Abstract
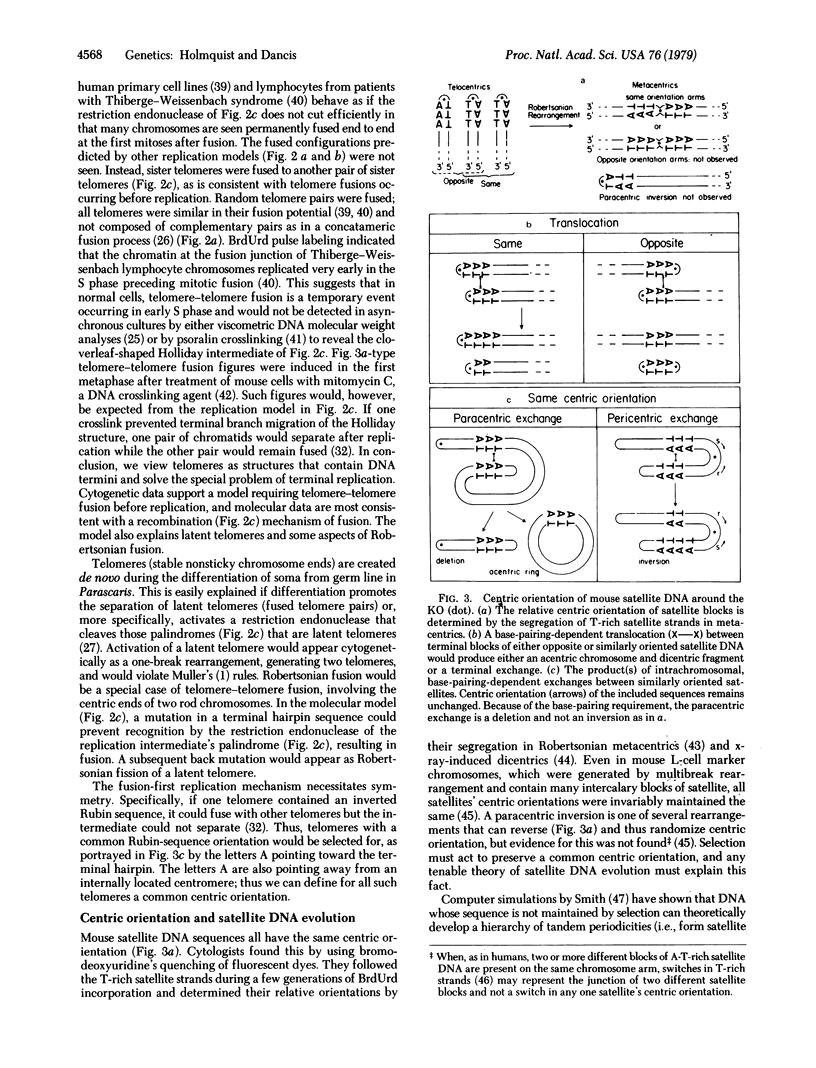
Robertsonian rearrangements demonstrate one-break chromosome rearrangement and the reversible appearance and disappearance of telomeres and centromeres. Such events are quite discordant with classical cytogenetic theories, which assume all chromosome rearrangements to require at least two breaks and consider centromeres and telomeres as immutable structures rather than structures determined by mutable DNA sequences. Cytogenetic data from spontaneous and induced telomere-telomere fusions in mammals support a molecular model of terminal DNA synthesis in which all telomeres are similar and recombine before replication and subsequent separation. This, along with evidence for a hypothetical DNA sequence, the kinetochore organizer, readily explains latent telomeres, latent centromeres, and reversible (one-break) Robertsonian rearrangements. A second model, involving simply recombination between like satellite DNA sequences on different chromosomes, explains not only how one satellite can simultaneously evolve on different chromosomes, but also why satellite DNA is usually located near centromeres or telomeres and why it maintains a preferred orientation with respect to the centromere.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angell R. R., Jacobs P. A. Lateral asymmetry in human constitutive heterochromatin. Chromosoma. 1975 Aug 11;51(4):301–310. doi: 10.1007/BF00326317. [DOI] [PubMed] [Google Scholar]
- Bateman A. J. Letter: Simplification of palindromic telomere theory. Nature. 1975 Jan 31;253(5490):379–380. doi: 10.1038/253379a0. [DOI] [PubMed] [Google Scholar]
- Benn P. A. Specific chromosome aberrations in senescent fibroblast cell lines derived from human embryos. Am J Hum Genet. 1976 Sep;28(5):465–473. [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Brutlag D. Cloning and characterization of a complex satellite DNA from Drosophila melanogaster. Cell. 1977 Jun;11(2):371–381. doi: 10.1016/0092-8674(77)90054-x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Pardue M. L. Electron microscopy of DNA crosslinked with trimethylpsoralen: test of the secondary structure of eukaryotic inverted repeat sequences. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2644–2648. doi: 10.1073/pnas.73.8.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D. E., Okada T. A. Whole-mount electron microscopy of the centromere region of metacentric and telocentric mammalian chromosomes. Cytogenetics. 1970;9(6):436–449. doi: 10.1159/000130113. [DOI] [PubMed] [Google Scholar]
- Dancis B. M., Holmquist G. P. Telomere replication and fusion in eukaryotes. J Theor Biol. 1979 May 21;78(2):211–224. doi: 10.1016/0022-5193(79)90265-0. [DOI] [PubMed] [Google Scholar]
- Daniel A., Lam-Po-Tang P. R. Structure and inheritance of some heterozygous Robertsonian translocation in man. J Med Genet. 1976 Oct;13(5):381–388. doi: 10.1136/jmg.13.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiberg H. New selective Giemsa technique for human chromosomes, Cd staining. Nature. 1974 Mar 1;248(5443):55–55. doi: 10.1038/248055a0. [DOI] [PubMed] [Google Scholar]
- Heumann J. M. A model for replication of the ends of linear chromosomes. Nucleic Acids Res. 1976 Nov;3(11):3167–3171. doi: 10.1093/nar/3.11.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist G. P., Comings D. E. Sister chromatid exchange and chromosome organization based on a bromodeoxyuridine Giemsa-C-banding technique (TC-banding). Chromosoma. 1975 Oct 14;52(3):245–259. doi: 10.1007/BF00332114. [DOI] [PubMed] [Google Scholar]
