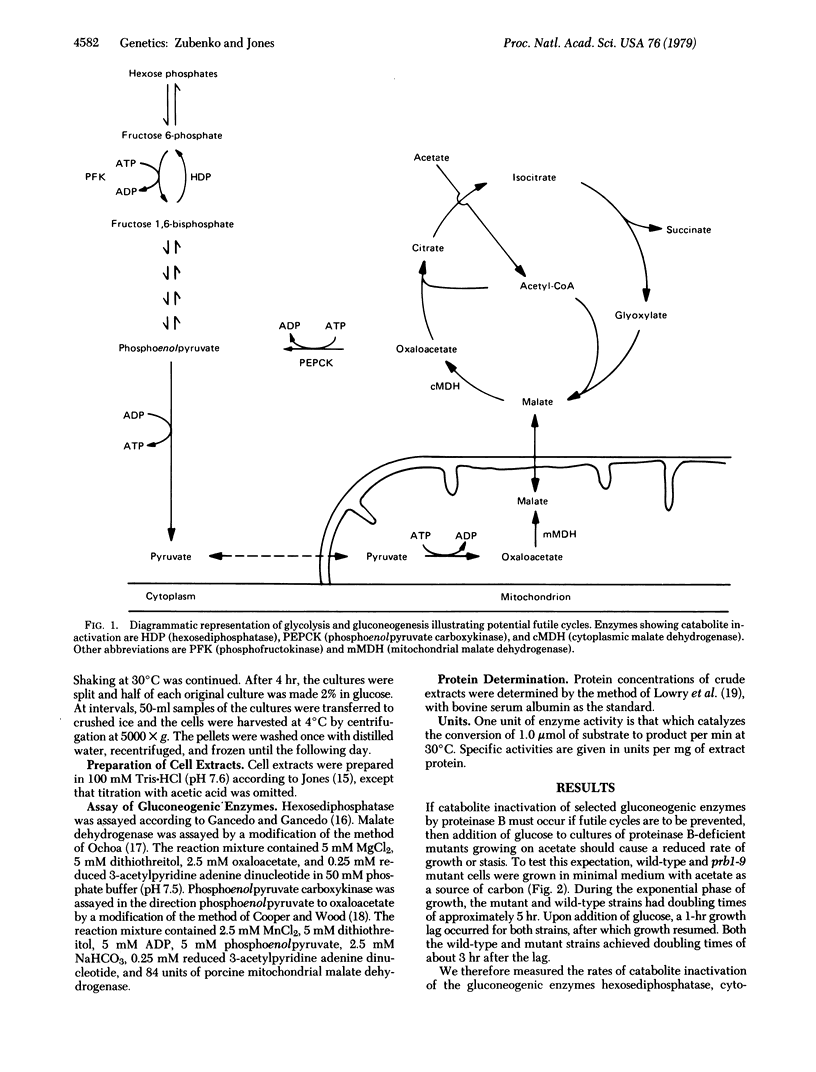
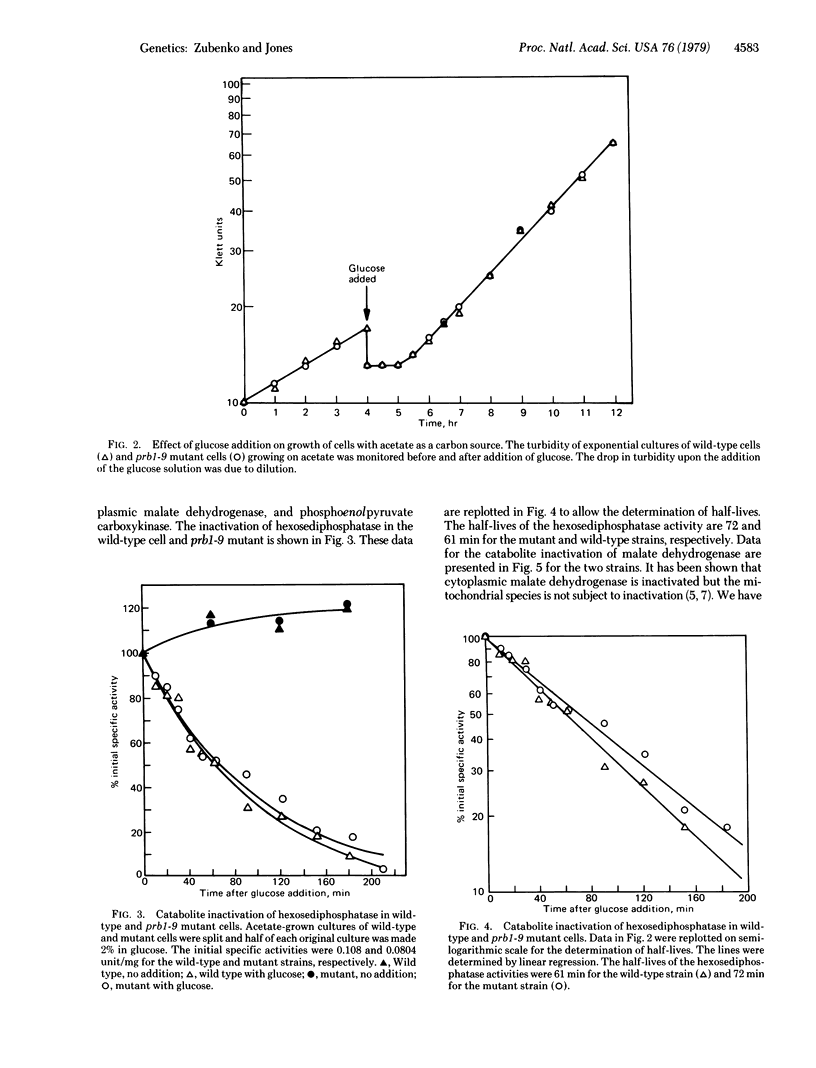
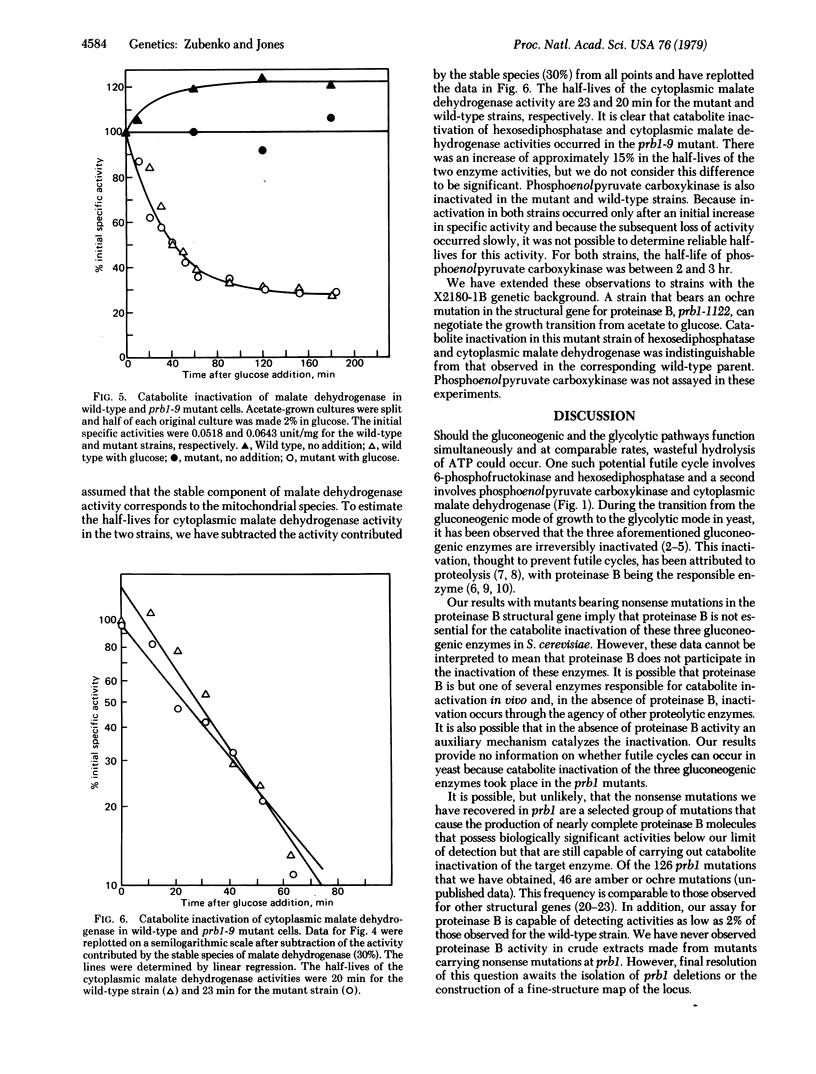
Abstract
Strains of Saccharomyces cerevisiae bearing nonsense mutations in the structural gene for proteinase B (EC 3.4.22.9) have been examined for the ability to make the transition from growth on acetate to growth on glucose and for the ability to inactivate three glucoeogenic enzymes during the transition because proteinase B has been proposed by others to be responsible for the inactivation of the three enzymes during the growth transition. The mutant strains make the growth transition normally. Catabolite inactivation of hexosediphosphatase (D-fructose-1,6-biphosphate 1-phosphohydrolase, EC 3.1.3.11), malate dehydrogenase (L-malate:NAD+ oxidoreductase, EC 1.1.1.37), and phosphoenolpyruvate carboxykinase (ATP) [ATP:oxaloacetate carboxy-lyase (transphosphorylating), EC 4.1.1.49] occurred in prb1 mutants with kinetics similar to those seen in wild-type strains. We infer that proteinase B activity is not essential for the process of catabolite inactivation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper T. G., Wood H. G. The carboxylation of phosphoenolpyruvate and pyruvate. II. The active species of "CO2" utilized by phosphoenlpyruvate carboxylase and pyruvate carboxylase. J Biol Chem. 1971 Sep 10;246(17):5488–5490. [PubMed] [Google Scholar]
