Abstract
Background
Limited information exists on adults ≥50 years receiving HIV care in sub-Saharan Africa.
Methodology
Using routinely-collected longitudinal patient-level data among 391,111 adults ≥15 years enrolling in HIV care from January 2005–December 2010 and 184,689 initiating ART, we compared characteristics and outcomes between older (≥50 years) and younger adults at 199 clinics in Kenya, Mozambique, Rwanda, and Tanzania. We calculated proportions over time of newly enrolled and active adults receiving HIV care and initiating ART who were ≥50 years; cumulative incidence of loss to follow-up (LTF) and recorded death one year after enrollment and ART initiation, and CD4+ response following ART initiation.
Findings
From 2005–2010, the percentage of adults ≥50 years newly enrolled in HIV care remained stable at 10%, while the percentage of adults ≥50 years newly initiating ART (10% [2005]-12% [2010]), active in follow-up (10% [2005]-14% (2010]), and active on ART (10% [2005]-16% [2010]) significantly increased. One year after enrollment, older patients had significantly lower incidence of LTF (33.1% vs. 32.6%[40–49 years], 40.5%[25–39 years], and 56.3%[15–24 years]; p-value<0.0001), but significantly higher incidence of recorded death (6.0% vs. 5.0% [40–49 years], 4.1% [25–39 years], and 2.8% [15–24 years]; p-valve<0.0001). LTF was lower after vs. before ART initiation for all ages, with older adults experiencing less LTF than younger adults. Among 85,763 ART patients with baseline and follow-up CD4+ counts, adjusted average 12-month CD4+ response for older adults was 20.6 cells/mm3 lower than for adults 25–39 years of age (95% CI: 17.1–24.1).
Conclusions
The proportion of patients who are ≥50 years has increased over time and been driven by aging of the existing patient population. Older patients experienced less LTF, higher recorded mortality and less robust CD4+ response after ART initiation. Increased programmatic attention on older adults receiving HIV care in sub-Saharan Africa is warranted.
Introduction
Recent UNAIDS and WHO data on the HIV epidemic show a considerable increase in the number of people receiving antiretroviral therapy (ART), and sharp declines in both the number of new infections and HIV-related deaths [1], [2]. Consequently, the number of people living with HIV (PLWH) [1], [2] and their life expectancy have increased to their highest levels [3]. These accomplishments bring hope in the fight against the epidemic but also new challenges.
Worldwide trends suggest a “graying” of the HIV epidemic with an increasing proportion of PLWH aged 50 years or older. In the United States, the proportion of older PLWH increased from 17% in 2001 to 24% in 2005 [4], and is projected to rise to 50% by 2015 [5]. This demographic shift is not limited to resource-rich settings. In 2011, 3.1 million adults 50 years or older were estimated to be living with HIV in sub-Saharan Africa, accounting for 13% of all infections in the region [6]. Given the aforementioned reduction in HIV-related mortality due to expansion of ART use [1], [2], coupled with the occurrence of secondary incidence peaks of HIV acquisition among older adults in sub-Saharan Africa [7], the number of older PLWH will likely continue to increase in this region [6], [8]–[10]. Despite this, information on what accounts for this shift and the characteristics of and outcomes among older PLWH in sub-Saharan Africa remains scant [8], [11]–[13].
Older PLWH face unique challenges that may hinder timely and effective HIV care and treatment. Symptoms of advanced HIV disease (e.g. fatigue, weight loss, cognitive deficits) resemble those associated with aging and therefore may result in delayed HIV diagnosis, enrollment in care and initiation of treatment, a common occurrence in sub-Saharan Africa and a leading cause of HIV-related death [2], [8], [14], [15]. Chronic conditions (e.g. hypertension, diabetes) associated with aging require treatment with medications, augmenting the likelihood of drug-drug interactions with ART and adverse drug reactions [16]–[18]. Furthermore, immune recovery decreases with age, particularly for HIV-positive individuals, as thymus function diminishes leading to increased risk of immunologic failure and death [19]–[21]. Lastly, in sub-Saharan Africa, older PLWH often face the financial and psychosocial responsibility of raising grandchildren orphaned by HIV [22], [23], which may compromise engagement and retention in HIV care.
We used routinely-collected patient-level data from a large, multi-country HIV program in sub-Saharan Africa to: (1) estimate the proportion of all newly enrolled and active adult patients receiving HIV care and initiating ART over time who were ≥50 years, and (2) compare key baseline characteristics and outcomes between PLWH ≥50 years and <50 years of age.
Materials and Methods
Study Population
The study population included patients ≥15 years of age enrolled into HIV care between January 01, 2005 and December 31, 2010 and followed through December 31, 2011 at 199 health facilities supported by ICAP at Columbia University through funding from the President's Emergency Plan for AIDS Relief (PEPFAR). These included 69 facilities in Kenya, 52 in Tanzania, 44 in Rwanda and 34 in Mozambique. Provision of services at each clinic was governed by respective national guidelines.
