This study used a multiplex high-throughput gene expression assay that simultaneously detects endogenous expression of multiple developmental, functional, and disease markers in induced pluripotent stem (iPS) cell-derived retinal pigment epithelium (RPE). This assay provides the basis to screen for compounds that improve RPE function and maturation and target disease pathways, thus providing the basis for effective treatments of several retinal degenerative diseases.
Keywords: Gene expression, Reprogramming, Retina, Stem cell, Induced pluripotency, iPS, Retinal pigmented epithelium
Abstract
There is continuing interest in the development of lineage-specific cells from induced pluripotent stem (iPS) cells for use in cell therapies and drug discovery. Although in most cases differentiated cells show features of the desired lineage, they retain fetal gene expression and do not fully mature into “adult-like” cells. Such cells may not serve as an effective therapy because, once implanted, immature cells pose the risk of uncontrolled growth. Therefore, there is a need to optimize lineage-specific stem cell differentiation protocols to produce cells that no longer express fetal genes and have attained “adult-like” phenotypes. Toward that goal, it is critical to develop assays that simultaneously measure cell function and disease markers in high-throughput format. Here, we use a multiplex high-throughput gene expression assay that simultaneously detects endogenous expression of multiple developmental, functional, and disease markers in iPS cell-derived retinal pigment epithelium (RPE). We optimized protocols to differentiate iPS cell-derived RPE that was then grown in 96- and 384-well plates. As a proof of principle, we demonstrate differential expression of eight genes in iPS cells, iPS cell-derived RPE at two different differentiation stages, and primary human RPE using this multiplex assay. The data obtained from the multiplex gene expression assay are significantly correlated with standard quantitative reverse transcription-polymerase chain reaction-based measurements, confirming the ability of this high-throughput assay to measure relevant gene expression changes. This assay provides the basis to screen for compounds that improve RPE function and maturation and target disease pathways, thus providing the basis for effective treatments of several retinal degenerative diseases.
Introduction
The recent use of induced pluripotent stem (iPS) cells to generate specific cell lineages has provided the opportunity to develop personalized therapies using two parallel approaches: (a) autologous cell transplantation to replace damaged tissue and (b) identification of new drugs using patient-specific “disease-in-a-dish” models [1, 2]. Several of the current in vitro lineage differentiation protocols rely on the knowledge of vertebrate embryonic development [3]. Differentiation of functional tissues during normal embryonic development is a step-wise process driven by precise gene expression changes produced by tightly controlled signaling pathways [4, 5]. Recapitulation of these developmental events in vitro requires distinct culture conditions including specific growth factors/cytokines and extracellular matrix molecules for each step of the differentiation process [3, 6–9]. However, the in vivo biological complexity of the differentiation processes has been difficult to duplicate in vitro. Differentiation of stem cells in vitro is still a low-efficiency process that produces heterogeneous cell populations with mixed lineages. Furthermore, for most lineages, gene expression and functional assays indicate that differentiated cells do not fully mature and continue to maintain a “fetal-like” phenotype [1, 10–12]. In cases in which mature phenotype can be achieved, the protocol extends over several weeks, making it expensive for clinical-grade and large-scale manufacturing. The concern remains that incompletely matured cells might be tumorigenic when implanted in vivo, thus obviating their use in cell-based therapies. Therefore, the goal is to establish differentiation protocols that are efficient in producing highly homogeneous pure populations of fully differentiated and mature cells. Establishing assays and screens that simultaneously monitor the developmental and functional state of a cell can help achieve this goal. In cases in which changes in endogenous expression of functional or developmental markers also represent a diseased state of cells, these assays will allow the possibility of identifying potential drugs using small molecule screens. For instance, in the case of recessive diseases, it is thought that artificially increasing the expression of a hypomorphic gene would provide benefit for the patient.
Major advances have been made in protocols to isolate, culture, and differentiate stem cells into retinal pigment epithelium (RPE) [1]. RPE can be easily obtained from human embryonic stem (ES) or iPS cells, but the differentiation efficiency from various iPS cell lines is often variable and low [1, 13]. Differentiated ES or iPS cell-derived RPEs do not express high levels of functional markers such as RPE65, RDH5, BEST1, and TYR and continue to express fetal-RPE genes such as SOX2 and PAX6 [14]. High-throughput assays that simultaneously measure the expression of these markers should provide a rather complete picture of the ES or iPS cell-derived RPE differentiation state. Because mutations in all these genes are associated with congenital or other eye malformations, these assays will also help identify potential therapeutic drugs for several potentially blinding eye diseases.
Here we describe protocols to produce and use fully authenticated iPS cell-derived RPE for a multiplex high-throughput gene expression assay. This multiplex gene expression assay reports on six RPE lineage genes, two stem/progenitor cell genes, and two housekeeping genes. It is based on the Panomics/Affymetrix technology coupled with Luminex fluorescent beads. We show proof of principle data that (a) the assay can be performed in 96-well and 384-well high-throughput modes, (b) the assay is able to measure subtle change in gene expression, and (c) the data obtained with the multiplex assay is highly correlated with quantitative reverse transcription (qRT)-polymerase chain reaction (PCR) data. This assay allows the possibility of identifying small molecules that can further enhance the efficiency of our current differentiation protocols toward fully mature RPE cells. In addition, it provides developmental and functional biomarkers that can be measured in a high-throughput mode. Small molecules that modulate the expression of these functional and disease biomarkers can provide potential therapeutic drugs for RPE-associated retinal degenerative diseases.
Materials and Methods
iPS Cell Derivation and Characterization
Human adult dermal fibroblasts (AG9309, female, 21 years old, toe biopsy) purchased from Coriell Institute for Medical Research (Camden, NJ, http://www.coriell.org) were reprogrammed as described previously [15].
iPS cell colonies were characterized by immunostaining for pluripotency markers (see below). qRT-PCR was used to detect silencing of transgenes and expression of endogenous genes from reprogrammed iPS cells using published primers [16]. For characterization, iPS cells were differentiated in vitro into the three germ layers using a previously published protocol [16]. To further demonstrate their pluripotency, undifferentiated and differentiated iPS cells were analyzed using the TaqMan hPSC Scorecard Panel (A15870; Life Technologies, Rockville, MD, http://www.lifetech.com) according to the manufacturer’s manual and published literature [17]. This TaqMan-based gene expression assay includes a panel of 93 genes, including 8 control/housekeeping genes, 9 self-renewal/pluripotency genes, 26 endoderm-specific genes, 22 mesoderm-specific genes, 22 ectoderm-specific genes, and 6 mesendoderm-specific genes. Pluripotency of an iPS cell line as well as its trilineage differentiation potential is determined by comparing Ct values obtained for each marker to the values obtained in reference standards (represented by nine undifferentiated pluripotent stem cell lines, including human embryonic stem cells and iPS cells). A proprietary algorithm (Life Technologies) calculates the relative score for iPS cells based on how well the expression of each gene correlates with reference lines. Scores closer to 0 indicate comparable expression between the iPS cell line tested and the reference controls; scores >1 indicate upregulation; scores <1 indicate downregulation. Karyotypic analysis of iPS cells was performed with Cytogenetics Laboratory (Albany, NY, http://www.opwdd.ny.gov/institute-for-basic-research/research/departments/human-genetics/cecl).
