The purpose of the study was to find out whether transurethral injections of autologous adipose stem cells (ASCs) are an effective and a safe treatment for female stress urinary incontinence (SUI). Validated questionnaires showed some subjective improvement in all patients. Thus far, the treatment with autologous ASCs has proven safe and well tolerated, but additional research is needed to develop SUI injection therapies.
Keywords: Stress urinary incontinence, Adipose stem cells, Female incontinence, Tissue engineering, Injection therapy
Abstract
The purpose of our study was to find out whether transurethral injections of autologous adipose stem cells (ASCs) are an effective and a safe treatment for female stress urinary incontinence (SUI). We treated five SUI patients with ASCs combined with bovine collagen gel and saline. Prior to the treatment, the ASCs were isolated from subcutaneous fat and expanded for 3 weeks in a good manufacturing practice-level laboratory. The mixture of ASCs and collagen was injected transurethrally via cystoscope. Additionally, viability, multipotency, and surface marker profile of ASCs were analyzed in vitro. We followed up with patients 3, 6, and 12 months after the injections. The primary endpoint was a cough test to measure objectively the effect of the treatment. Validated questionnaires were used to determine the subjective cure rate. After 6 months, 1 of 5 patients displayed a negative cough test with full bladder filled with 500 ml of saline. At 1 year, the cough test was negative with three patients; two of them were satisfied with the treatment and did not wish further treatment for SUI. Validated questionnaires showed some subjective improvement in all five patients. This is the first study describing the use of autologous ASCs in combination with collagen gel for female SUI treatments. Thus far, the treatment with autologous ASCs has proven safe and well tolerated. However, the feasibility and efficacy of the treatment were not optimal; therefore, additional research is needed to develop SUI injection therapies.
Introduction
Urinary incontinence is a common health problem affecting a large number of women. Approximately 35% of women over 18 years in Europe reported involuntary urine loss [1]. Stress urinary incontinence (SUI) is the most common type, and it is defined as involuntary loss of urine on effort or physical exertion. Urgency urinary incontinence is associated with a feeling of urgency, and mixed urinary incontinence (MUI) is a combination of the two aforementioned types [2]. SUI is further categorized as urethral hypermobility, intrinsic sphincter deficiency, or both [3]. Furthermore, the prevalence of incontinence increases with age [4].
The urethral continence control system is a vital component in stress urinary incontinence. It consists of the sphincteric unit and the vaginal support system. The urethra is a multilayered structure consisting of striated muscle, smooth muscle, connective tissue, submucosal vascular plexus, and a lining epithelium. The combined actions of these tissues serve to create a wall tension that compresses the lumen closed. Striated muscle has been shown to be responsible for one-third of the total intraurethral pressure, with another third being exerted by the urethral vascular bed, and the remaining third controlled by the smooth musculature and connective tissues [5, 6].
Midurethral slings have become first-line surgical treatments for SUI because they are minimally invasive, have excellent short-term success rates and good long-term success rates, and have a short learning curve [7]. However, there are patients who have a SUI unresponsive to operative treatment and patients who are not suitable for midurethral sling operation. Additionally, there are patients who do not want artificial synthetic material as their treatment.
Tissue engineering offers an attractive treatment method to regenerate sphincter muscle. Previously, various different cell sources, such as skeletal muscle-derived stem cells (SkMSCs), mesenchymal stem cells (derived from bone marrow [BMSCs]), and adipose stem cells (ASCs), have been studied for treating urinary incontinence. The SkMSCs and BMSCs would be a potential alternative for incontinence therapy. However, when compared with ASCs, the major limitation of SkMSCs and BMSCs is the difficulty to obtain these cells in large quantities. Furthermore, the isolation site injury for ASCs is minimal, and they are readily expanded in vitro. These features are important when considering the cell source for clinical applications. ASCs, like BMSCs, have been shown to undergo myogenic, adipogenic, osteogenic, and chondrogenic differentiation [8]. Moreover, the ASCs have been used for clinical applications in different surgical areas and are considered safe for clinical use [9].
The aim of this study was to find out whether adipose stem cells could provide a new, and possibly effective, alternative to invasive surgical treatment of SUI. To our knowledge, this was the first study of transurethral injections of autologous ASCs in combination with collagen (Contigen) in treatment of SUI or MUI.
Materials and Methods
Patients were recruited from the Tampere University Hospital outpatient clinic, and primarily they did not want the midurethral sling operation. Two of them had been operated on previously (Table 1) with unsuccessful results. The Ethics Committee of Pirkanmaa Hospital District approved the study pilot. Furthermore, all patients signed a written informed consent for the study.
Table 1.