- Esposito M. S. X-ray and meiotic fine structure mapping of the adenine-8 locus in Saccharomyces cerevisiae. Genetics. 1968 Apr;58(4):507–527. doi: 10.1093/genetics/58.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. J., Jr, Boll M., Holzer H. Yeast malate dehydrogenase: enzyme inactivation in catabolite repression. Eur J Biochem. 1967 Mar;1(1):21–25. doi: 10.1007/978-3-662-25813-2_4. [DOI] [PubMed] [Google Scholar]
- Fink G. R. A cluster of genes controlling three enzymes in histidine biosynthesis in Saccharomyces cerevisiae. Genetics. 1966 Mar;53(3):445–459. doi: 10.1093/genetics/53.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J Bacteriol. 1971 Aug;107(2):401–405. doi: 10.1128/jb.107.2.401-405.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76(2):132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- HAWTHORNE D. C., MORTIMER R. K. Super-suppressors in yeast. Genetics. 1963 Apr;48:617–620. doi: 10.1093/genetics/48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägele E., Neeff J., Mecke D. The malate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, characterisation and studies on their regulation. Eur J Biochem. 1978 Feb 1;83(1):67–76. doi: 10.1111/j.1432-1033.1978.tb12069.x. [DOI] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusić M., Hinze H., Holzer H. Inactivation of yeast enzymes by proteinase A and B and carboxypeptidase Y from yeast. Hoppe Seylers Z Physiol Chem. 1976 May;357(5):735–740. doi: 10.1515/bchm2.1976.357.1.735. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANNEY T. R. ACTION OF A SUPER-SUPPRESSOR IN YEAST IN RELATION TO ALLELIC MAPPING AND COMPLEMENTATION. Genetics. 1964 Jul;50:109–121. doi: 10.1093/genetics/50.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano J., Gancedo C. Specific inactivation of fructose 1,6-bisphosphatase from Saccharomyces cerevisiae by a yeast protease. Eur J Biochem. 1974 May 2;44(1):213–217. doi: 10.1111/j.1432-1033.1974.tb03475.x. [DOI] [PubMed] [Google Scholar]
- Neeff J., Hägele E., Nauhaus J., Heer U., Mecke D. Application of an immunoassay to the study of yeast malate dehydrogenase inactivation. Biochem Biophys Res Commun. 1978 Jan 13;80(1):276–282. doi: 10.1016/0006-291x(78)91133-6. [DOI] [PubMed] [Google Scholar]
- Ulane R. E., Cabib E. The activating system of chitin synthetase from Saccharomyces cerevisiae. Purification and properties of the activating factor. J Biol Chem. 1976 Jun 10;251(11):3367–3374. [PubMed] [Google Scholar]
- Witt I., Kronau R., Holzer H. Isoenzyme der malatdehydrogenase und ihre regulation in Saccharomyces cerevisiae. Biochim Biophys Acta. 1966 Oct 17;128(1):63–73. [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Septum formation, cell division, and sporulation in mutants of yeast deficient in proteinase B. Proc Natl Acad Sci U S A. 1979 May;76(5):2395–2399. doi: 10.1073/pnas.76.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]