iPS Cell to RPE Differentiation and Primary RPE Cultures
Prior to differentiation, iPS cells were dissociated using CTK solution [16] and passaged 1:2 onto Matrigel-coated plates (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) in TeSR medium (Stem Cell Technologies, Vancouver, Canada, http://www.stemcell.com) in the presence of ROCK inhibitor (Stemgent, San Diego, CA, https://http://www.stemgent.com). To drive neural induction of iPS cells, 70%–80% confluent stage cells were transferred to knockout serum replacement (KSR) medium supplemented with 500 ng/ml Noggin (R&D System) and 10 μM SB431542 (Tocris, Bristol, U.K., http://www.tocris.com) every day, for 3 days [18]. At day 5, to specify RPE differentiation, 1 mM nicotinamide (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and 150 ng/ml ACTIVIN A (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) were added to KSR medium [19, 20]. Colonies with characteristic “cobblestone” RPE morphology and pigmentation appeared at an early stage (days 25–35). RPE colonies were manually picked and trypsinized, and 125 × 103 cells were plated per well of a 24-well Primaria plate (BD Biosciences) in RPE-taurine, hydrocortisone, triiodothyronine (THT) medium [21]. Once confluent, cells were trypsinized again, and 2 × 106 cells were plated per T25 Primaria flask (BD Biosciences) in RPE-THT medium [21] and grown to form a highly enriched RPE monolayer. For the experiments described here, iPS cell-derived RPE were then trypsinized and replated in 96-well or 384-well plates in RPE-THT medium [21] according to the QuantiGene Plex assay protocol described below.
Primary RPE cells were isolated from 16–18-week-old human fetal eyes as described previously [21] and cultured in RPE-THT medium [21]. Primary RPE cells were passaged twice before being used for the multiplex assay.
Immunostaining, Flow Cytometry, Electron Microscopy, and qRT-PCR Analysis
Immunostaining and qRT-PCR analysis was performed as described previously [15, 22]. Primary RPE and iPS cell-derived RPE cultured on semipermeable cell culture inserts (Transwells; Corning, Corning, NY, http://www.corning.com) were used for gene expression studies. Validated primer sets for qRT-PCR of human RPE-specific genes were purchased from SABioscience/Qiagen (Valencia, CA, http://www.sabiosciences.com; catalog no. CAPH10651A). Fold change in gene expression was calculated using the ΔΔCt method. Two genes, B2M and HPRT1, were used to normalize the data and calculate ΔCt values. Normalized ΔCt values of iPS cell-derived RPE were used to calculate ΔΔCt values for iPS cells and primary RPE samples. −2^ln of ΔΔCt values was used to calculate fold change. For flow cytometry analysis, iPS cells were dissociated with Accutase (Life Technologies) for 5 min at room temperature. Cell suspension was washed with iPS cell culture medium and incubated with human Alexa Fluor 488-anti-SSEA4 antibody (1:50, BD Biosciences, catalog no. 560308) for 30 min at room temperature. As control, we used unstained cells. Cells were analyzed on a FACS Aria II Cell Sorter (BD Biosciences) with 10,000 events acquired for each sample. The data were analyzed with FlowJo software.
The following antibodies were used: Alexa Fluor 488 anti-Tra-1-60 (1:50, BD Biosciences, catalog no. 560173); Alexa Fluor 488 anti-SSEA4 (1:50, BD Biosciences, catalog no. 560308); OCT4 (1:400, Cell Signaling, Danvers, MA, http://www.cellsignal.com, catalog no. C30A3); NANOG (1:100, R&D Systems, catalog no. AF1997); SOX2 (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com, catalog no. sc-17319); KLF4 (1:50, Santa Cruz, catalog no. sc-20691); c-MYC (1:50, Santa Cruz, catalog no. sc-764); AFP (1:75, Thermo Scientific, Waltham, MA, http://www.thermoscientific.com, catalog no. RB-365-A1); TUJ1 (1:400, Sigma-Aldrich, catalog no. T8660); aSMA (1:500, Thermo Scientific, catalog no. MS-113-P0); DCT (1:100, Bioworld Antibodies, Mt. Airy, MD, http://bioworldantibodies.com, catalog no. BS3320); PAX6 (1:200, Covance, Chantilly, VA, http://www.covance.com, catalog no. PRB-27P); EZRIN (1:200, Sigma-Aldrich, catalog no. E8897); CLCN2 (1;200, Abgent, San Diego, CA, http://www.abgent.com, catalog no. AT1550a); SLC16A1 (1:200, Abcam, Cambridge, U.K., http://www.abcam.com, catalog no. ab90582); and ZO1 (1:100, Life Technologies, catalog no. 339111).
Intracellular Calcium Measurements and Electrophysiology
To assess calcium signaling in the RPE cells, ATP (200 µM; Sigma-Aldrich) and cyclopiazonic acid (10 µM; EMD Millipore) were used to modulate intracellular calcium concentration. Calomel electrodes in series with Ringer solutions and agar bridges were used to measure the transepithelial potential (TEP). The signals from intracellular microelectrodes were referenced to the basal bath to measure the apical and basal membrane potentials, VA and VB, where TEP = VB − VA. The total transepithelial resistance (RT) and the ratio of the apical to basolateral membrane resistance (RA/RB) were obtained by passing 2–4 μA current pulses (peak to peak) across the tissue and measuring the resultant changes in TEP, VA, and VB (see [21, 23] for further details).
Principle of Quantigene Plex Technology
Cell lysates are transferred to a hybridization plate containing QuantiGene Plex probe and Luminex beads sets. Each bead type was coated with a different single-strand DNA capture probe (CP). Several other components of the QuantiGene Probe set are also comprised of single-strand DNA oligonucleotides including the capture extenders (CEs), label extenders (LEs), and blocking probes (BPs). Parts of CE oligonucleotides are complementary to the target mRNA (covering 200–600 bases), and parts are complementary to the CPs bound on Luminex beads. This interaction facilitates capture of specific target mRNAs to specific Luminex beads. LEs include target mRNA specific sequences and a binding site for the preamplifier, the first component used for signal amplification. BPs bind to any sequences on the target mRNA that are not bound by the CEs and LEs. A typical probe set for a single target mRNA consists of a family of four or more different CEs and LEs, usually covering approximately 500 bases within the target mRNA. After washing off excess probes and remaining cell lysate, the signal amplification reagents, consisting of the preamplifier (PreAmp), amplifiers (Amp), and label probes (LPs), are sequentially hybridized to the mRNAs. The LP also include a biotin molecule, which in turn is a binding site for the final signal amplification reagent, streptavidin-conjugated R-phycoerythrin (SAPE). Each amplifier binds up to 400 SAPE. The resulting fluorescence signal associated with individual capture beads is read on a Luminex instrument. Signal is reported as median fluorescence intensity (MFI) and is proportional to the number of target RNA molecules present in the sample. MFI is calculated by measuring signals on 50–100 beads/gene. MFI values obtained from background blank wells with no target RNA were subtracted from MFI of each target reading.