Data Sources
Patient information routinely collected at each clinic visit was documented by clinicians on national patient forms, and regularly entered into on-site electronic databases by data clerks. Information was collected at enrollment and subsequent follow-up visits, with visit frequency depending on patient CD4+ count and whether they were receiving ART. Data quality assessments were done at least annually at each health facility. Each quarter, these electronic data were de-identified, encrypted, and imported into a common-format database for analyses. Use of these de-identified patient-level data for research purposes was approved by the Columbia University Medical Center IRB and ethics committees in each of the four countries where patients were enrolled.
Outcome definitions
The proportion of newly enrolled patients into HIV care and ART initiation who were ≥50 years were calculated for each quarter from 2005–2010. Patients were considered “active in care” if they were not documented as dead, transferred, or lost to follow-up (LTF) and if they had a recorded visit during the quarter (ART patients) or during the current or prior three quarters (pre-ART patients). Patients were followed until death, loss to follow-up, transfer, or December 31, 2011, whichever occurred first. Patients were considered LTF if they were not recorded as dead or transferred and did not have a recorded visit date for six months for ART patients or 12 months for pre-ART patients with no subsequent recorded visit. ART patients LTF were censored 15 days after their last recorded visit and pre-ART patients were censored 3 months after their last recorded visit. Patients with a documented transfer to another facility were censored at their transfer date.
Measures of HIV disease status (CD4+ cell count and WHO stage) at enrollment into HIV care and ART initiation were determined by assigning the closest recorded measure taken within three months prior to or one month after enrollment or ART initiation, as appropriate.
Statistical Analysis
Trends over time in the proportion of patients active in HIV care or on ART were conducted after calculating each active patient's age at the beginning of a given quarter. For all other analyses, patients were classified into four age groups (15–24, 25–39, 40–49, and ≥50 years of age) based on their age at the time of enrollment into care or ART initiation.
We assessed trends over time in the proportion of all adults who were ≥50 years among the following groups using unadjusted log-binominal regression: (1) patients newly enrolling in HIV care, (2) patients newly initiating ART, (3) patients currently active in HIV care, and (4) patients currently on ART.
Baseline characteristics and outcomes (LTF and recorded death) through one year after enrollment into HIV care and ART initiation were compared between age groups using generalized linear mixed models and survival analytic techniques (Kaplan-Meier and Cox proportional models with robust sandwich estimators of variance), respectively. Comparisons of CD4+ cell count at enrollment into care and ART initiation were performed after log-transformation. CD4+ cell count response after ART initiation was compared between age groups for the subset of patients with a recorded CD4+ cell count at ART initiation and at least one follow-up CD4+ cell count. Change in CD4+ cell count after ART initiation was plotted as the median increase in CD4+ cell count by time after ART initiation, lagging each patient's recorded CD4+ count for up to three months or until the patient had another CD4+ cell count or was censored from the analysis. Statistical tests for differences in absolute overall change in CD4+ cell count after ART initiation by age group were conducted using repeated-measures linear regression, adjusting for country, sex, CD4+ count at ART initiation, year of ART initiation, facility type and location.
IRB and ethical board approval for use of this routinely-collected de-identified patient-level data has been given by the Columbia University Medical Center IRB, the Kenya Medical Research Institute Ethics Review Committee, the Comité Nacional de Bioética para Saúde (Mozambican National Ethic Committee), the Comite National de Pilotage des Recherches dans le Domaine du VIH/SIDA in Rwanda (National Steering Committee for HIV/AIDS Research in Rwanda), and the National Institute for Medical Research in Tanzania. This study involved the use of existing, routinely-collected de-identified patient-level data and consequently was given a non-research designation by the Columbia University Medical Center IRB and country ethical review boards. No interviews were conducted, and no data was collected independently for this study. Consequently, informed consent was not necessary or obtained.
Results
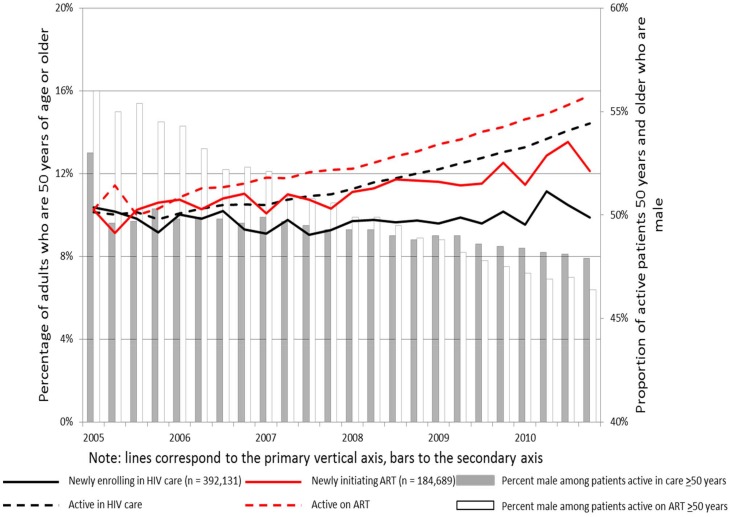
Over the six-year period of observation, 392,131 patients ≥15 years of age enrolled in HIV care (Table 1), 184,689 (47%) of whom initiated ART across the 199 health facilities (Table 2). 10% (N = 38,337) of the patients enrolling in HIV care and 11% (N = 21,108) of those initiating ART were ≥50 years of age. The percentage of patients newly enrolling into HIV care who were ≥50 years of age remained stable over time at around 10% (P = 0.33), while the percentage of patients remaining in HIV care who were ≥50 years increased from 10% to 14% over follow-up period (P<0.0001). The percentage of patients newly initiating ART who were ≥50 years increased from 10% to 12% (P<0.0001) over the study period, and the percentage of patients remaining active on ART who were ≥50 years increased from 10% to 16% (P<0.0001) (Figure 1).
