited to confirm that NRT was offered. The number of counseling sessions provided and their duration were recorded by the TTS. In addition, counseling sessions were audio-recorded and 12% of the participants were randomly sampled and their counseling sessions checked for adherence to the content of the clinical intervention checklist. For patients assigned to SRS, records of QuitPal training were audited for training adherence.
Patient reports on the use of NRT and the level of adherence were collected at each counseling session. The number of days of SRS use and the percentage of cigarettes actually smoked on schedule compared to the total number of scheduled cigarettes planned were computed. A program evaluation including a 15-item (for BP) and a 27-item (BP+SRS) survey assessed patients' perceptions of treatment quality and satisfaction.
Statistical Methods
To address the primary study aim, a series of Fisher's exact test statistics were used to compare biochemically-verified abstinence between the treatment conditions at hospital admission, and the 3- and 6-month follow-up assessment points. A participant was considered to be a current smoker unless his or her self-reported 24-hour or 7-day point abstinence was biochemically verified. Consistent with Intention-To-Treat (ITT) principles (Nagelkerke, Fidler, Bernsen, & Borgdorff, 2000), group comparisons were made based on the initial group assignment, regardless of intervention completion.
Comparisons of baseline differences across the two intervention conditions were based on independent-sample t-tests and exact tests of sample proportions. Treatment efficacy over time was evaluated by a Generalized Estimating Equation (GEE) model of longitudinal smoking abstinence (Zeger & Liang, 1986) as a function of the intervention condition and discrete time points, using a logit link for the binomial distributional family data, assuming unstructured working correlations over time. Robust variance estimates for the coefficients were calculated to guard against inflated Type-I error in an over-dispersed correlation matrix (Williams, 2000). In the secondary analyses, reduction in cigarette consumption from baseline to the three follow-up points was assessed with paired t-tests. Self-reported smoking outcomes were also examined. All statistical analyses were conducted in SAS version 9.2. (SAS Institute Inc., 2000-2008) and R version 2.13.1 (R Development Core Team, 2011) software packages.
Results
Participant enrollment and retention data are presented in Figure 1. Newly diagnosed cancer patients seeking surgical consultation were screened for smoking status, and 264 patients meeting all eligibility criteria were identified. Of those, 79 (29.9%) declined participation. Refusers were older (M = 69.3, SD = 13.9, p < 0.001) and less likely to be diagnosed with either lung or head and neck cancers (p < .001). The most prevalent reasons for refusal (more than one could be reported) included: not interested in study (54.4%), not motivated to quit smoking before surgery (38.0%), not motivated to quit smoking (35.4%), wants to quit on own (24.1%), and too stressed (20.3%). There was no significant difference between group sizes at allocation. Retention at the 6-month follow-up was high and comparable across the two conditions (84% BP vs 85% BP+SRS).
Figure 1. CONSORT flow diagram of the BP vs BP+SRS intervention for cancer patients scheduled for hospitalization for cancer surgery.
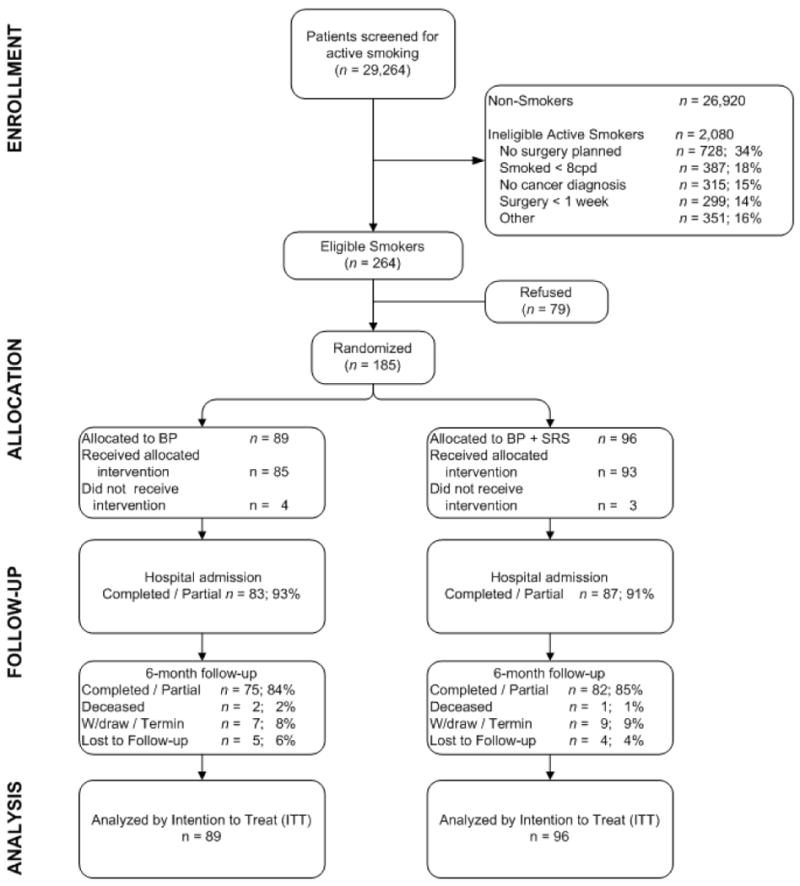
Participant characteristics are presented in Table 1. The sample was predominantly white (87%), with a mean age of 55.9 years, about half were women (53%) and lung cancer was the most prevalent diagnosis (30%). At baseline, the mean number of cigarettes smoked daily was 19.5 (SD = 10.5) and the overall mean pack-years was 35.4 (SD = 12.2). The mean Fagerstrom score was 4.9 (SD =1.9), indicating moderate nicotine dependence, and 38% reported smoking their first cigarette within 5 minutes of waking. There were no statistically significant differences in baseline characteristics between the intervention conditions. Internal consistency for the baseline Confidence Questionnaire Form assessment was 0.88.