QuantiGenePlex 2.0 Reagent System: 96-Well Assay Protocol
The QuantiGene Plex 96-well Assay was performed according to the manufacturer’s manual (Quantigene Plex 2.0 Assay, Affymetrix, Santa Clara, CA, http://www.affymetrix.com). Twenty-five thousand and 50,000 cells were seeded per well and cultured for 14 days at 37°C, 5% CO2. Cells were lysed in 200 μl of working lysis mixture for 30 minutes at 50°C. Eighty microliters of cell lysate were transferred to the assay’s hybridization plate (96-well clear polypropylene plate Abgene, Pittsburg, PA, http://www.thermoscientificbio.com/abgene/, catalog no. AB0796), where each well already contained 20 μl of working bead mix (6.6 μl of lysis mixture, 5.2 μl of nuclease free water, 0.2 μl of proteinase K solution, 2 μl of blocking reagent, 5 μl of probe set, 1 μl of magnetic Luminex beads; QuantiGene Plex Set Panel no. 11828). Hybridization was done for 18–22 hours at 54°C ± 1°C, with shaking at 600 rpm. The hybridization mixtures were then transferred to a 96-well magnetic separation plate (96-well flat-bottom microplate Nunc catalog no. 269620). An Affymetrix handheld magnetic bead washer (Affymetrix P/N QP0702) was used to wash the beads, thus removing all unbound materials. 100 μl of 2.0 preamplifier working reagent (3:1,000 dilution using PreAmp solution plus Amp diluent provided by manufacturer) was added to each assay well. The magnetic separation plate was sealed with adhesive backed foil and incubated for 1 hour at 50°C ± 1°C and 600 rpm. The unbound 2.0 preamplifier was removed, and beads were washed three times with 100 μl of wash buffer (provided by manufacturer) using the handheld magnetic washer. This was followed by incubation with 2.0 Amplifier working reagent, followed by label probe working reagent, and finally followed by SAPE working reagent (all three solutions were 100 μl at 3:1,000 dilution, manufacturer provided). Incubation and washing were done as described above. Signals from the beads were measured with a Luminex FlexMap three-dimensional instrument (Luminex Corp., Austin, TX, http://www.luminexcorp.com), after resuspending the beads in 130 μl of SAPE wash buffer, using dd gate settings of 5,000–25,000. Per target, 50–100 beads were measured in a sample volume of 100 μl.
QuantiGene Plex 384-Well Assay Protocol
The QuantiGene Plex 384-well Assay was optimized using technical support from Affymetrix and using the 96-well assay protocol provided by the manufacturer. Then 6,000 and 12,000 cells were seeded per well of a 384-well plate. Proportional volumes of reagents/well were used for the 384-well plate. Signal was measured as described above.
Results
Generation of iPS Cell-Derived RPE
In this study, we describe a high content gene expression screening assay that simultaneously monitors developmental and functional features of stem cell-derived RPE. Figure 1A provides an overview of the experimental strategy for obtaining pure cultures of iPS cell-derived RPE, as well as the steps required to prepare cells for functional authentication and use in a high-throughput screen. iPS cells were generated from human adult female dermal fibroblasts, using retroviral vectors expressing OCT3/4, c-MYC, SOX2, and KLF4 [15]. Pluripotency of selected iPS cell colonies was determined using the following four pluripotency validation assays: (a) Positive immunostaining for pluripotency markers NANOG, SSEA4, TRA1-60, OCT3/4, c-MYC, SOX2, and KLF4 (Fig. 1B; supplemental online Fig. 1A). (b) Comparison of mRNA expression of NANOG, OCT3/4, c-MYC, SOX2, and KLF4 to an embryonic stem cell line and to dermal fibroblasts. The transduced viral vector also expressed OCT3/4, c-MYC, SOX2, and KLF4; therefore specific primer sets were used to distinguish expression from the endogenous locus to the total expression (viral vector + endogenous) (Fig. 1C). Expression of pluripotency markers from endogenous loci in iPS cells was significantly higher compared with dermal fibroblasts and comparatively higher or equal to undifferentiated ES cells (Fig. 1C). (c) Ability of iPS cells to differentiate into all three germ layers. As shown in Figure 1D, this iPS cell line differentiates into all three germ layers. (d) Confirmation of the expression of 93 genes, including markers for pluripotency and differentiation potential (ectoderm, endoderm, mesoderm, and mesendoderm derivatives), using a commercially available quantitative TaqMan-based gene expression assay (TaqMan hPSC Scorecard), based on the work of Bock et al. [17]. The iPS cell line was compared with a list of nine reference standard cell lines, including six human embryonic stem cell lines and three iPS cell lines, and was found to be pluripotent, with higher propensity to differentiate into ectoderm and mesoderm lineages and less propensity to differentiate toward endoderm (supplemental online Fig. 1B). Karyotypic analysis shows that the iPS cell line contains the correct number and normal G-banding of all chromosomes, suggesting that no major structural abnormalities occurred during the reprograming process (supplemental online Fig. 1C).
Figure 1.