Table 1. Characteristics of adult patients enrolling in HIV care by age group, 2005–2010.
| Overall | ≥50 years | 40–49 years | 25–39 years | 15–24 years | |||||||
| N | % | N | % | N | % | N | % | N | % | ||
| 392,131 (100%) | 38,337 (10%) | 77,457 (20%) | 211,842 (54%) | 64,495 (16%) | |||||||
| Country | Kenya | 87,992 | 22% | 10,427 | 27% | 19,980 | 26% | 48,453 | 23% | 9,132 | 14% |
| Mozambique | 211,849 | 54% | 18,640 | 49% | 36,797 | 48% | 112,392 | 53% | 44,020 | 68% | |
| Rwanda | 38,038 | 10% | 3,642 | 9% | 8,582 | 11% | 20,634 | 10% | 5,180 | 8% | |
| Tanzania | 54,252 | 14% | 5,628 | 15% | 12,098 | 16% | 30,363 | 14% | 6,163 | 10% | |
| Facility location | Urban | 197,588 | 50% | 18,080 | 47% | 37,160 | 48% | 106,225 | 50% | 36,123 | 56% |
| Semi-Urban | 109,037 | 28% | 11,571 | 30% | 23,434 | 30% | 61,059 | 29% | 12,973 | 20% | |
| Rural | 85,506 | 22% | 8,686 | 23% | 16,863 | 22% | 44,558 | 21% | 15,399 | 24% | |
| Facility type | Primary | 139,245 | 36% | 12,668 | 33% | 25,500 | 33% | 74,565 | 35% | 26,512 | 41% |
| Secondary/Tertiary | 252,886 | 64% | 25,669 | 67% | 51,957 | 67% | 137,277 | 65% | 37,983 | 59% | |
| Enrollment year | 2005 | 35,404 | 9% | 3,468 | 9% | 8,422 | 11% | 19,173 | 9% | 4,341 | 7% |
| 2006 | 59,210 | 15% | 5,820 | 15% | 12,654 | 16% | 32,117 | 15% | 8,619 | 13% | |
| 2007 | 74,738 | 19% | 6,951 | 18% | 14,409 | 19% | 40,580 | 19% | 12,798 | 20% | |
| 2008 | 78,454 | 20% | 7,622 | 20% | 14,934 | 19% | 42,453 | 20% | 13,445 | 21% | |
| 2009 | 75,526 | 19% | 7,400 | 19% | 14,129 | 18% | 40,976 | 19% | 13,021 | 20% | |
| 2010 | 68,799 | 18% | 7,076 | 18% | 12,909 | 17% | 36,543 | 17% | 12,271 | 19% | |
| Point of Entry | PMTCT | 36,685 | 9% | 311 | 0.8% | 1,653 | 2% | 21,053 | 10% | 13,668 | 21% |
| TB/HIV | 9,419 | 2% | 1,212 | 3% | 2,340 | 3% | 5,045 | 2% | 822 | 1% | |
| VCT | 147,340 | 38% | 14,769 | 39% | 30,954 | 40% | 79,508 | 38% | 22,109 | 34% | |
| Inpatient | 20,307 | 5% | 2,409 | 6% | 4,195 | 5% | 10,663 | 5% | 3,040 | 5% | |
| Outpatient | 27,048 | 7% | 3,343 | 9% | 6,057 | 8% | 14,238 | 7% | 3,410 | 5% | |
| Other* | 117,510 | 30% | 12,820 | 33% | 25,348 | 33% | 62,991 | 30% | 16,351 | 25% | |
| Unknown | 33,822 | 9% | 3,473 | 9% | 6,910 | 9% | 18,344 | 9% | 5,095 | 8% | |
| Sex | Male | 130,928 | 33% | 19,058 | 50% | 34,945 | 45% | 66,942 | 32% | 9,983 | 15% |
| Female | 261,203 | 67% | 19,279 | 50% | 42,512 | 55% | 144,900 | 68% | 54,512 | 85% | |
| Median (IQR) CD4+ Cell Count (% missing) | 259 (117–460) (49%) | 224 (109–400) (47%) | 216 (97–394) (46%) | 256 (113–454) (49%) | 377 (199–593) (55%) | ||||||
| N (%) WHO stage III/IV (% missing) | 132,752 (47%) (28%) | 15,499 (53%) (24%) | 30,553 (53%) (25%) | 71,225 (47%) (28%) | 15,475 (37%) (35%) | ||||||
| On TB Treatment at enrollment | 12,952 | 3% | 1,459 | 4% | 3,019 | 4% | 7,255 | 3% | 1,219 | 2% | |
*“Other” category includes mostly transfers from other facilities.