Table 1. Patient Demographic, Disease, and Tobacco-Use Characteristics at Study Enrollment.
| Characteristics | BP N = 89 |
BP+SRS N = 96 |
Total N = 185 |
|
|---|---|---|---|---|
| Gender (Female) | 51 (57%) | 47 (49%) | 98 (53%) | |
| Age (yrs. mean, SD) | 55.4 (10.1) | 56.4 (10.3) | 55.9 (10.2) | |
| Ethnicity (White) | 79 (89%) | 82 (85%) | 161 (87%) | |
| Marital status (Married or living with a partner) 1 | 48 (54%) | 58 (62%) | 106 (58%) | |
| Education (High school graduate or below) 1 | 34 (38%) | 37 (39%) | 71 (39%) | |
| Income (below $50,000/year) 1 | 38 (44%) | 34 (37%) | 72 (40%) | |
| Employment status (Employed) 1 | 47 (53%) | 52 (54%) | 99 (54%) | |
| Cancer site 2 | Thoracic | 30 (34%) | 25 (26%) | 55 (30%) |
| Head & Neck | 9 (10%) | 8 (8%) | 17 (9%) | |
| Breast | 8 (9%) | 14 (14%) | 22 (12%) | |
| GYN | 11 (12%) | 11 (12%) | 22 (12%) | |
| Urology | 18 (20%) | 20 (21%) | 38 (20%) | |
| Other | 13 (15%) | 18 (19%) | 31 (17%) | |
| Time since diagnosis (Months mean, SD) 3 | 2.4 (7.9) | 2.0 (5.0) | 2.2 (6.5) | |
| Treatment | Surgery | 79 (89%) | 76 (79%) | 155 (84%) |
| Neoadjuvant Chemotherapy + Surgery | 1 (1%) | 5 (5%) | 6 (3%) | |
| Unresectable tumor | 3 (3%) | 4 (4%) | 7 (4%) | |
| Smoking-related variables | Cigarettes per day (mean, SD) 1 | 20.6 (12.1) | 18.5 (8.7) | 19.5 (10.5) |
| Fagerstrom Test for Nicotine Dependence (mean, SD) 1 | 5.2 (2.1) | 4.7 (1.7) | 4.9 (1.9) | |
| Smoke first cigarette within 5 minutes after wake up 1 | 36 (40%) | 34 (34%) | 70 (38%) | |
| Number of years smoking (mean, SD) 1 | 35.5 (11.7) | 35.3 (12.7) | 35.4 (12.2) | |
| Quitting self-efficacy (mean, SD) | 47.6 (20.1) | 52.3 (19.9) | 50.0 (20.0) | |
| Change in smoking rate since diagnosis 1 | ||||
| About the same | 33 (37%) | 45 (48%) | 78 (43%) | |
| Reduced | 45 (51%) | 34 (37%) | 79 (43%) | |
| Increased | 11 (12%) | 14 (15%) | 25 (14%) | |
| Commitment to Quit Smoking 1 | 37 (44.0) | 41 (44.1) | 78 (44.1) | |
Statistics are N and percentages unless otherwise noted. GYN=gynecological cancers
No statistically significant differences in baseline characteristics were found between the two intervention conditions.
Frequency of missing data on patient characteristics: education (2 patients), marital status (2), income (7), employment (3), cigarettes per day (6), Fagerstrom (8), number of years smoking (9), quitting self-efficacy (8), commitment to smoking abstinence (8), and change in smoking rate since diagnosis (3).
Twenty-eight patients (15%) were ultimately found to have a nonmalignant mass, but are included in the analyses because inclusion criteria specified eligible pre-surgical patients as having a solid mass deemed by their oncologist as “likely” to be cancer.
If a definitive diagnosis was not possible, we used the date of first consultation with oncologist as the presumptive date of diagnosis.
Based on biochemical verification, only 13 (7.5%) participants misreported smoking status at hospital admission, 5 (3.4%) at the 3-month follow-up and 4 (2.5%) at the 6-month follow-up. There were no group differences in misreporting of smoking status.
Treatment Implementation Fidelity, Usage, and Program Evaluation
Overall, 84% of patients reported discussing their smoking with their surgeon, and 78% reported being advised to quit prior to surgery. NRT was offered to 99.4%, and 72.5% reported actual usage of NRT during the trial. Of those using NRT, 91% reported full adherence to the standard dose and usage recommendations.
