Abstract
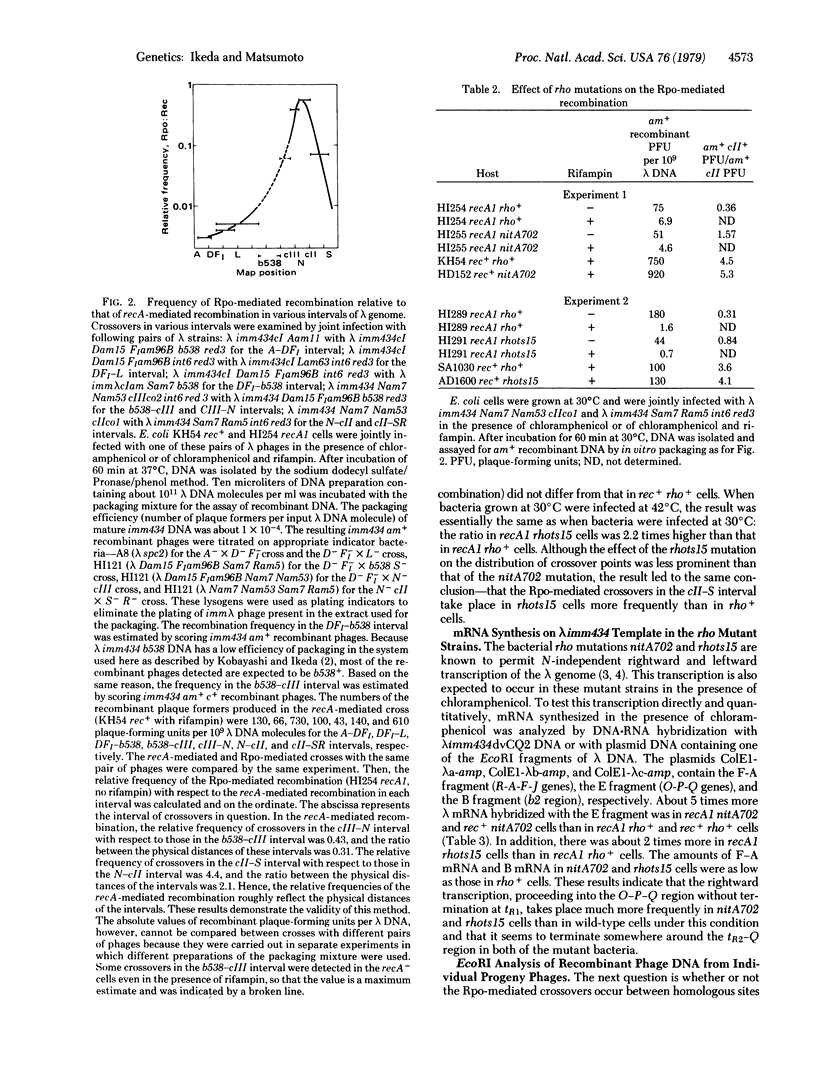
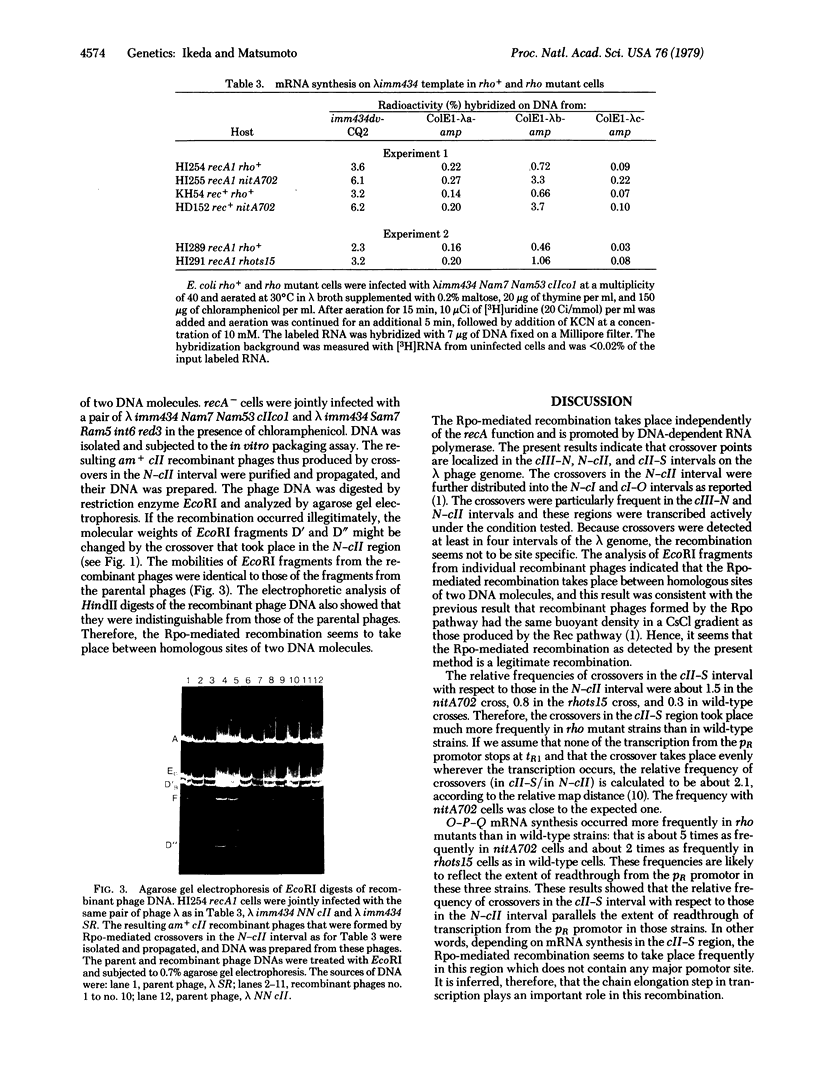
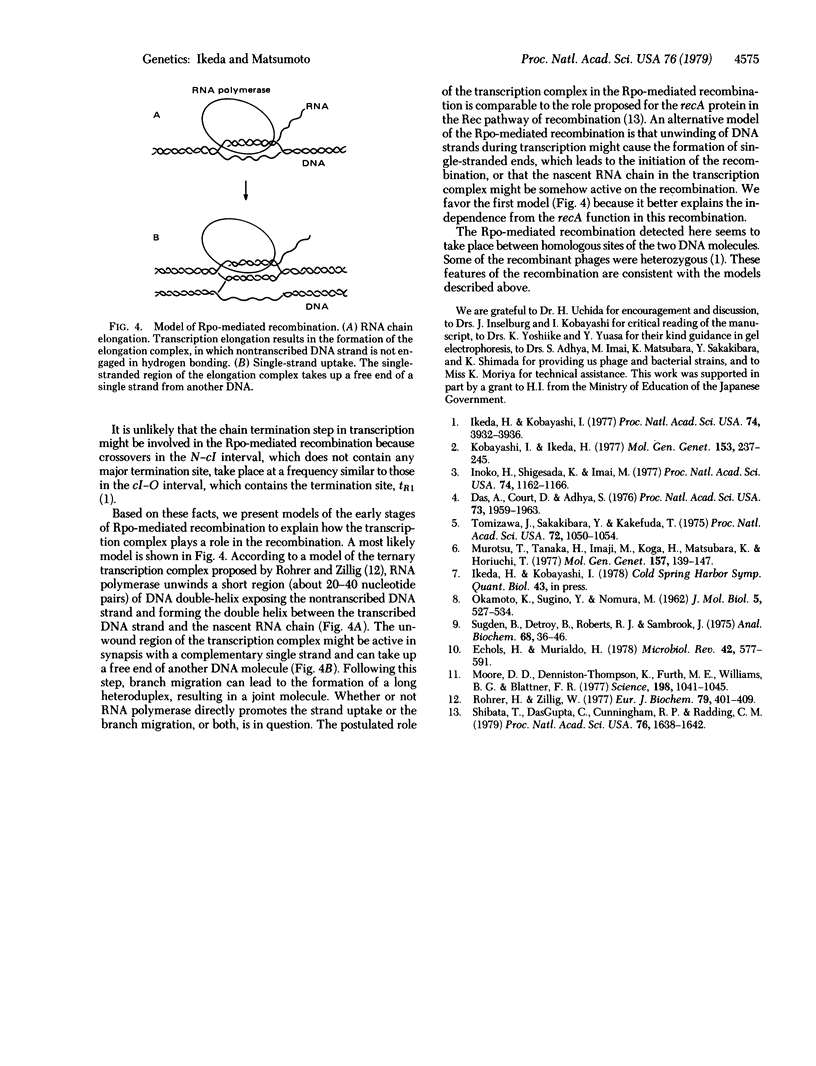
The Rpo-mediated recombination of phage lambda takes place independently of the recA function and is promoted by DNA-dependent RNA polymerase of Escherichia coli [Ikeda, H. & Kobayashi, I. (1977) Proc. Natl. Acad Sci. USA 74, 3932--3936]. The crossovers were particularly frequent to the cIII-N and N-cII regions which are transcribed actively. To determine whether the transcription process required for the recombination is the initiation step or the chain elongation step, we have examined the effect of bacterial rho mutation, which affects transcription termination, on the distribution of crossover points in the lambda phage genome. The crossovers in the cII-S interval took place more frequently in rho mutant strains than in wild-type strains. Analysis of lambda mRNA showed that much more O-P-Q mRNA is synthesized in the rho mutant cells than in the wild-type cells and is largely produced by the readthrough from the PR promotor. These results strongly suggest that the chain elongation in transcription plays an essential role in this recombination. Physical analysis of the recombinant phage DNA showed that this recombination is a legitimate type. Models are presented to explain how the transcription complex can promote this recA-independent recombination.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