Table 2. Characteristics of adult patients initiating ART by age group, 2005–2010.
| Overall | ≥50 years | 40–49 years | 25–39 years | 15–24 years | |||||||
| N | % | N | % | N | % | N | % | N | % | ||
| Patients initiating ART | 184,689 | 21,108 (11%) | 43,955 (24%) | 101,682 (55%) | 17,944 (10%) | ||||||
| Year of ART initiation | |||||||||||
| 2005 | 10,822 | 6% | 1,097 | 5% | 3,107 | 7% | 5,894 | 6% | 724 | 4% | |
| 2006 | 24,789 | 13% | 2,663 | 13% | 6,325 | 14% | 13,763 | 14% | 2,038 | 11% | |
| 2007 | 33,981 | 18% | 3,585 | 17% | 7,823 | 18% | 18,976 | 19% | 3,597 | 20% | |
| 2008 | 36,025 | 20% | 4,121 | 20% | 8,236 | 19% | 19,997 | 20% | 3,671 | 20% | |
| 2009 | 36,510 | 20% | 4,291 | 20% | 8,429 | 19% | 20,091 | 20% | 3,699 | 21% | |
| 2010 | 35,219 | 19% | 4,406 | 21% | 8,379 | 19% | 18,950 | 19% | 3,484 | 19% | |
| 2011* | 7,343 | 4% | 945 | 4% | 1,656 | 4% | 4,011 | 4% | 731 | 4% | |
| Sex | |||||||||||
| Male | 65,188 | 35% | 10,405 | 49% | 19,646 | 45% | 32,037 | 32% | 3,100 | 17% | |
| Female | 119,501 | 65% | 10,703 | 51% | 24,309 | 55% | 69,645 | 68% | 14,844 | 83% | |
| Median (IQR) CD4 cell count at ART initiation, cells/mm3 | |||||||||||
| Overall | CD4 count | 161 (77–243) | 166 (90–245) | 154 (75–234) | 159 (74–242) | 182 (89–272) | |||||
| % missing | 33% | 33% | 32% | 33% | 37% | ||||||
| Males | CD4 count | 144 (63–229) | 152 (78–234) | 140 (62–223) | 141 (59–230) | 159 (62–249) | |||||
| % missing | 32% | 32% | 31% | 32% | 36% | ||||||
| Females | CD4 count | 170 (86–250) | 177 (103–256) | 165 (88–242) | 166 (82–247) | 187 (95–276) | |||||
| % missing | 32% | 34% | 34% | 34% | 37% | ||||||
| WHO stage III/IV at ART initiation | |||||||||||
| Overall | % WHO III/IV | 66,672 64% | 8,166 66% | 16,660 66% | 36,128 63% | 5,718 60% | |||||
| % missing | 43% | 41% | 42% | 43% | 47% | ||||||
| Males | % WHO III/IV | 25,762 70% | 4,089 67% | 7,774 70% | 12,752 71% | 1,147 70% | |||||
| % missing | 43% | 41% | 43% | 44% | 47% | ||||||
| Females | % WHO III/IV | 40,910 60% | 4,077 62% | 8,886 61% | 23,376 58% | 4,571 56% | |||||
| % missing | 43% | 40% | 42% | 43% | 47% | ||||||
| Documented tuberculosis treatment at ART initiation | 4,522 (2%) | 470 (2%) | 1,149 (3%) | 2,580 (3%) | 323 2(%) | ||||||
*ART initiation in 2011 was through June 30th only for those enrolling in care by the end of 2010.
Figure 1. Proportion of adult patients 50 years and older, and proportion male, over time.