- Holmquist G. Organisation and evolution of Drosophila virilis heterochromatin. Nature. 1975 Oct 9;257(5526):503–506. doi: 10.1038/257503a0. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Pathak S., Basen B. M., Stark G. J. Induced Robertsonian fusions and tandem translocations in mammalian cell cultures. Cytogenet Cell Genet. 1978;21(1-2):86–98. doi: 10.1159/000130881. [DOI] [PubMed] [Google Scholar]
- Hsu T. C., Pathak S., Chen T. R. The possibility of latent centromeres and a proposed nomenclature system for total chromosome and whole arm translocations. Cytogenet Cell Genet. 1975;15(1):41–49. doi: 10.1159/000130497. [DOI] [PubMed] [Google Scholar]
- John B., Freeman M. Causes and consequences of Robertsonian exchange. Chromosoma. 1975 Sep 26;52(2):123–136. doi: 10.1007/BF00326262. [DOI] [PubMed] [Google Scholar]
- Jokelainen P. T. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967 Jul;19(1):19–44. doi: 10.1016/s0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- Kato H., Sagai T., Yosida T. H. Stable telocentric chromosomes produced by centric fission in Chinese hamster cells in vitro. Chromosoma. 1973;40(2):183–192. doi: 10.1007/BF00321463. [DOI] [PubMed] [Google Scholar]
- Kavenoff R., Klotz L. C., Zimm B. H. One the nature of chromosome-sized DNA molecules. Cold Spring Harb Symp Quant Biol. 1974;38:1–8. doi: 10.1101/sqb.1974.038.01.003. [DOI] [PubMed] [Google Scholar]
- Kurnit D. M., Brown F. L., Maio J. J. Mammalian repetitive DNA sequences in a stable Robertsonian system. Characterization, in situ hybridizations, and cross-species hybridizations of repetitive DNAs in calf, sheep, and goat chromosomes. Cytogenet Cell Genet. 1978;21(3):145–167. doi: 10.1159/000130888. [DOI] [PubMed] [Google Scholar]
- Lau Y. F., Hsu T. C. Variable modes of Robertsonian fusions. Cytogenet Cell Genet. 1977;19(4):231–235. doi: 10.1159/000130813. [DOI] [PubMed] [Google Scholar]
- Lin M. S., Davidson R. L. Centric fusion, satellite DNA, and DNA polarity in mouse chromosomes. Science. 1974 Sep 27;185(4157):1179–1181. doi: 10.1126/science.185.4157.1179. [DOI] [PubMed] [Google Scholar]
- Lin M. S., Davidson R. L. Centromeric asymmetry and induction of translocations and sister chromatid exchanges in mouse chromosomes. Nature. 1975 Mar 27;254(5498):354–356. doi: 10.1038/254354a0. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- McClintock B. The Production of Homozygous Deficient Tissues with Mutant Characteristics by Means of the Aberrant Mitotic Behavior of Ring-Shaped Chromosomes. Genetics. 1938 Jul;23(4):315–376. doi: 10.1093/genetics/23.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M., Brinkley B. R. Human chromosomes and centrioles as nucleating sites for the in vitro assembly of microtubules from bovine brain tubulin. J Cell Biol. 1975 Oct;67(1):189–199. doi: 10.1083/jcb.67.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagome Y., Teramura F., Katoaka K., Hosono F. Mental retardation, malformation syndrome andpartial 7p monosomy [45, XX, tdic (7;15) (p21;p11)]. Clin Genet. 1976 Jun;9(6):621–624. [PubMed] [Google Scholar]
- Rubin G. M. Isolation of a telomeric DNA sequence from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1041–1046. doi: 10.1101/sqb.1978.042.01.104. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Telzer B. R., Moses M. J., Rosenbaum J. L. Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4023–4027. doi: 10.1073/pnas.72.10.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]