Generation and characterization of induced pluripotent stem (iPS) cells for use in a multiplex screening assay. (A): Schematic of the step-wise protocol for differentiation of iPS cells into retinal pigment epithelium (RPE), authentication of RPE cells, and their use for a multiplex screening. The different types of media used throughout the process and the timeline of the process are shown. (B): Characterization of the iPS cell line used for generating RPE for screening. Fully confluent iPS cells were stained with antibodies against indicated pluripotency markers. Cells express high levels of all these markers. Bright-field images show iPS cell colony morphology. Scale bar = 100 μm. (C): qRT-PCR analysis of pluripotency markers NANOG, OCT4, SOX2, c-MYC, and KLF4 in iPS cell line used for RPE differentiation. Fibroblasts and embryonic stem cell RNA are used for comparison. (D): Immunostaining of iPS cells spontaneously differentiated in vitro after basic fibroblast growth factor withdrawal. iPS cells are able to generate cells from the three germ layers (AFP = endoderm; TUJ1 = ectoderm; SMA = mesoderm). Scale bars = 50 μm. Abbreviations: EB, embryoid bodies; endo, endoderm; ESC, embryonic stem cell; Fibr, fibroblast; hESC, human embryonic stem cell; iPSC, induced pluripotent stem cell; KSR, knockout serum replacement-containing medium; MEF, mouse embryonic fibroblast; NIC, nicotinamide.
iPS cells were differentiated into RPE using a protocol combining the dual SMAD inhibition that induces neuroectoderm at a very high efficiency [18] and recombinant ACTIVIN A and nicotinamide treatment that induces RPE fate from the neuroectoderm lineage [19, 20]. Pigmented cell clusters with RPE-like cobblestone morphology were manually picked and expanded in a T25 flask to obtain pure cultures of RPE cells. Purified RPE cells were reseeded onto Transwell filters to obtain polarized confluent electrically stable (TEP/RT) RPE monolayers in approximately 6–8 weeks. Immunostaining performed at this stage for epithelial marker ZO1 confirmed the typical epithelial morphology of the cells (Fig. 2A; supplemental online Fig. 2; data not shown). iPS cell-derived RPE also expressed EZRIN, a protein localized in the apical processes; DCT, an enzyme important for RPE pigmentation; CLCN2, a chloride channel critical for volume regulation of the RPE; and SLC16A1, an apical membrane monocarboxylate transporter required for lactate transport across the RPE monolayer and involved in intracellular pH regulation. Analysis of transmission electron microscopy images revealed several typical RPE features in these cells such as cigar- and oval-shaped melanosomes localized predominantly apically, tight junctions between adjoining cells, and apical processes (Fig. 2B) [21].
Figure 2.
Authentication of induced pluripotent stem (iPS) cell-derived RPE. (A–D): RPE cells were grown on semipermeable Transwells until fully confluent and characterized by immunostaining for RPE markers (A), electron microscopy (B), intracellular calcium responses (C), and electrophysiological responses (D). (A): Cells were stained for RPE markers EZRIN, DCT, SLC16A1, CLCN2 (red), and ZO1 (green). Scale bar = 20 μm. (B): Electron micrograph of a section of RPE growing on a semipermeable Transwell. Cells show several features typical of RPE including extensive apical process, apically localized pigmented melanosomes, and tight junctions between adjoining cells. Scale bar = 2 μm. (C): Similar to primary fetal RPE cells, iPS cell-derived RPEs show a baseline calcium concentration of 110 nM. Addition of ATP to the apical bath activates apical P2Y2 receptors, resulting in a spike in intracellular calcium concentration. (D): Apical and basolateral membrane resting potentials were measured in response to changing potassium and ATP concentrations in the apical bath. Similar to the primary cultures of human RPE, these cells hyperpolarize when apical potassium concentration is reduced from 5 to 1 mM, and they depolarize following the addition of ATP to the apical bath. Abbreviations: ap, apical; CPA, cyclopiazonic acid; iPSC, induced pluripotent stem cell; me, melanosome; RPE, retinal pigment epithelium; TEP, transepithelial potential; Tj, tight junction.
Many characteristic physiological responses of human native and cultured RPE cells can be measured in vitro [21, 23]. iPS cell-derived RPE monolayer cultures display several of these features. These cells are electrically intact displaying a transepithelial resistance of 170–200 Ω⋅cm2 (Fig. 2C, 2D) and a steady-state intracellular calcium concentration of ≈110 nM. Similar to native RPE, iPS cell-derived RPE stimulation of apical membrane P2Y2 receptors by ATP leads to a typical biphasic response. The initial response signals the release of intracellular calcium from the endoplasmic reticulum into the cytoplasm, and the second phase is dependent on the presence of extracellular Ca2+ [21]. Application of cyclopiazonic acid blocks the sarco/endoplasmic reticulum Ca2+ ATPase, an ATP-dependent calcium reuptake mechanism located on the ER membrane, which increases cytoplasmic steady-state calcium and blunts the ATP response (Fig. 2C). The resting membrane potentials of apical and basal membranes are approximately −55 mV. However, because the basolateral membrane is more depolarized than the apical membrane, this causes a TEP across the monolayer of ≈2.5 mV, apical side positive (Fig. 2D). A reduction of the apical bath [K+]ο from 5 to 1 mM mimics the in vivo alterations in subretinal space [K+]ο following a dark-to-light transition and significantly hyperpolarizes the apical membrane by 10–20 mV, causing a sharp decrease in TEP [21, 23]. Application of ATP to the apical membrane significantly depolarizes the basolateral membrane following the release of calcium from intracellular stores and activation of basolateral membrane Ca2+-activated Cl− channels [21, 23, 24]. These findings confirm that iPS cell-derived RPE duplicate key functions of native human RPE.