The timing for the delivery of the five cessation counseling sessions was planned relative to each patient's surgery date. The median intake counseling session occurred 12 days before surgery. Of the planned sessions, 98% of scheduled calls were made by the TTS. Patients received on average 4.1 counseling sessions (SD = 1.3) with a mean duration of 16.7 minutes each. At least four of the five counseling sessions were received by 71.3% of BP and 76.3% of BP+SRS participants. Only 13.8% of BP and 9.7% of BP+SRS participants received two or fewer sessions. In addition, 89% of the targeted content from the treatment checklist was verified in the sampled calls. There were no statistically significant group differences found in either rates of quitting advice, use of NRT, or the number, duration, and satisfaction with counseling sessions.
In the BP+SRS condition, 96.7% of patients received the planned QuitPal training. In terms of SRS adherence, participants smoked 50% of the scheduled cigarettes, on average, with daily within-person variability averaging 24%.3 Patients used the QuitPal for a median of 16 days (inter-quartile range: 12 to 28 days) prior to surgery. Completion of a morning start-up routine was considered a minimal indicator of daily use of the QuitPal. Overall, a median of 73% of participants used the QuitPal daily (inter-quartile range: 36-93%). However, 22 patients assigned to the BP+SRS condition did not have evaluable QuitPal use data because of technical problems (n=3), withdrawal from the study prior to using the QuitPal (n=5), or battery depletion (n=14). Patients reported that QuitPal was helpful in quitting smoking and was not particularly inconvenient (M = 3.8, SD = 1.2 and M = 3.2, SD = 1.3, respectively, on a 1 to 5 scale).
The assessment of patient satisfaction with the quality of cessation counseling had high internal consistency (standardized Cronbach's alpha of 0.83 and 0.88 for the BP and BP+SRS groups, respectively). The overall average score was high (M = 3.44 on a 1 to 4 scale, SD = 0.70). Almost all (97%) reported that they would recommend the cessation counseling to others.
Effect of the Intervention on Primary and Secondary Smoking Cessation Outcomes
Table 2 summarizes results of the ITT analyses of abstinence rates for treatment conditions and assessment points. At hospital admission, 40 of 89 patients (45%) in the BP condition were verified 24-hour abstainers, compared to 43 of 95 patients (45%) in the BP+SRS condition (p = 1.0 by Fisher's test, OR=0.987, 95% CI: 0.530, 1.839). At 3-month follow-up, the 7-day point abstinence rates were also similar between the conditions, with 34% and 36% abstinent in the BP and BP+SRS conditions, respectively (p = 0.878, OR=0.944, 95% CI: 0.490, 1.816). At 6-month follow-up, the 7-day point abstinence rates were 32% for both treatment conditions (p = 1.0, OR=1.028, 95% CI: 0.525, 2.012). The analysis of self-reported smoking status yielded comparable null results. These outcome analyses were based on unadjusted Fisher's tests comparing ITT abstinence rates across treatment conditions without covariates.
Table 2. Comparison of Smoking Cessation Outcomes by Treatment Conditions.
| BP | BP+SRS | OR | 95% CI | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Sample Size | Outcome | Sample Size | Outcome | |||
| Surgery admission | ||||||
| Intention-to-treat analysis (N, %)1 | 89 | 40 (45%) | 95 5 | 43 (45%) | 0.98 | 0.53 – 1.84 |
| Self-report (N, %)1,2 | 85 | 49 (58%) | 89 | 47 (53%) | 1.21 | 0.64 – 2.31 |
| Cigarettes per day (mean, SD)2 | 60 | 6.1 (8.1) | 58 | 4.4 (4.6) | - | - |
| Self-reported reduction4 (N, %) | 46 | 18 (39%) | 42 | 26 (62%) | 0.40 | 0.15 – 1.01 |
| 3 Month follow-up | ||||||
| Intention-to-treat analysis3 | 87 5 | 30 (34%) | 95 5 | 34 (36%) | 0.94 | 0.49 – 1.82 |
| Self-report2,3 | 69 | 30 (43%) | 78 | 37 (47%) | 0.85 | 0.42 – 1.72 |
| Cigarettes per day (mean, SD)2 | 46 | 8.4 (8.8) | 51 | 6.2 (7.6) | - | - |
| Self-reported reduction4 (N, %) | 39 | 26 (67%) | 41 | 31 (76%) | 0.65 | 0.22 – 1.90 |
| 6 Month follow-up | ||||||
| Intention-to-treat analysis3 | 87 5 | 28 (32%) | 95 5 | 30 (32%) | 1.03 | 0.52 – 2.01 |
| Self-report 2,3 | 75 | 28 (37%) | 82 | 34 (41%) | 0.84 | 0.42 – 1.68 |
| Cigarettes per day (mean, SD)2 | 56 | 11.3 (11.4) | 56 | 9.3 (8.8) | - | - |
| Self-reported reduction4 (N, %) | 57 | 27 (47%) | 48 | 29 (60%) | 0.59 | 0.25 – 1.38 |
24 hour point prevalence abstinence;
Participants were excluded from the denominator if they provided no usable follow-up data;
7 day point prevalence abstinence;
Participants self-reported that they were smoking regularly. Abstainers were excluded;
One patient in BP+ SRS arm died prior to data collection at hospital admission, two patients in BP arm died prior to the 3 month follow-up data collection.