Characteristics of the patients treated with transurethral injections of adipose stem cells + Contigen
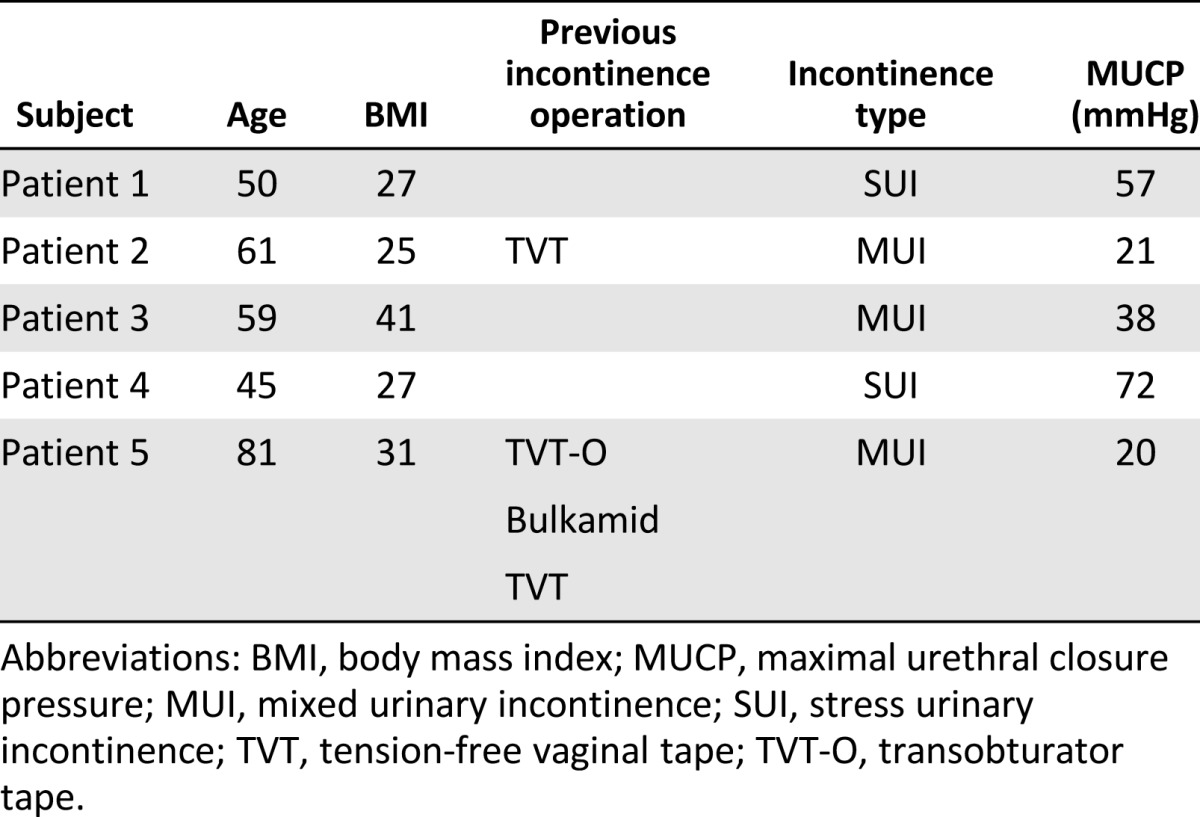
After gynecological examination, diagnosis of either pure SUI or predominantly stress MUI was made according to anamnesis, a positive cough test, and urodynamic evaluations. Validated questionnaires—Urinary Inventory Stress test (UISS), Detrusor Instability Score, Incontinence Impact Questionnaire-short form (IIQ-7), and Urogenital Distress Inventory-short form (UDI-6, with scores 0–100, lower scores reflecting better quality of life) and the bother of incontinence in patients’ lives according to visual analog scale (VAS) (ranging 0–10) [10–12]—were filled, and exclusion of infectious diseases (hepatitis B and C, HIV, syphilis) was conducted.
The subcutaneous fat was collected from patients’ lower abdomens under local anesthesia. Approximately 0.3–0.5 dl of fat was obtained from six patients. However, because of bacterial contamination (Propionibacterium acnes in repeated samples), one patient did not receive treatment. In addition, 50 ml of autologous serum was obtained for the expansion of clinically used ASCs. The ASCs were then isolated and augmented as described later in this article. A mixture of ASCs and collagen (Contigen; Bard Medical, Covington, GA, http://www.bardmedical.com) was injected transurethrally via cystoscope under local anesthesia. The injections were placed directly under mucosa: 1.5 cm distal from the urethral neck at 3 and 9 o’clock, injected volume being 2.4–4 ml per patient. Two additional concomitant injections of ASCs mixed with saline solution (volume 2 ml) were performed 2 mm more distally to bring the ASCs in contact with the urethral musculature.