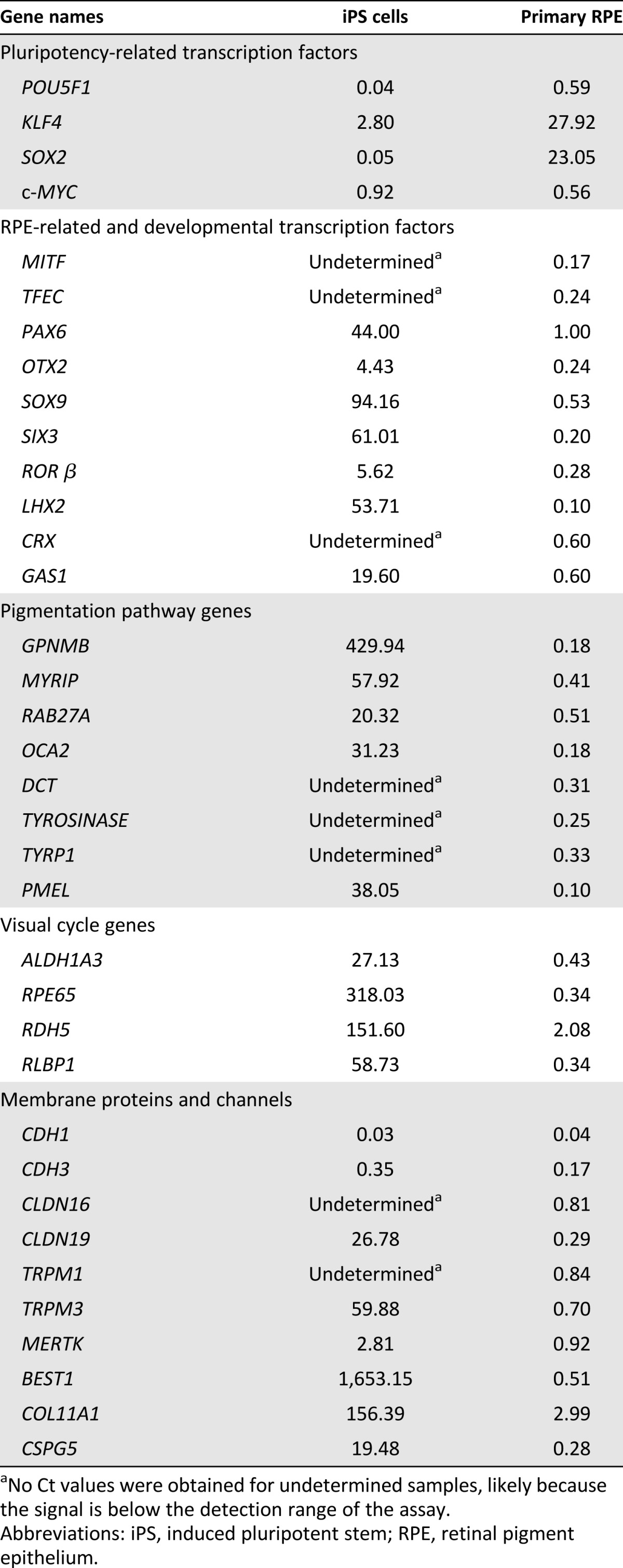
We next compared the expression of pluripotency and RPE signature genes in undifferentiated iPS cells, iPS cell-derived RPE, and primary human fetal RPE using qRT-PCR [14, 25]. Two major pluripotency genes, OCT3/4 (also called POU5F1) and SOX2, are downregulated in iPS cell-derived RPE as compared with undifferentiated iPS cells, suggesting a loss of the pluripotent state. However, several other RPE-specific transcription factors, pigmentation pathways genes, visual cycle genes, structural proteins, and channels were expressed at significantly higher levels in iPS cell-derived RPE as compared with undifferentiated iPS cells and at a lower or at similar levels compared with the primary RPE (Table 1). The data in Table 1 show that the expression of the following genes is similar between iPS cell-derived RPE and primary RPE: transcription factors POU5F1, c-MYC, PAX6, SOX9, CRX, and GAS1; pigmentation gene RAB27A; visual cycle gene RDH5; and membrane-associated proteins CLDN16, TRPM1, TRPM3, MERTK, and BEST1. Other genes continue to be dysregulated in iPS cell-derived RPE as compared with primary RPE including: transcription factors KLF4, SOX2, MITF, TFEC, OTX2, SIX3, ROR β, and LHX2; pigmentation genes GPNMB, MYRIP, OCA2, DCT, TYROSINASE, TYRP1, and PMEL; visual cycle genes ALDH1A3, RPE65, and RLBP1; and membrane-associated proteins CDH1, CDH3, CLDN19, COL11A1, and CSPG5. These results indicate that although iPS cell-derived RPE cells have attained an RPE-like phenotype, they are still not in a mature state. It is noteworthy that despite the fact that iPS cell-derived RPEs express higher levels of fetal or progenitor genes (SOX2 and KLF4; see Table 1), the overall physiological responses of these cells are not significantly compromised. However, the transepithelial resistance and physiological responses of iPS cell-derived RPE appear to be somewhat smaller compared with primary human RPE [21].
Table 1.
Fold difference of gene expression between iPS cell-derived RPE, iPS cells, and primary human RPE derived-RPE
A Proof-of-Principle High-Throughput Scalable Multiplex Gene Expression Assay Using Authenticated iPS Cell-Derived RPE
Our goals were to develop a high-throughput screening assay that can simultaneously monitor developmental, functional, and disease markers for RPE and demonstrate that the assay is able to detect gene expression changes at two different differentiation stages of iPS cell-derived RPE. In designing the screening assay platform, we chose a multiplex gene expression assay for two main reasons: (a) it is a high-content assay that allows the simultaneous detection of genes involved in RPE development, differentiation, function, and pathology, and (b) it is readily amenable for a high-throughput screening using standard screening instrumentation. We selected genes that measured different RPE cellular processes: RPE development and differentiation (SOX2 and PAX6), RPE function (TYROSINASE, RPE65, RDH5, TRPM1, CSPG5, and BEST1), and RPE pathology (TYROSINASE, RPE65, RDH5, and BEST1). Figure 3A summarizes the outline of the multiplex assay, Figure 3B schematically illustrates the assay principle, and Figure 3C provides the list of genes used in this multiplex assay, their accession numbers, respective length of their mRNA, and the regions to which capture extenders and label extenders were hybridized. Assay protocol is described in detail in the Materials and Methods section. In brief, mRNA isolated in cell lysate prepared in microtiter plates is bound to Luminex beads using the antisense oligonucleotide technology. Detection probe (biotin-streptavidin-phycoerythrin) is hybridized to specific mRNA also using the antisense oligonucleotide technology. mRNA quantification is performed using flow cytometry-based equipment that recognizes the specific fluorescent label on each bead and measures signal intensity per bead.
Figure 3.
Schematic of the multiplex assay performed in a 96/384-well plate format. (A): Brief summary of the protocol assay and timeline for the multiplex assay performed using induced pluripotent stem (iPS) cell-derived retinal pigment epithelium (RPE). Assay can be performed in a day and a half with full automation. (B): Magnetic beads labeled with unique fluorophores capture specific mRNAs through antisense oligonucleotide interactions of capture probe (CP) and multiple capture extenders (CEs). CE is a branched oligonucleotide; one side of it is antisense to CP, and the other side has variable sequence that is antisense to the specific mRNA. Label extender is also a branched oligonucleotide that binds to different regions of the mRNA and allows the binding of preamplifier and amplifier oligonucleotides through antisense interactions. Biotin-containing label probes and streptavidin-conjugated phycoerythrin bind to amplifier oligonucleotides and are detected using the Luminex flow reader. (C): Table summarizes mRNA detected in this assay, their accession numbers, length, and the region where capture extenders and label extenders bind. Abbreviation: PE, phycoerthyrin.