Daily cigarette smoking reduction was observed in both treatment conditions. Smokers randomized to BP substantially reduced their daily smoking rate from 20.6 to 6.1 cpd between baseline and hospital admission. BP+SRS participants reduced from 18.5 to 4.4 cpd during the same time period. The overall reduction averaged over hospital admission, 3- and 6-month follow-up time points was 12.2 cpd for BP and 12.2 cpd for BP+SRS, respectively. A repeated-measures ANOVA comparing the two profiles of cpd reduction over the three assessment time points, controlling for baseline cpd, yielded no significant difference (p= 0.091). At enrollment, 51% of BP and 37% of BP+SRS participants reported that they had reduced their overall smoking rate since cancer diagnosis (Table 1). Whereas at hospital admission, BP+SRS participants were more likely to report that they had reduced their daily smoking than those in the BP only condition (62% vs. 39%; OR = 2.5, 95% CI: 0.98 – 6.53, p=0.054) (Table 2).
The GEE model on the longitudinal abstinence outcomes over time shows that none of the coefficients and corresponding odds ratios were statistically significant. The Treatment-by-Time interaction terms show no treatment differences for longitudinal abstinence rates (Table 3).
Table 3. Coefficients of the GEE model on the longitudinal Intention-To-Treat abstinence outcomes as a function of treatment assignment and time (maximum of 3 longitudinal observations per each of the n=185 randomized participants).
| Parameter | Effects | Coef | SE | Odds Ratio | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| OR | OR 95% CI | |||||
| Intercept | -0.20 | 0.21 | - | - | - | |
| Treatment (Tx) | ||||||
| BP +SRS vs. BP at hospital admission | 0.01 | 0.30 | 1.01 | 0.56, 1.81 | 0.965 | |
| Time | ||||||
| 3 month effect (vs. hospital admission) 1 | -0.42 | 0.28 | 0.65 | 0.38, 1.13 | 0.128 | |
| 6 month effect (vs. hospital admission) 1 | -0.52 | 0.28 | 0.59 | 0.34, 1.03 | 0.063 | |
| Time * Tx | ||||||
| 3 month effect for BP+SRS (vs. BP) | 0.03 | 0.38 | 1.03 | 0.49, 2.15 | 0.933 | |
| 6 month effect for BP+SRS (vs. BP) | -0.06 | 0.36 | 0.94 | 0.46, 1.92 | 0.874 | |
|
| ||||||
| Unstructured working correlation | Admission | 3 months | 6 months | |||
|
|
||||||
| Admission | 1.0 | |||||
| 3 months | 0.24 | 1 | ||||
| 6 months | 0.31 | 0.53 | 1 | |||
The GEE coefficients for the discrete time effects can only be evaluated when treatment has a coding of zero. Thus, this 3 months effect represents the drop in abstinence rates in the BP group as compared to hospital admission.
Baseline predictors of smoking abstinence at six months following hospitalization
Given the lack of an overall treatment effect, participants from both intervention conditions were combined and sociodemographic, disease, and baseline tobacco-use variables were examined as predictors of smoking abstinence at the 3-month and 6-month follow-up time points. Gender, age, education, income, employment status, cancer site (thoracic vs others), time since diagnosis, time to first cigarette, baseline cpd, baseline quitting self-efficacy, and baseline commitment to abstinence on smoking abstinence were examined as potential predictors of smoking abstinence. Only age was significantly related to smoking abstinence at both 3-month and 6-month follow-up time points, such that older patients were more likely to be abstinent (OR at 3 months = 1.045, CI: 1.012 – 1.080, p = 0.007; OR at 6 months = 1.036, CI: 1.003 – 1.070, p = 0.032). Cancer site (thoracic vs other sites) also had a significant association with abstinence at 3 months (OR = 2.16, CI: 1.116 – 4.183, p = 0.022) such that patients diagnosed with thoracic cancers were more likely to quit smoking. At the 6 months follow-up, the association between cancer site and smoking abstinence remained marginally significant (OR = 1.915, CI: 0.979 – 3.746, p = 0.058).
Discussion
This randomized controlled trial tested the efficacy of adding a scheduled reduced smoking (SRS) component to best practices (BP) for cancer patients who smoke and were awaiting hospitalization for surgical treatment. Smokers were randomly assigned to receive either BP only (counseling sessions and cessation pharmacotherapy) or BP+SRS. Although relatively high rates of biochemically verified smoking abstinence were found, the SRS intervention in addition to BP did not yield superior quit rates at either 3 or 6 months following hospitalization. Nevertheless, as this was the first known study to test SRS with medically ill patients in a pre-surgical context, the study contributes to knowledge on the therapeutic benefit of SRS.