Fifty percent of patients aged ≥50 were male compared with 45%, 32% and 15%, of those aged 40–49 years, 25–39 years, and 15–24 years, respectively. The proportion of patients aged ≥50 active in HIV care and on ART who were male decreased over time (P<0.0001 for trend) (Figure 1). CD4+ cell counts at enrollment into care and ART initiation were available for 51% and 67% of all patients, respectively (Tables 1 and 2). At enrollment into HIV care, median CD4+ cell count among older patients was 224 cells/mm3 (IQR: 109–400) and was similar to those aged 40–49 years (216 cells/mm3 [97–394]) but lower than those aged 25–39 years (256 cells/mm3 [113–454]) and 15–24 years (377 cells/mm3 [199–593]) (P<0.0001 for overall difference in medians) (Table 1). At ART initiation (Table 2), older patients had slightly higher median CD4+ cell count (166 cells/mm3 [90–245]) compared with adults aged 40–49 years (154 cells/mm3 [75–234]) and those aged 25–39 years (159 cells/mm3 [74–242]), but significantly lower than those aged 15–24 years (182 cells/mm3 [89–272]) (P<0.0001). Information on WHO stage was available at enrollment into care and ART initiation for 72% and 57% of all patients, respectively. Nearly two-thirds of patients with WHO stage initiated treatment at WHO stage III or IV, with no substantial differences between age groups. For each age stratum, male patients initiated treatment at more advanced stages of HIV disease as measured by CD4+ cell count. The proportion of patients on treatment for tuberculosis at enrollment and at ART initiation was similar across all age strata (Tables 1 and 2).
Half of the patients received HIV care in urban areas, and 64% attended secondary or tertiary facilities, with little substantive difference by age group. Point of entry into HIV care was similar across age groups with the exception of those from prevention of mother to child transmission (PMTCT) programs. Less than 1% of patients ≥50 years of age were referred from PMTCT settings, compared with 2%, 10%, and 21% of those aged 40–49, 25–39 and 15–24 years (Table 1).
Table 3 shows the cumulative incidence of LTF and recorded death through one year after enrollment into HIV care and ART initiation by age group. Cumulative incidence of LTF through one year after enrollment into HIV care among patients ≥50 years of age was similar (33.1%) to that observed among patients 40–49 years of age (32.6%), but significantly lower than that of younger age groups (40.5% for adults 25–39 years and 56.3% for adults 15–24 years; P<0.0001 for difference between groups). Incidence of recorded death was significantly higher among older patients (6.0%) versus 5.0% for 40–49 years, 4.1% for 25–39 years, and 2.8% for 15–24 years (P<0.0001 for difference between groups). Across all age groups, men experienced higher incidence of LTF and death compared with women.
Table 3. Loss to follow-up and death one year after enrollment into care and ART initiation, by age group.
| Cumulative incidence* of loss to follow-up and recorded death one year after enrollment into HIV care** n = 392,131 | ||||||
| % Loss to follow-up | % Recorded Dead | |||||
| Age Group (years) | Overall | Males | Females | Overall | Males | Females |
| ≥50 | 33.1 | 35.9 | 30.5 | 6.0 | 7.3 | 4.7 |
| 40–49 | 32.6 | 36.5 | 29.4 | 5.0 | 6.5 | 3.9 |
| 25–39 | 40.5 | 46.1 | 38.0 | 4.1 | 5.9 | 3.3 |
| 15–24 | 56.3 | 58.5 | 55.9 | 2.8 | 4.3 | 2.5 |
*Cumulative incidence of loss to follow-up and death estimated using Kaplan-Meier survival analytic techniques.
**Log-rank test for equality over strata statistically significant for all models at an alpha of 0.05.
LTF after ART initiation was lower than after enrollment into HIV care for all age and sex strata. Among patients who initiated ART, cumulative incidence of LTF was similar among adults ≥50 years and those 40–49 years of age (16.1% and 15.3%, respectively) but significantly lower than among younger age groups (19.3% for adults 25–39 years and 28.0% for adults 15–24 years); P<0.0001 for difference between groups. Recorded death through one year after ART initiation was highest among the oldest age group (6.0%) compared with adults 40–49 years (5.1%), 25–39 years (4.5%), and 15–24 years (4.4%); P<0.0001 for difference between groups. For each age-category, male patients were more likely to be LTF and to have died within a year after enrollment or ART initiation (Table 3).
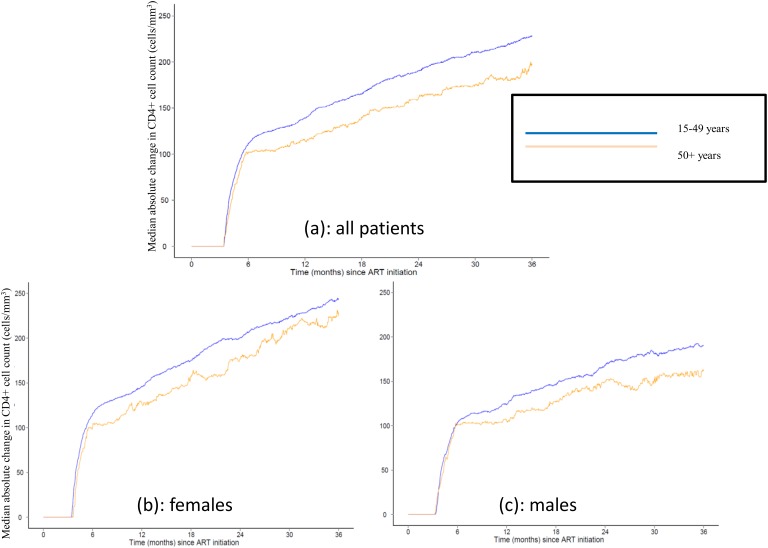
Figure 2 presents CD4+ cell count response after ART initiation for the 85,763 (46%) patients with CD4+ cell counts at ART initiation and at least one follow-up CD4+ cell count. Among these patients, older adults experienced smaller increases in CD4+ cell count after ART initiation than all other age groups. This result was observed overall and when stratified by gender. After adjustment for baseline CD4+ cell count at ART initiation, sex, year of ART initiation, facility type, location, and country, compared to adults 25–39 years of age, the average CD4+ cell increase at 12 months among adults ≥50 years was 20.6 cells/mm3 lower (95% CI: 17.1 to 24.1 cells/mm3) (Table 4). The full results of the regression results are in Table S1.