As a proof of principle, we demonstrated the feasibility of the multiplex gene expression assay to simultaneously detect differential levels of multiple genes in a 96- or 384-well plate between undifferentiated iPS cells, iPS cell-derived RPEs, and primary human fetal RPEs. A total of nine genes were selected for the optimization. HPRT1 and B2M, two housekeeping genes, were used for data normalization because previous work suggested that the expression of these two genes does not differ significantly among the three cell types used in this assay [14, 25]. To analyze RPE differentiation, functioning, and pathology, we monitored the expression of SOX2, PAX6, RPE65, RDH5, CSPG5, TRPM1, and BEST1. For in vitro culture, cells seeded at higher density mature faster compared with cells seeded at lower density. In the present experiments, we chose to induce faster maturation by seeding cells at higher density rather than culturing them for a longer period of time. To analyze changes in RPE gene expression with cell maturity, both iPS cell-derived RPEs and primary fetal RPEs were seeded at two cell densities, 25,000 or 50,000 cells per well (Fig. 4B–4D, lower density for primary RPE not shown). Undifferentiated iPS cells were used as an additional control (Fig. 4A). iPS cell-derived RPE and primary RPE were cultured for 2 weeks to generate confluent pigmented monolayers. To perform the assay at the same time for all cells types, iPS cells were cultured later and reached confluence in only 5 days. A linear range of detection was calculated for each probe set using purified RNA and lysates from undifferentiated iPS cells, iPS cell-derived RPE (low and high cell density), and primary RPE (low and high cell density) (supplemental online Fig. 3). Signal above the background over a 16-fold sequential dilution was detected for all the probes. The linear regression plot generated from this serial dilution generated a coefficient of correlation value of more than .97 in all cases, indicating that the probes were able to detect a signal over a large range of mRNA concentrations.
Figure 4.
Proof of principle for multiplex gene expression assay in 96-well plates. (A–D): Bright-field images of iPS and RPE cells growing in a 96-well plate. (A): iPS cells. (B, C): iPS cell-derived RPE seeded at two different cell densities (25,000 and 50,000 cells per well). (D): Primary human fetal RPE (50,000 cells per well). Scale bar = 100 μm. (E–H): Results obtained in the multiplex assay. (E, G): Expression of indicated genes was normalized to geomean of HPRT1 and B2M genes. Results are shown as fold change in gene expression in iPS cell-derived RPE seeded at lower cell density (blue bars) and seeded at higher cell density (red bars) normalized either to undifferentiated iPS cells (E) or to the primary fetal RPE (G). (F, H): Pearson’s correlation analysis between gene expression results obtained from the qRT-PCR assay and the results obtained from the multiplex gene expression assay performed in 96-well plates. The correlation is significant for iPS cells (r = .687) and highly significant for primary RPE cells (r = .751). (F): TRPM1 is not included in Pearson’s correlation because its expression was undetectable by qRT-PCR in iPS cells. Abbreviations: iPS, induced pluripotent stem; iPSC, induced pluripotent stem cell; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; RPE, retinal pigment epithelium.
The results obtained from this assay are presented as fold change in gene expression in iPS cell-derived RPE at two different cell densities as compared with undifferentiated iPS cells (Fig. 4E, 4F) and as compared with primary fetal RPE at high cell density (Fig. 4G, 4H). As expected, compared with undifferentiated iPS cells, iPS cell-derived RPE expresses lower levels of neural progenitor factor SOX2 and much higher levels of RPE-specific genes PAX6, RPE65, RDH5, TRPM1, and BEST1. CSPG5, an extracellular protein, is expressed at similar levels in the two cell types. An important goal of this optimization was to compare results obtained from the multiplex assay with those obtained from a standard qRT-PCR assay (Table 1). Therefore, we determined the Pearson’s correlation coefficient (r) between fold change in gene expression obtained from the multiplex assay and from the qRT-PCR assay. Our results show that the two assays are significantly correlated (r value = .69 and .75). This provides confidence that the multiplex assay measures relevant gene expression changes and can be used for a high-throughput screen. In conclusion, we show here that this high-content gene expression assay can be performed in 1.5 days in a high-throughput fashion on undifferentiated iPS cells, iPS cell-derived RPE at two different differentiation stages, and primary human RPE. When compared with primary fetal RPEs, iPS cell-derived RPEs express lower levels of differentiation RPE markers (RPE65, RDH5, CSPG5, and BEST1) and higher levels of progenitor transcription factors PAX6 and SOX2, suggesting a relatively immature state of these cells. It is important to note that the results obtained by comparison of primary RPE and iPS cell-derived RPE in the multiplex assay are strongly correlated with the standard qRT-PCR assay presented in Table 1.
Evaluating the Limit of Detection and Increasing the Throughput of the Assay
We next tested the limit of detection for each transcript in a given assay well, another important aspect of the multiplex assay. The vendor reported that the lowest limit of detection for the multiplex gene expression assay is less than or equal to 200 transcripts when analyzing one target per well of a 96-well plate. The following formula was used to calculate the limit of detection (LOD) of our probe set: LOD = average background value of four wells + 3× standard deviation of the average background value (Affymetrix, manufacturer’s manual). Our assay is significantly more sensitive than the described limit, and the following values of detection were obtained: SOX2 = 41, PAX6 = 32, RPE65 = 24, RDH5 = 10, CSPG5 = 19, TRPM1 = 25, BEST1 = 11, HPRT1 = 55, and B2M = 30. These transcript numbers encouraged us to test the assay in a 384-well format to increase screening throughput and reduce reagent and screening costs.
When optimizing the assay for a 384-well format, we also tested assay versatility by including a new probe for the gene TYROSINASE. To test the linearity of the range of signal detection, we performed the assay using two cell densities: 6,000 and 12,000 cells per well in a 384-well plate. As expected, after 2 weeks of culture in 384-well plates, the culture with lower cell density produced a less confluent and less pigmented cell layer both for the primary fetal RPE cells and the iPS cell-derived RPE (Fig. 5A–5D). This resulted in RPE cells with two different stages of differentiation. We also tested two different bead concentrations: 700 beads per well per probe, as recommended for a 96-well plate, and 375 beads per well per probe. Figure 5 shows that the assay was successfully optimized in 384-well plate using both bead concentrations. These data support the following five conclusions: (a) results obtained in 384-well plates are similar to those obtained in 96-well plates; for example, when compared with primary RPEs, iPS cell-derived RPEs express higher levels of SOX2 and PAX6 and lower levels of RPE differentiation/functioning genes; (b) the assay can detect subtle changes in gene expression at different stages of RPE differentiation; (c) the two bead concentrations give almost identical results in the 384-well plate format; (d) the assay easily incorporates new genes; and (e) the multiplex assay in 384-well plates correlates better (Pearson’s correlation coefficients of .91 and .89, respectively, for high and low beads) with the qRT-PCR data compared with the 96-well plate assay. In conclusion, the present results demonstrate that changes in expression of up to 10 different genes can be measured simultaneously from iPS cell-derived RPE in 384-well assay plates.
Figure 5.
Proof of principle for multiplex gene expression assay in 384-well plates. (A–D): Bright-field images of iPS cell-derived RPE (A, B) and human fetal RPE (C, D) seeded at two different cell densities growing in 384-well plates. Scale bar = 100 μm. (E, G): Fold change in gene expression in iPS cell-derived RPE seeded at lower cell density (blue bars) and seeded at higher cell density (red bars) normalized to the higher cell density of primary fetal RPE. Expression of indicated genes was normalized to geomean of HPRT1 and B2M housekeeping genes. Almost identical results were obtained with high bead number (E) and low bead number (G). (F, H): Pearson’s correlation analysis shows a very high correlation between results obtained from the qRT-PCR assay and the results obtained from 384-well multiplex gene expression assay. The coefficient values are r = .908 and r = .891, respectively, for different numbers of beads. Abbreviations: iPS, induced pluripotent stem; iPSC, induced pluripotent stem cell; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; RPE, retinal pigment epithelium.