What factors might explain the null findings in contrast to previous work demonstrating efficacy of SRS? First, despite efforts to maintain treatment integrity, participants in both treatment conditions substantially reduced their daily smoking rate immediately following diagnosis of cancer, most rapidly prior to hospitalization. Unlike Cinciripini's prior study (1995) conducted with healthy volunteers, participants in the current trial were newly diagnosed cancer patients advised to quit by their surgical oncologists. Second, as all patients were scheduled for cancer surgery, there was a somewhat shorter duration of time between the start of the SRS intervention and hospitalization resulting in a shorter duration of SRS intervention than delivered in prior studies (approximately 2 versus 4 weeks). Further, whereas a prior SRS study required smokers to deposit $110, with repayment contingent on SRS adherence (Cinciripini et al., 1995), the current study was conducted without monetary incentive for adherence. Cinciripini and colleagues (1995) observed high (93%) SRS adherence (defined as the proportion of actual cigarettes smoked out of the total number of cigarettes scheduled to smoke). In comparison, SRS adherence for cancer patients enrolled in our study was lower, averaging 50%, which may have also contributed to the lack of intervention effect. This observed difference in SRS adherence rates may be due to the lack of monetary incentives for SRS adherence as well as the potential burden on medically ill smokers to adhere to the SRS regimen in the context of numerous medical care appointments and life disruptions that restricted opportunities to smoke on schedule. Finally, while participants in the prior SRS study received no cessation medications, all participants in this trial were offered NRT at no cost and were strongly encouraged to use cessation medications following their quit date.
Regarding the current study's trial design, the “control” group received an unusually robust treatment dose compared to prior hospital-based, cessation studies (Rigotti, Clair, Munafo, & Stead, 2012). It was deemed unethical to withhold best practices for tobacco cessation treatment from pre-surgical cancer patients with a medical urgency for quitting. Consequently, this trial design set a high standard for testing the superior efficacy of an experimental intervention. With few exceptions, prior cessation studies conducted with cancer patients have typically used usual care (no-treatment) or physician advice as control conditions (Duffy et al., 2006; Griebel, Wewers, & Baker, 1998; Gritz et al., 1993; Park et al., 2011; Schnoll et al., 2005; Schnoll et al., 2003; Wakefield, Olver, Whitford, & Rosenfeld, 2004; Wewers, Bowen, Stanislaw, & Desimone, 1994). For example, using a no-treatment control condition, Wewers et al. showed that a nurse-managed, smoking cessation counseling intervention yielded a superior quit rate among tobacco dependent cancer patients (Griebel, Wewers, & Baker, 1998; Stanislaw & Wewers, 1994; Wewers, Jenkins, & Mignery, 1997). Similarly, Park et al. demonstrated higher (though nonsignificant) quit rates among thoracic cancer patients who received varenicline and intensive counseling compared with those patients who received usual (unspecified) care (34.4% vs. 14.3% abstinence at 3-month follow-up, p = .18) (Park et al., 2011). In contrast, current study participants assigned to the BP condition received physician advice to quit, multiple counseling sessions by a trained TTS, and cessation pharmacotherapy consistent with evidence-based guidelines (USDHHS, 2008). Thus, the BP condition of this trial was more akin to intensive experimental intervention conditions offering evidence-based counseling and cessation pharmacotherapy that have been found to be efficacious in prior clinical trials with hospitalized smokers (Rigotti, Munafo, & Stead, 2008). In fact, the 32% overall biochemically-verified abstinence rate observed at 6 months represents one of the highest cessation rates found in a cessation trial with tobacco-dependent cancer patients (Nayan, Gupta, & Sommer, 2011) and is also consistent with the highest cessation rates reported with hospitalized smokers (Rigotti, Munafo, & Stead, 2008). In summary, participant characteristics, the absence of participant incentives for SRS adherence, unanticipated similarities in the rate of pre-surgical smoking reduction, and the intensity of the best practices control group may have contributed to cessation outcomes divergent from earlier SRS studies.
Despite the null trial results, the study contributes to the knowledge base on behavioral interventions for smoking cessation and the clinical care of recently diagnosed, tobacco-dependent cancer patients. First, the results argue for current implementation of best practices for treating tobacco dependence among hospitalized smokers. These findings underscore the value of integrating tobacco treatment into standards of quality cancer care (American Society for Clinical Oncology, 2009; Morgan et al., 2011) and support the pre-hospitalization period as a feasible entry point for delivery of smoking cessation interventions among medically ill smokers. However, there remains considerable room for improvement in increasing adherence to smoking cessation interventions (implementation) and smoking relapse prevention (maintenance) following hospital discharge (France, Glasgow, & Marcus, 2001), and this remains a high priority area for future research. Although most oncology provider believe that tobacco cessation should be a standard part of clinical care (Warren et al., 2013), most cancer care settings have not yet established tobacco cessation treatment as standard care (Goldstein, Ripley-Moffitt, Pathman, & Patsakham, 2012), and oncology providers miss many opportunities to promote tobacco cessation (Coups, Dhingra, Heckman, & Manne, 2009; Sabatino et al., 2007). Our findings support the need for continued development and evaluation of novel smoking cessation interventions that are acceptable and efficacious for cancer patients. Consistent with a recent publication by Berg and colleagues (Berg, Carpenter, Jardin, & Ostroff, 2013), the observation of marked smoking reduction from enrollment (cancer diagnosis) to hospitalization suggests cancer patients' acceptability of behavioral cessation treatment efforts that allow for tapering and reducing daily smoking, perhaps as preparation for quitting. In a review of smoking reduction studies with healthy smokers, the authors emphasize the potential benefit of behavioral treatment that emphasizes gradual tapering of smoking as a prerequisite for quit attempt (Hughes & Carpenter, 2006). Second, this study is noteworthy for its inclusion of smokers with tobacco-related as well as non-tobacco-related cancers, a subgroup of smokers typically under-represented in prior work that has focused predominantly on cessation outcomes in lung or head and neck cancer patients.