Figure 2. Change in CD4+ cell count after ART initiation, by age category.
2a. All Patients. 2b. Males. 2c. Females.
Table 4. Multivariate regression: Relative increase in CD4+ cell count (cells/mm3) after ART initiation, n = 85,763.
| Model11 | Model22 | |||
| Age Group | Change in CD4 | 95% CI | Change in CD4 | 95% CI |
| ≥50 years | −33.9 | (−30.2 to −37.6) | −20.6 | (−17.1 to −24.1) |
| 40–49 years | −12.7 | (−9.8 to −15.6) | −8 | (−5.2 to −10.7) |
| 25–39 years | Reference | |||
| 15–24 years | 1.3 | (−4.3 to 6.8) | 5.5 | (0.4 to 10.5) |
Controls for timing of CD4 measure after ART initiation and within-clinic correlation.
Additionally controls for clinic type and location, country, sex, year of ART initiation, and CD4 at ART initiation.
Discussion
Our study—which reports on the largest number of PLWH ≥50 years of age enrolled in HIV programs in sub-Saharan Africa to date—showed that the percentage of older adults newly initiating ART and active in HIV care or on ART increased significantly from 2006 to 2010, while the percentage of such patients newly enrolling in HIV care remained stable during that period. These findings suggest that the observed increases in the percentage of patients aged ≥50 years initiating ART, and remaining active in care is resulting from a “graying” of the existing patient population rather than new enrollment of older patients. We found that approximately 14% and 16% of adult patients active in HIV care and on ART, respectively, were ≥50 years of age at the end of 2010, similar to population estimates of the percentage of older PLWH in sub-Saharan Africa [24]. Furthermore, in our analyses, 12% of the adult population newly initiating ART were ≥50 years of age at the end of 2010, similar to estimates from a recent study which included data from nine countries in sub-Saharan Africa [25].
We observed that patients ≥50 years of age enrolling into HIV care and initiating ART were more likely to be male compared to younger adults, which were disproportionately female. Our findings of disproportionately higher proportions of males among older adults compared to younger adults accessing HIV care is consistent with findings from a retrospective cohort study in Zomba District, Malawi [26], which compared adults 25–49 years of age at ART initiation to those 50 years and older, and the DART clinical trial in Zimbabwe and Uganda [27], which compared symptomatic (WHO stage 2–4) adults at ART initiation with CD4+ cell counts <200 cells/mm3 18–49 years of age to those 50 years and older. We extend these studies by additionally presenting higher proportions of older adults enrolling in HIV care who are male, in addition to the findings at ART initiation, and by further stratifying into age categories of 15–24 years, 25–39 years and 40–49 years to highlight that the proportion male increases with each increasing age category.
The predominance of active males among patients ≥50 years in HIV care or on ART remained even though males were more likely to die or become LTF. This gender differential may reflect a higher prevalence of HIV among males ≥50 years, consistent with epidemiologic surveys in South Africa [28], and/or delayed engagement in care among men. As shown in our analyses and the published literature, male patients in resource-limited settings present for care at more advanced stages of HIV disease and therefore are more likely to initiate treatment late [29]–[31].
The imbalance in gender distribution by age does not completely explain the disparity in mortality between older and younger adults. Age-stratified analyses showed that mortality was higher and CD4+ cell response was less robust among older adults for both men and women when compared to younger PLWH, similarly to results described elsewhere [25]. Information on cause of death was unavailable, and it is likely that at least some of the increased mortality among older adults is due to increased background risk of death from natural aging processes. Additionally, differences in CD4+ cell count response may contribute to the higher mortality observed among older patients. Indeed, although older adults had slightly higher average CD4+ cell counts at ART initiation compared to adults 40–49 years and adults 25–39 years, older adults experienced significantly lower CD4+ cell count response than younger patients after ART initiation. Studies have shown discrepant findings, with some showing similar results to ours [25], [32], and others finding comparable CD4+ cell count responses in older and younger adults [33], [34]. However, with the exception of the study by Grieg et al [25], all of the latter studies were conducted in resource-rich countries where adults may have a different baseline clinical profile than that of our population. Our findings on reduced CD4+ cell response after ART initiation among older adults are similar to those noted by Grieg et al [25] by demonstrating that this decreased response remains up to 36 months after ART initiation, and that the decreased response remained even after adjustment for factors potentially associated with differential CD4+ cell response post ART initiation.