Discussion
The goal of this study was to develop and demonstrate a proof-of-principle high-content multiplex gene expression assay that simultaneously monitors developmental, functional, and disease biomarkers in iPS cell-derived RPE. The assay design is based on our ability to systematically generate authentic and pure RPE cells in large numbers and to consistently and simultaneously measure multiple RPE features in a high-throughput fashion. Here we report the development of a platform and show proof-of-principle data that meet both of these criteria. This assay is also able to distinguish differential expression for selected genes in iPS cell-derived RPE at two maturation stages, producing data that correlate well with qRT-PCR studies. In addition, we describe a method to generate sufficient quantities of pure RPE cells that are molecularly and functionally authenticated. These cells were successfully used to measure several RPE features in a screening assay.
Results obtained in a high-throughput screen critically depend on the purity and differentiated phenotype of cells used, which in turn fundamentally depend on the design of the differentiation protocol. In this study, we have used a combination of two previously published, developmentally guided protocols. Chambers et al. [18] showed that the combined use of two SMAD signaling inhibitors (NOGGIN and SB431542) induce robust neuroectoderm phenotype in iPS cells within 5 days. Idelson et al. [20] used a TGF-β pathway activator ACTIVIN A to induce ES cell-derived neuroectoderm cells into RPE fate. We adapted a combined protocol with sequential addition of NOGGIN plus SB431542 and ACTIVIN A on human iPS cells to strongly induce an RPE phenotype in these cells.
The output of a small molecule screen critically depends on the phenotype of the cells used in the assay. Using functionally authenticated cells increases the likelihood of identifying small molecules with physiologically relevant effects on RPE cells. Authentication of iPS cell-derived RPE is determined using a combination of molecular, morphological, and functional assays including gene expression, electron microscopy, and physiological analysis [21]. The two functional assays used in this analysis focus on electrical properties of the intact RPE monolayer and on the polarization of the apical and basolateral cell membranes. These RPE properties depend on fully developed junctional complexes between neighboring cells and the presence of functional signaling cascades within the cells. The tight junctions help ensure differential localization of different functional channels on the apical and the basolateral sides of the cells. These channels mediate the ability of the RPE to regulate the volume and the chemical milieu on both sides of the cell [21, 23, 24]. For example, ATP added to the apical bath binds to purinergic receptors and activates intracellular calcium stores through inositol trisphosphate-mediated cytoplasmic signaling (Fig. 2C). Blockade of the ATP response by cyclopiazonic acid helps confirm the specificity of these measurements, but also suggests that the calcium uptake via the sarco/endoplasmic reticulum calcium ATPase ATPase located on the ER surface is functional in these cells [21, 23, 24]. ATP-induced depolarization of RPE, the concomitant TEP increase, and membrane resistance changes (RT, RA/RB) taken together (Fig. 2D) provide strong evidence for the activation of basolateral membrane calcium-activated chloride channels. In vivo it has been shown that the apical membrane of human RPE is hyperpolarized following a light-induced decrease in subretinal space [24]. These responses are mimicked in vitro in human iPS cell-derived RPE as shown in Figure 2D. It is noteworthy that physiological responses of iPS cell-derived RPE are somewhat smaller than primary human RPE cultures [21]. This is likely due to continued high expression of developmental factors and reduced expression of RPE differentiation genes (Figs. 4, 5E, 5G; Table 1). Taken together, the data in Figure 2 demonstrate the structural, morphological, and functional properties of iPS cell-derived RPE and provide the basis for their authenticity and use in high-throughput screens.
Initially, the multiplex assay was performed in 96-well microtiter plates according to the manufacturer’s manual, but to increase the throughput of this assay, it was optimized for a 384-well microtiter plate. In the case of 384-well assay plate, we reduced assay buffer volumes to one-fourth and tested magnetic beads at two different concentrations: identical to the bead number used in 96-well plates and half that number. This allowed us to reduce the screen cost by one-half. In addition, in the 384-well format, we demonstrated the versatility of the assay by including a new probe for the gene TYROSINASE. To check whether the data obtained from 96- and 384-well assay plates correlated well with qRT-PCR data, we determined Pearson’s correlation coefficient between the two data sets (Figs. 4, 5F, 5H). In all cases, a significant correlation was seen between qRT-PCR data and the multiplex data, and the correlation coefficient for 384-well plates was slightly better than for 96-well assay plates. This outcome probably results from the smaller surface area of 384-well assay plates, that allows RPE cells to form confluent monolayers much more quickly and with stronger cell-cell adhesion. Importantly, there was no significant difference in the quality of data obtained in 384-well plates using half the number of magnetic beads that were used in 96-well plates. The present data support the use of 384-well plates for simultaneously measuring the expression of multiple genes in iPS cell-derived RPE.
The genes used in this study were carefully chosen as disease markers and to reflect the developmental and maturation state of RPE cells. RPE65, BEST1, TYR, and RDH5 are associated with RPE-associated autosomal retinal dystrophies [26, 27]. It is suggested that compounds that change the expression of these genes would provide potential therapeutics for patients affected with mutations in these genes. SOX2 and PAX6 are developmental transcription factors, and their haploinsufficiency is associated with congenital eye abnormalities with defects in the RPE [28]. It is possible that drugs that change their expression in the RPE are also able to rescue developmental eye defects when used in utero. Examples of genes whose change in expression can indirectly affect RPE phenotype include RPE-expressed genes TRPM1 and TRPM3, which cotranscribe microRNAs miR-211 and miR-204, respectively [29, 30]. Both miR-204 and miR-211 are critical for maintaining epithelial phenotype in RPE cells [31]. Therefore, it is expected that small molecules that increase TRPM1/miR-211 and TRPM3/miR-204 expression will help induce mature RPE phenotype in iPS cell-derived RPE.
This multiplex assay is also useful for in vitro maturation of RPE cells. Previous data showed higher expression of progenitor genes like SOX2 and PAX6 in ES or iPS cell-derived RPE and lower expression of RPE-specific differentiation genes (TYR, RPE65, RDH5, CSPG5, BEST1, and TRPM1), as compared with primary fetal/adult human RPE cells [14]. This suggests that pluripotent stem cell-derived RPE has “fetal-like” properties. We confirmed these findings using both standard qRT-PCR assay and the multiplex gene expression assay in both the 96- and 384-well formats. Therefore, compounds that change the expression of these genes may also change the maturation status of iPS cell-derived RPE in cultures.