Limitations and Recommendations for Future Studies
Although the study had a relatively low refusal rate (29%), younger patients and to some extent those diagnosed with tobacco-related cancers were more likely to enroll. Future trials should address barriers to participation in cessation trials among older patients and those diagnosed with non-tobacco related cancers who may be less cognizant of the risks of persistent smoking. In our sample, 30% of patients were diagnosed with lung cancer. These patients tend to quit smoking at higher rates than those diagnosed with other cancers (Cox et al., 2002) and their inclusion may have created a potential ceiling for finding experimental treatment effects. Future cessation trials should address cessation among patients diagnosed with tobacco- and non-tobacco-related cancers. Although we found low rates of misreporting, another study limitation is that not all participants (5% missing data) provided samples for biochemical verification of self-reported smoking abstinence. The two-arm study design did not enable isolated examination of the potential benefit of self-monitoring of daily cigarette smoking. Ideally, future SRS trials should include a third study condition in which participants would self-monitor their daily rate and patterns of cigarette smoking without following a structured smoking reduction regimen. While the SRS intervention was designed to improve quitting self-efficacy, daily interaction with the QuitPal may have been taxing for some patients dealing with the life disruptions associated with diagnosis and imminent surgical treatment.
These limitations should be balanced against the study's considerable methodological strengths and clinical importance including the randomized design, relatively high rate of enrollment, inclusion of newly diagnosed patients with non-tobacco-related cancers typically under-represented in cessation trials, relatively large sample size, biochemical verification of smoking abstinence, and the use of best practices well-integrated into the cancer treatment setting as the control condition. To facilitate advances in tobacco cessation treatment for hospitalized cancer patients, it is recommended that future cessation trials benchmark their proposed treatment enhancements with best practices (USDHHS, 2008) rather than no treatment controls. Given the risks of persistent smoking, further research is needed to develop and evaluate cessation intervention components that enhance current best practices for treating tobacco dependent cancer patients.
Acknowledgments
This research was supported by grants from the National Cancer Institute R01CA90514 and T32CA009461.
Author Note: We gratefully acknowledge Nathan Cooper, Lonette Sandy, and Joshua Mann for their assistance with participant recruitment and data collection; Maureen O'Brien, RN, CTTS and Sheila Keaveney, NP, CTTS for delivering the smoking cessation counseling intervention; Nian Wu, PhD and the facilities of the Analytic Pharmacology Lab at Sloan-Kettering Institute for performing the salivary cotinine assays; Drs. Valerie Rusch, Carol Brown, Yuman Fong, Jay Boyle, and Paul Russo from the MSK Department of Surgery for their clinical leadership in promoting smoking cessation as the standard of cancer care for pre-surgical cancer patients; Victoria Mills for her assistance with manuscript preparation, Todd Ricker for naming the “QuitPal”, and the patients who participated in this trial.
Footnotes
Patients were surgical candidates at the time of study entry and some were later determined not to be candidates for imminent surgical treatment. Of this group, six patients received neoadjuvant chemotherapy prior to surgery and seven were unresectable during surgery. These patients were retained in the study. To align the delivery of the intervention components and assessment timelines for surgically resected and non-surgical patients, we treated non-surgically treated patients' treatment start date as the equivalent of the hospitalization date.
Available by request from the authors.
Adherence to SRS intervention did not impact primary cessation outcome.