Our study has a number of strengths. To our knowledge, it is the largest multi-country study investigating characteristics and outcomes of older PLWH enrolling in HIV care in sub-Saharan Africa. The data used in this study were derived from health facilities that are in many ways typical of HIV care settings in the region. Some limitations must be noted, however. Only 67% of patients had a CD4+ cell count at ART initiation and of these, only 67% had at least one post-ART CD4+ cell count. Patients missing CD4+ cell counts at ART initiation were similar to patients with CD4+ cell counts at ART initiation in facility location, year of ART initiation, point of entry, sex, and WHO stage at ART initiation. However, patients initiating ART in Rwanda were significantly more likely to have ART initiation CD4+ cell counts (86%) compared to patients enrolling in Kenya (72%), Mozambique (62%), and Tanzania (61%). While it is possible that individuals with ART initiation and follow-up CD4+ cell counts differ on CD4 response from those with missing ART initiation and/or follow-up CD4+ cell counts, it is unlikely that this difference would be substantially differential by age category, suggesting this is an unlikely explanation for the observed difference in CD4 response between older and younger adults. Further, our findings are similar to those of another multi-country analysis in Africa [25]. Additionally, we lacked information on co-morbidities which may increase with age, and thus were unable to explore whether they contributed to the higher death rates observed among patients aged ≥50 years of age. Lastly, a large proportion of patients were LTF, especially among younger adults. Whether these patients stopped treatment, transferred to another facility or died is unknown. Since some proportion of patients deemed LTF in our analyses are likely to be unrecorded deaths [35], [36], we expect that the true death rate in our population is likely higher than documented in our data.
Conclusions
This analysis found that, across a large population including 199 health facilities in 4 sub-Saharan African countries, increasing proportions of adults newly initiating ART, and active in HIV care and on ART, are 50 years and older. These older adults are at increased risk of death, at lower risk of loss to follow-up and have less robust CD4+ cell count response after initiation of ART. Given trends in increasing representation of older adults among the population seeking HIV care in sub-Saharan Africa, there is the need to better understand CD4+ cell count response and to identify programmatic features associated with enhanced outcomes of older patients on treatment.
Supporting Information
Complete results of regression analysis: change in CD4+ cell count after ART initiation.
(DOCX)
Acknowledgments
We thank all patients and staff at the HIV care and treatment clinics included in this analysis. We would also like to thank Ministries of Health in Kenya, Mozambique, Rwanda, and Tanzania for their continued collaboration, and all ICAP and clinic staff responsible for collecting the data used in this analysis.
Disclaimers
A preliminary analysis of data included in this manuscript was presented at the XIX International AIDS Conference in Washington, DC, July 22–27, 2012.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
U.S. Centers for Disease Control and Prevention, Identifying Optimal Models of HIV Care and Treatment, Grant Number 5U2GPS001537-03. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.UNAIDS (2013) Global Report: UNAIDS Report on the Global AIDS Epidemic New York: UNAIDS. [Google Scholar]
- 2.WHO (2013) Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3. Nakagawa F, May M, Phillips A (2013) Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis 26: 17–25. [DOI] [PubMed] [Google Scholar]
- 4.CDC (2008) HIV/AIDS Among Persons Aged 50 and Older. U.S. Centers for Disease Control and Prevention.
- 5. Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, et al. (2008) Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 47: 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hontelez JA, de Vlas SJ, Baltussen R, Newell ML, Bakker R, et al. (2012) The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS 26 (Suppl 1) S19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaba B, Todd J, Biraro S, Shafer L, Lutalo T, et al. (2008) Diverse age patterns of HIV incidence rates in Africa [Abstract]. XVII Internation AIDS Conference. Mexico City.
- 8. Manfredi R (2002) HIV disease and advanced age: an increasing therapeutic challenge. Drugs Aging 19: 647–669. [DOI] [PubMed] [Google Scholar]
- 9. Nguyen N, Holodniy M (2008) HIV infection in the elderly. Clin Interv Aging 3: 453–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stoff DM, Khalsa JH, Monjan A, Portegies P (2004) Introduction: HIV/AIDS and Aging. AIDS 18 (Suppl 1) S1–2. [PubMed] [Google Scholar]
- 11. Fang CT, Chang YY, Hsu HM, Twu SJ, Chen KT, et al. (2007) Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM 100: 97–105. [DOI] [PubMed] [Google Scholar]
- 12. Pontali E, Vareldzis B, Perriens J, Narain J (2003) Antiretroviral Treatment in Resource-limited Settings. Journal of Health Management 2003: 315–326. [Google Scholar]
- 13.UNAIDS (2007) AIDS epidemic update: December 2007. 1–43.