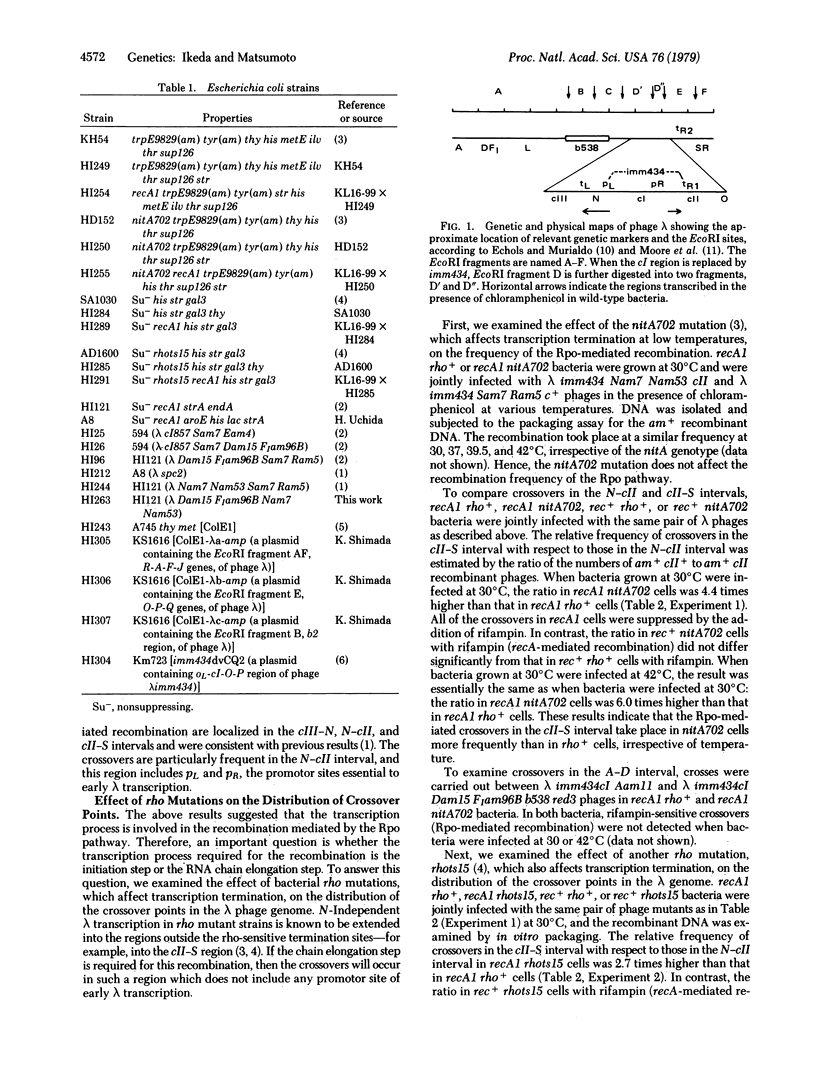
- Echols H., Murialdo H. Genetic map of bacteriophage lambda. Microbiol Rev. 1978 Sep;42(3):577–591. doi: 10.1128/mr.42.3.577-591.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Kobayashi I. Involvement of DNA-dependent RNA polymerase in a recA-independent pathway of genetic recombination in Escheria coli. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3932–3936. doi: 10.1073/pnas.74.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoko H., Shigesada K., Imai M. Isolation and characterization of conditional-lethal rho mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1162–1166. doi: 10.1073/pnas.74.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Ikeda H. Formation of recombinant DNA of bacteriophage lambda by recA function of Escherichia coli without duplication, transcription, translation, and maturation. Mol Gen Genet. 1977 Jun 24;153(3):237–245. doi: 10.1007/BF00431589. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Denniston-Thompson K., Furth M. E., Williams B. G., Blattner F. R. Construction of chimeric phages and plasmids containing the origin of replication of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1041–1046. doi: 10.1126/science.929185. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Tanaka H., Imaji M., Koga H., Matsubara K., Horiuchi T. Purification and properties of a lambda operator-binding protein which is expected to be autorepressor (tof protein) from E. coli carrying lambdadv plasmid. Mol Gen Genet. 1977 Nov 29;157(2):139–147. doi: 10.1007/BF00267391. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., SUGINO Y., NOMURA M. Synthesis and turnover of phage messenger RNA in E. coli infected with bacteriophage T4 in the presence of chloromycetin. J Mol Biol. 1962 Nov;5:527–534. doi: 10.1016/s0022-2836(62)80126-0. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Zillig W. Studies on the transcription complex of Escherichia coli RNA polymerase. Eur J Biochem. 1977 Oct 3;79(2):401–409. doi: 10.1111/j.1432-1033.1977.tb11822.x. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., De Troy B., Roberts R. J., Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975 Sep;68(1):36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA added to cell extracts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1050–1054. doi: 10.1073/pnas.72.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]