In conclusion, the approach presented here provides a well-defined method for identifying small molecules that downregulate the expression of progenitor genes and up-regulate the expression of differentiation genes, thus allowing systematic maturation of pluripotent stem cell-derived RPE in culture. This iPS cell-derived RPE will be potentially more effective in therapeutic intervention of macular degenerative diseases and also serve as an effective disease model. This screening method also can be used to identify small molecules that alter the activity of putative disease-causing pathways and lead to the discovery of therapeutic drugs.
Conclusion
In this study, we demonstrate newly optimized protocols for a high content high-throughput screening assay that simultaneously monitors developmental, functional, and disease biomarkers in iPS cell-derived RPE. We developed highly efficient methods to differentiate, expand, and authenticate iPS cell-derived RPE in parallel to their use in high-throughput assay. Such cells are more likely to provide successful screening outcomes. We performed optimization of a multiplex gene expression assay using iPS cell-derived RPE cultured in 96- and 384-well microtiter plates. We show data for simultaneously monitoring of up to 10 different genes in iPS cells, primary RPE, and iPS cell-derived RPE. Our multiplex assay performed in the high-throughput format correlates well with standard qRT-PCR assay. This screening assay provides the basis to develop potential drugs for RPE-associated retinal degenerative diseases and helps improve iPS cell to RPE differentiation and maturation protocols.
Supplementary Material
Acknowledgments
We thank Dr. Rong Li, Fang Hua, and Omar Memon (National Eye Institute) and Patricia Lederman (NSCI) for technical assistance and Dr. Sunita D’Souza (Mt. Sinai School of Medicine) for reagents. This work was supported by National Eye Institute intramural funds and NIH CRM awards to Drs. Kapil Bharti and Sheldon Miller and funding from the NIH Common Fund Molecular Libraries and Imaging Program, Grant U54 MH084681 to NIH Center for Advancing Translational Sciences.
Author Contributions
M.F.: conception/design, data analysis and interpretation, manuscript writing; B.C., Q.W., K.J.M., R.K., A.M., J.M., and R.S.: collection and/or assembly of data; J.D. and M.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.T.: conception/design; S.M.: conception/design, financial support; K.B.: conception/design, data analysis and interpretation, manuscript writing, financial support.
Disclosure of Potential Conflicts of Interest
M.S. has compensated employment from Affymetrix.
References
- 1.Bharti K, Miller SS, Arnheiter H. The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24:21–34. doi: 10.1111/j.1755-148X.2010.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry AB, Daley GQ. Reprogrammed cells for disease modeling and regenerative medicine. Annu Rev Med. 2013;64:277–290. doi: 10.1146/annurev-med-050311-163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Huangfu D. Human pluripotent stem cells: An emerging model in developmental biology. Development. 2013;140:705–717. doi: 10.1242/dev.086165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takaoka K, Hamada H. Cell fate decisions and axis determination in the early mouse embryo. Development. 2012;139:3–14. doi: 10.1242/dev.060095. [DOI] [PubMed] [Google Scholar]
- 5.Zernicka-Goetz M, Morris SA, Bruce AW. Making a firm decision: Multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet. 2009;10:467–477. doi: 10.1038/nrg2564. [DOI] [PubMed] [Google Scholar]
- 6.Chailangkarn T, Acab A, Muotri AR. Modeling neurodevelopmental disorders using human neurons. Curr Opin Neurobiol. 2012;22:785–790. doi: 10.1016/j.conb.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther. 2011;89:655–661. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- 8.Knollmann BC. Induced pluripotent stem cell-derived cardiomyocytes: Boutique science or valuable arrhythmia model? Circ Res. 2013;112:969–976; discussion 976. doi: 10.1161/CIRCRESAHA.112.300567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsden CM, Powner MB, Carr AJ, et al. Stem cells in retinal regeneration: Past, present and future. Development. 2013;140:2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hick A, Wattenhofer-Donzé M, Chintawar S, et al. Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich’s ataxia. Dis Model Mech. 2013;6:608–621. doi: 10.1242/dmm.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan JM, Lyon AR, Harding SE. The case for induced pluripotent stem cell-derived cardiomyocytes in pharmacological screening. Br J Pharmacol. 2013;169:304–317. doi: 10.1111/j.1476-5381.2012.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Doering LC. Induced pluripotent stem cells to model and treat neurogenetic disorders. Neural Plast. 2012;2012:346053. doi: 10.1155/2012/346053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland TJ, Blaschke AJ, Buchholz DE, et al. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J Tissue Eng Regen Med. 2013;7:642–653. doi: 10.1002/term.1458. [DOI] [PubMed] [Google Scholar]
- 14.Liao JL, Yu J, Huang K, et al. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010;19:4229–4238. doi: 10.1093/hmg/ddq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvajal-Vergara X, Sevilla A, D’Souza SL, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Narita M, Yokura M, et al. Human induced pluripotent stem cells on autologous feeders. PLoS ONE. 2009;4:e8067. doi: 10.1371/journal.pone.0008067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock C, Kiskinis E, Verstappen G, et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- 20.Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharti K, Gasper M, Ou J, et al. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet. 2012;8:e1002757. doi: 10.1371/journal.pgen.1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3513–3527. [PubMed] [Google Scholar]
- 24.Steinberg RH. Interactions between the retinal pigment epithelium and the neural retina. Doc Ophthalmol. 1985;60:327–346. doi: 10.1007/BF00158922. [DOI] [PubMed] [Google Scholar]
- 25.Strunnikova NV, Maminishkis A, Barb JJ, et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet. 2010;19:2468–2486. doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Hollander AI, Roepman R, Koenekoop RK, et al. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari S, Di Iorio E, Barbaro V, et al. Retinitis pigmentosa: Genes and disease mechanisms. Curr Genomics. 2011;12:238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardakjian TM, Schneider A. The genetics of anophthalmia and microphthalmia. Curr Opin Ophthalmol. 2011;22:309–313. doi: 10.1097/ICU.0b013e328349b004. [DOI] [PubMed] [Google Scholar]
- 29.Janas MM, Khaled M, Schubert S, et al. Feed-forward microprocessing and splicing activities at a microRNA-containing intron. PLoS Genet. 2011;7:e1002330. doi: 10.1371/journal.pgen.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaham O, Gueta K, Mor E, et al. Pax6 regulates gene expression in the vertebrate lens through miR-204. PLoS Genet. 2013;9:e1003357. doi: 10.1371/journal.pgen.1003357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang FE, Zhang C, Maminishkis A, et al. (2010) MicroRNA-204/211 alters epithelial physiology FASEB J 2010;24:1552–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