We followed up with patients at 3, 6, and 12 months after the injections by a gynecological examination, a vaginal ultrasonography, a cough test, a 24-hour pad test, standardized questionnaires, and urodynamic evaluations (at 6 months).
The primary outcome measure was the cough test. Other outcome measures were the 24-hour pad test, urodynamic evaluations (maximal urethral closure pressure [MUCP], and urethral stress profile), and patients’ evaluations of their quality of life.
Stem Cell Isolation and Preparation for Injection
The isolation and expansion of ASC was done in a validated cleanroom (BioMediTech, University of Tampere) following European Union good manufacturing practice (GMP) quality system guidelines.
The cell isolation, expansion, karyotyping, sterility, endotoxin, and mycoplasma testing were performed as described previously [9, 13]. Briefly, the adipose tissue was minced into small pieces and digested with collagenase NB-6 (GMP grade; Serva, Heidelberg, Germany, http://www.serva.de) in a 37°C incubator for 60 minutes while mixing by pipetting up and down every 20 minutes. After centrifuging and lysing the red blood cells, the pellet was suspended in the basal medium (BM) containing 15% of autologous serum in Dulbecco’s modified Eagle’s medium/F-12 (DMEM/F-12; Life Technologies, Rockville, MD, http://www.lifetech.com). The isolated cells were expanded for 3–4 weeks in BM. When nearly confluent (90%), the cells were mechanically detached using a cell scraper (Nunc, Rochester, NY, http://www.nuncbrand.com; Life Technologies) and passaged. For the injection therapies, we used passages 3–4, which were the lowest possible passages to get an adequate amount of cells.
Half of the freshly isolated ASCs were blended with 2.1 ml of collagen (Contigen), and the rest of the ASCs were blended with 0.9% NaCl. The amount of cells used for injection therapy varied from 2.5 × 106 to 8.5 × 106 cells, depending on the patient. The live/dead staining was used to evaluate the viability of ASCs in Contigen prior to the injection therapy as previously described [13].
In Vitro Analyses
Cell Expansion
For the following in vitro analyses, the cells were expanded in vitro in BM consisting of DMEM/F-12 supplemented with 15% human serum (Lonza, Walkersville, MD, http://www.lonza.com) and 1% GlutaMAX (Life Technologies).
Flow Cytometric Surface Marker Expression Analysis
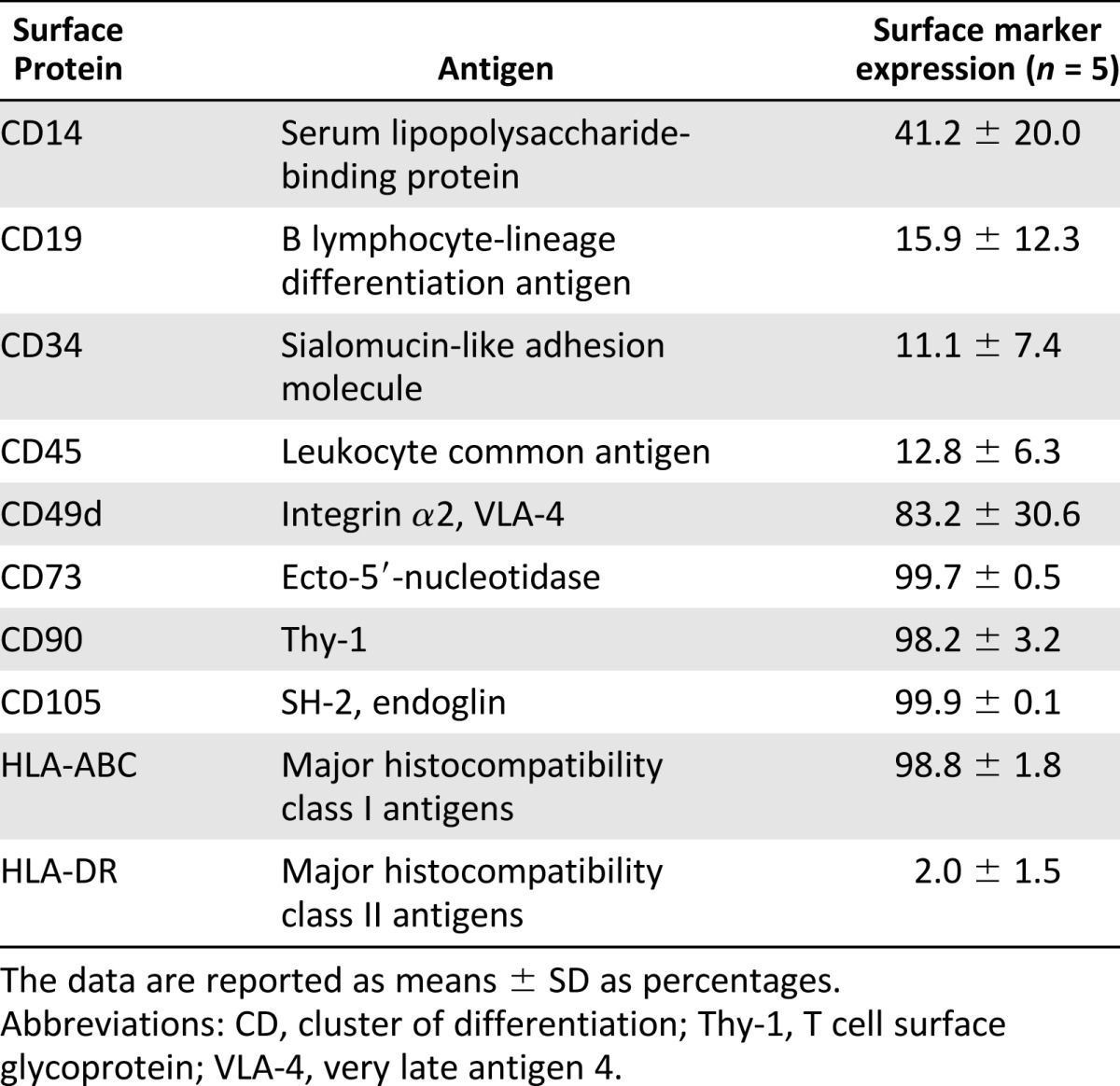
The ASCs (n = 5) at passages 5–6 were analyzed with a fluorescence-activated cell sorter (FACSAria; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). Antibodies against CD14-PECy7 (BD Biosciences), CD19-PECy5 (BD Biosciences), CD34-APC (Immunotools GmBH, Friesoythe, Germany, http://www.immunotools.de), CD45-PE, CD49d-PE (BD Biosciences), CD73-PE (BD Biosciences), CD90-PE, CD105-PE, HLA-ABC-PE (Immunotools), and HLA-DR-PE (Immunotools) were used. The analysis was performed on 10,000 cells per sample, and unstained cell samples were used to compensate for the background autofluorescence levels. The surface marker expression of >2% was defined as a positive expression.