References
- American Society for Clinical Oncology. Tobacco cessation and quality cancer care. Journal of Oncology Practice. 2009;5(1):2–5. doi: 10.1200/JOP.0913501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56(1):104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Czajkowski S. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology. 2004;23(5):443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Carpenter MJ, Jardin B, Ostroff JS. Harm reduction and cessation efforts and interest in cessation resources among survivors of smoking-related cancers. J Cancer Surviv. 2013 doi: 10.1007/s11764-012-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Grobe JE. Using basic laboratory research to understand scheduled smoking: a field investigation of the effects of manipulating controllability on subjective responses to smoking. Health Psychology. 2008;27(3 suppl):S189–S196. doi: 10.1037/0278-6133.27.3(Suppl.).S189. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Lapitsky LG, Wallfisch A, Mace R, Nezami E, Van Vunakis H. An evaluation of a multicomponent treatment program involving scheduled smoking and relapse prevention procedures: initial findings. Addictive Behaviors. 1994;19(1):13–22. doi: 10.1016/0306-4603(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Lapitsky LL, Seay SS, Wallfisch AA, Kitchens KK, Van Vunakis HH. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? Journal of Consulting and Clinical Psychology. 1995;63(3):388–399. doi: 10.1037//0022-006x.63.3.388. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, McClure JB. Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. Addictive Behaviors. 1997;22(6):759. doi: 10.1016/s0306-4603(97)00061-0. [DOI] [PubMed] [Google Scholar]
- Coups EJ, Dhingra LK, Heckman CJ, Manne SL. Receipt of provider advice for smoking cessation and use of smoking cessation treatments among cancer survivors. J Gen Intern Med. 2009;24 Suppl 2:S480–486. doi: 10.1007/s11606-009-0978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Preventive Medicine. 2005;40(6):702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98(3):632–644. doi: 10.1002/cncr.11538. [DOI] [PubMed] [Google Scholar]
- Cox LS, Patten CA, Ebbert JO, Drews AA, Croghan GA, Clark MM, Hurt RD. Tobacco use outcomes among patients with lung cancer treated for nicotine dependence. Journal of Clinical Oncology. 2002;20(16):3461–3469. doi: 10.1200/Jco.2002.10.085. [DOI] [PubMed] [Google Scholar]
- de Moor JS, Elder K, Emmons KM. Smoking prevention and cessation interventions for cancer survivors. Seminars in Oncology Nursing. 2008;24(3):180–192. doi: 10.1016/j.soncn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. Journal of clinical oncology. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110(5):1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, Terrell JE. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer epidemiology, biomarkers & prevention. 2006;15(11):2203–2208. doi: 10.1158/1055-9965.epi-05-0880. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear, Nose & Throat Journal. 1990;69(11):763–765. [PubMed] [Google Scholar]
- Fleshner N, Garland J, Moadel A, Herr H, Ostroff J, Trambert R, Russo P. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86(11):2337–2345. [PubMed] [Google Scholar]
- France EK, Glasgow RE, Marcus AC. Smoking cessation interventions among hospitalized patients: What have we learned? Preventive Medicine. 2001;32(4):376–388. doi: 10.1006/pmed.2000.0824. [DOI] [PubMed] [Google Scholar]
- Garces YI, Yang P, Parkinson J, Zhao X, Wampfler JA, Ebbert JO, Sloan JA. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126(6):1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- Goldstein AO, Ripley-Moffitt CE, Pathman DE, Patsakham KM. Tobacco Use Treatment at the U.S. National Cancer Institute's Designated Cancer Centers. Nicotine & Tobacco Research. 2012 doi: 10.1093/ntr/nts083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel B, Wewers ME, Baker CA. The effectiveness of a nurse-managed minimal smoking-cessation intervention among hospitalized patients with cancer. Oncology nursing forum. 1998;25(5):897. [PubMed] [Google Scholar]
- Gritz ER, Carr CR, Rapkin D, Abemayor E, Chang LJ, Wong WK, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiology Biomarkers & Prevention. 1993;2(3):261–270. [PubMed] [Google Scholar]
- Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiology Biomarkers & Prevention. 2005;14(10):2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer. 2006;106(1):17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. Journal of Consulting & Clinical Psychology. 1990;58(2):175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res. 2006;8(6):739–749. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine and Tobacco Research. 2003;5(1):13–25. [PubMed] [Google Scholar]
- IOM. Reducing Tobacco-Related Cancer Incidence and Mortality - Workshop Summary. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Chan JM, Giovannucci E. Smoking and prostate cancer survival and recurrence. JAMA : The Journal of the American Medical Association. 2011;305(24):2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DK, Carlson J. Smoking patterns in cancer survivors. Nicotine Tob Res. 2011;13(1):34–40. doi: 10.1093/ntr/ntq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DK, Terrin NC, Menon U, Kreps GL, McCance K, Parsons SK, Mooney KH. Health behaviors in cancer survivors. Oncology Nursing Forum. 2007;34(3):643–651. doi: 10.1188/07.ONF.643-651. [DOI] [PubMed] [Google Scholar]
- McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer control. 2003;10(4):325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]
- Moller AM. Preoperative smoking intervention. Journal of Clinical Anesthesia. 2006;18(8):561–562. doi: 10.1016/j.jclinane.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Morgan G, Schnoll RA, Alfano CM, Evans SE, Goldstein A, Ostroff J, Cox LS. National Cancer Institute Conference on Treating Tobacco Dependence at Cancer Centers. Journal of Oncology Practice. 2011;7(3):178–182. doi: 10.1200/jop.2010.000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest. 2001;119(6):1635–1640. doi: 10.1378/chest.119.6.1635. [DOI] [PubMed] [Google Scholar]
- Nagelkerke N, Fidler V, Bernsen R, Borgdorff M. Estimating treatment effects in randomized clinical trials in the presence of non-compliance. Statistics in Medicine. 2000;19(14):1849–1864. doi: 10.1002/1097-0258(20000730)19:14<1849::aid-sim506>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN practice guidelines in oncology - v.1.2007: Genetic/familial high-risk assessment: breast and ovarian. 2007 Retrieved May 20, 2007, from http://www.nccn.org/professionals/physician_gls/default.asp.