- 14. el-Sadr W, Gettler J (1995) Unrecognized human immunodeficiency virus infection in the elderly. Arch Intern Med 155: 184–186. [PubMed] [Google Scholar]
- 15. Valcour V, Paul R (2006) HIV infection and dementia in older adults. Clin Infect Dis 42: 1449–1454. [DOI] [PubMed] [Google Scholar]
- 16. Goodkin K, Heckman T, Siegel K, Linsk N, Khamis I, et al. (2003) “Putting a face” on HIV infection/AIDS in older adults: a psychosocial context. J Acquir Immune Defic Syndr 33 (Suppl 2) S171–184. [PubMed] [Google Scholar]
- 17. Orlando G (2006) Antiretroviral Treatment and Age-related Comorbidities in a Cohort of Older HIV-infected Patients. British HIV Association HIV Medicine 7: 549–557. [DOI] [PubMed] [Google Scholar]
- 18. Stoff DM (2004) Mental health research in HIV/AIDS and aging: problems and prospects. AIDS 18 (Suppl 1) S3–10. [PubMed] [Google Scholar]
- 19. Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, et al. (2004) Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS 18: 2029–2038. [DOI] [PubMed] [Google Scholar]
- 20. Perez JL, Moore RD (2003) Greater effect of highly active antiretroviral therapy on survival in people aged > or = 50 years compared with younger people in an urban observational cohort. Clin Infect Dis 36: 212–218. [DOI] [PubMed] [Google Scholar]
- 21. Tuboi SH, Pacheco AG, Harrison LH, Stone RA, May M, et al. (2010) Mortality associated with discordant responses to antiretroviral therapy in resource-constrained settings. J Acquir Immune Defic Syndr 53: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kautz T, Bendavid E, Bhattacharya J, Miller G (2010) AIDS and declining support for dependent elderly people in Africa: retrospective analysis using demographic and health surveys. BMJ 340: c2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muga GO, Onyango-Ouma W (2009) Changing Household Composition and Food Security Among the Elderly Caretakers in Rural Western Kenya. J Cross Cult Gerontol 24: 259–272. [DOI] [PubMed] [Google Scholar]
- 24.Negin J, Cumming RG (2010) HIV Infection in Older Adults in sub-Saharan Africa: Extrapolating Prevalence from Existing Data. Bulleting of the World Health Organization. [DOI] [PMC free article] [PubMed]
- 25. Greig J, Casas EC, O'Brien DP, Mills EJ, Ford N (2012) Association between older age and adverse outcomes on antiretroviral therapy: a cohort analysis of programme data from nine countries. AIDS 26 (Suppl 1) S31–37. [DOI] [PubMed] [Google Scholar]
- 26. Negin J, van Lettow M, Semba M, Martiniuk A, Chan A, et al. (2011) Anti-retroviral treatment outcomes among older adults in Zomba district, Malawi. PLoS One 6: e26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parikh SM, Obuku EA, Walker SA, Semeere AS, Auerbach BJ, et al. (2013) Clinical differences between younger and older adults with HIV/AIDS starting antiretroviral therapy in Uganda and Zimbabwe: a secondary analysis of the DART trial. PLoS One 8: e76158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorrington R, Johnson L, Bradshaw D, Daniel T (2006) The Demographic Impact of HIV/AIDS in South Africa. National and Provincial Indicators for 2006. Cape Town, South Africa: Center for Actuarial Research, South African Medical Research Council and Actuarial Society of South Africa. [Google Scholar]
- 29. Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, et al. (2008) Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 17: 47–55. [DOI] [PubMed] [Google Scholar]
- 30. Mayhew S (1996) Integrating MCH/FP and STD/HIV services: current debates and future directions. Health Policy Plan 11: 339–353. [DOI] [PubMed] [Google Scholar]
- 31. Remien RH, Berkman A, Myer L, Bastos FI, Kagee A, et al. (2008) Integrating HIV care and HIV prevention: legal, policy and programmatic recommendations. AIDS 22 (Suppl 2) S57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manrique L, Aziz M, Adeyemi OM (2010) Successful immunologic and virologic outcomes in elderly HIV-infected patients. J Acquir Immune Defic Syndr 54: 332–333. [DOI] [PubMed] [Google Scholar]
- 34. Nogueras M, Navarro G, Anton E, Sala M, Cervantes M, et al. (2006) Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis 6: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dalal RP, Macphail C, Mqhayi M, Wing J, Feldman C, et al. (2008) Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in johannesburg, South Africa. J Acquir Immune Defic Syndr 47: 101–107. [DOI] [PubMed] [Google Scholar]
- 36. Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, et al. (2007) True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ 85: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete results of regression analysis: change in CD4+ cell count after ART initiation.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.