- Nayan S, Gupta MK, Sommer DD. Evaluating smoking cessation interventions and cessation rates in cancer patients: a systematic review and meta-analysis. ISRN oncology. 2011;2011:849023. doi: 10.5402/2011/849023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff J, Burkhalter J, O'Brien M, Hay J, Dhingra L. Smoking Cessation Guide for Cancer Patients and their Families. Memorial Sloan-Kettering Cancer Center. 2005:1–26. [Google Scholar]
- Ostroff J, Garland J, Moadel A, Fleshner N, Hay J, Cramer L, Russo P. Cigarette smoking patterns in patients after treatment of bladder cancer. Journal of cancer education. 2000;15(2):86–90. doi: 10.1080/08858190009528663. [DOI] [PubMed] [Google Scholar]
- Park ER, Japuntich SJ, Rigotti NA, Traeger L, He Y, Wallace RB, Keating NL. A snapshot of smokers after lung and colorectal cancer diagnosis. Cancer. 2012 doi: 10.1002/cncr.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ER, Japuntich S, Temel J, Lanuti M, Pandiscio J, Hilgenberg J, Rigotti NA. A smoking cessation intervention for thoracic surgery and oncology clinics: a pilot trial. Journal of Thoracic Oncology. 2011;6(6):1059–1065. doi: 10.1097/JTO.0b013e318215a4dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. British Medical Journal. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppone LJ, Mustian KM, Morrow GR, Dozier AM, Ossip DJ, Janelsins MC, McIntosh S. The effect of cigarette smoking on cancer treatment-related side effects. The Oncologist. 2011;16(12):1784–1792. doi: 10.1634/theoncologist.2011-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing (Version 2 13 1) Vienna, Austria: R Foundation for Statistical Computing; 2011. Retrieved from http://www.R-project.org. [Google Scholar]
- Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;5:CD001837. doi: 10.1002/14651858.CD001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: a systematic review. Archives of Internal Medicine. 2008;168(18):1950–1960. doi: 10.1001/archinte.168.18.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. Journal of Clinical Oncology. 2007;25(15):2100–2106. doi: 10.1200/Jco.2006.06.6340. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS System for Windows (Version 9.2) Cary, NC: SAS Institute Inc.; 2000-2008. [Google Scholar]
- Schnoll RA, Rothman RL, Wielt DB, Lerman C, Pedri H, Wang H, Cheng J. A randomized pilot study of cognitive-behavioral therapy versus basic health education for smoking cessation among cancer patients. Annals of Behavioral Medicine. 2005;30(1):1–11. doi: 10.1207/s15324796abm3001_1. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Zhang B, Rue M, Krook JE, Spears WT, Marcus AC, Engstrom PF. Brief physician-initiated quit-smoking strategies for clinical oncology settings: a trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2003;21(2):355–365. doi: 10.1200/JCO.2003.04.122. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on biochemical verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stanislaw AE, Wewers ME. A smoking cessation intervention with hospitalized surgical cancer patients: a pilot study. Cancer Nursing. 1994;17(2):81–86. [PubMed] [Google Scholar]
- Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tobacco Control. 2006;15(5):352–358. doi: 10.1136/tc.2005.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood JM, Townsend JS, Stewart SL, Buchannan N, Ekwueme DU, Hawkins NA, Fairley TL. Surveillance of demographic characteristics and health behaviors among adult cancer survivors - behavioral risk factor surveillance system, United States, 2009. MMWR Surveillance summaries : Morbidity and mortality weekly report Surveillance summaries/CDC. 2012;61 Suppl 1:1–23. [PubMed] [Google Scholar]
- Underwood JM, Townsend JS, Tai E, White A, Davis SP, Fairley TL. Persistent cigarette smoking and other tobacco use after a tobacco-related cancer diagnosis. J Cancer Surviv. 2012;6(3):333–344. doi: 10.1007/s11764-012-0230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDHHS. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. 2008 Retrieved May, 2008, from http://www.ncbi.nlm.nih.gov/books/NBK63952/
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychological Bulletin. 1992;111(1):23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- Waggoner SE, Darcy KM, Fuhrman B, Parham G, Lucci J, 3rd, Monk BJ, Moore DH. Association between cigarette smoking and prognosis in locally advanced cervical carcinoma treated with chemoradiation: a Gynecologic Oncology Group study. Gynecologic Oncology. 2006;103(3):853–858. doi: 10.1016/j.ygyno.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Wakefield M, Olver I, Whitford H, Rosenfeld E. Motivational interviewing as a smoking cessation intervention for patients with cancer: Randomized controlled trial. Nursing Research. 2004;53(6):396–405. doi: 10.1097/00006199-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Warner DO. Perioperative abstinence from cigarettes: physiologic and clinical consequences. Anesthesiology. 2006;104(2):356–367. doi: 10.1097/00000542-200602000-00023. 00000542-200602000-00023. pii. [DOI] [PubMed] [Google Scholar]
- Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. International Journal of Cancer. 2012 doi: 10.1002/ijc.27617. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Warren GW, Marshall JR, Cummings KM, Toll B, Gritz ER, Hutson A, Dresler C. Practice Patterns and Perceptions of Thoracic Oncology Providers on Tobacco Use and Cessation in Cancer Patients. J Thorac Oncol. 2013;8(5):543–548. doi: 10.1097/JTO.0b013e318288dc96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients--influence of a smoking-related diagnosis: a pilot study. Heart & lung. 1994;23(2):151–156. [PubMed] [Google Scholar]
- Wewers ME, Jenkins L, Mignery T. A nurse-managed smoking cessation intervention during diagnostic testing for lung cancer. Oncology nursing forum. 1997;24(8):1419. [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]