Differentiation Analyses
The myogenic, adipogenic, osteogenic, and chondrogenic differentiation analyses were carried out to verify the multidifferentiation potential of ASCs (n = 5, passages 7–10). All the cultures were maintained for 14 days in differentiation conditions. The BM supplemented with antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin; Life Technologies) served as a control medium.
For myogenic differentiation, the ASCs were plated onto fibronectin-coated (Sanquin, Amsterdam, The Netherlands, http://www.sanquin.nl) wells with a density of 2,631 cells per cm2. The ASCs were cultured in myogenic medium, containing 5 ng/ml of transforming growth factor β1 (hBA-112; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) in BM for 14 days. The myogenic differentiation was verified by immunostaining using smooth muscle protein 22-α (SM22-α, 1:100; Abcam, Cambridge, U.K., http://www.abcam.com), α smooth muscle actin (α-SMA, 1:100; Abcam), and myosin heavy chain II (MHCII, 1:200; Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com) as primary antibodies. The cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and incubated in primary antibodies. Thereafter, secondary antibodies from goat, donkey, and donkey (1:200, Life Technologies), respectively, were conjugated to primary antibodies. Finally, the cells were mounted with Vectashield (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) and imaged with the fluorescent microscope (Olympus).
The adipogenic, osteogenic, and chondrogenic differentiation have been described in detail previously [13]. Briefly, the adipogenic differentiation was performed by culturing ASCs in adipogenic medium at a density of 2 × 104 cells per cm2. After 14 days, adipogenesis was verified with Oil Red O staining (Sigma-Aldrich). For the osteogenic differentiation, 2,500 cells per cm2 were incubated in osteogenic medium, and the osteogenic capacity was studied using alkaline phosphatase (ALP) staining (Sigma-Aldrich). A micromass culture method was used for chondrogenic differentiation, and the chondrogenic potential was verified by Alcian blue staining (Sigma-Aldrich).
Results
Clinical Results
Five patients were followed-up 1 year after the procedure. At 6 months, 1 of 5 patients displayed a negative cough test with full bladder filled with 500 ml of saline. At 1 year, the cough test was negative for three patients, and two of them were satisfied and did not wish further treatment for SUI. There was also improvement according to the 24-hour pad test with the objectively cured patients (Table 2). There were no changes in either urodynamic parameters or in urine residual volume in any of the patients.
Table 2.
Objective findings of urinary incontinence in patients treated with transurethral injections of adipose stem cells + Contigen
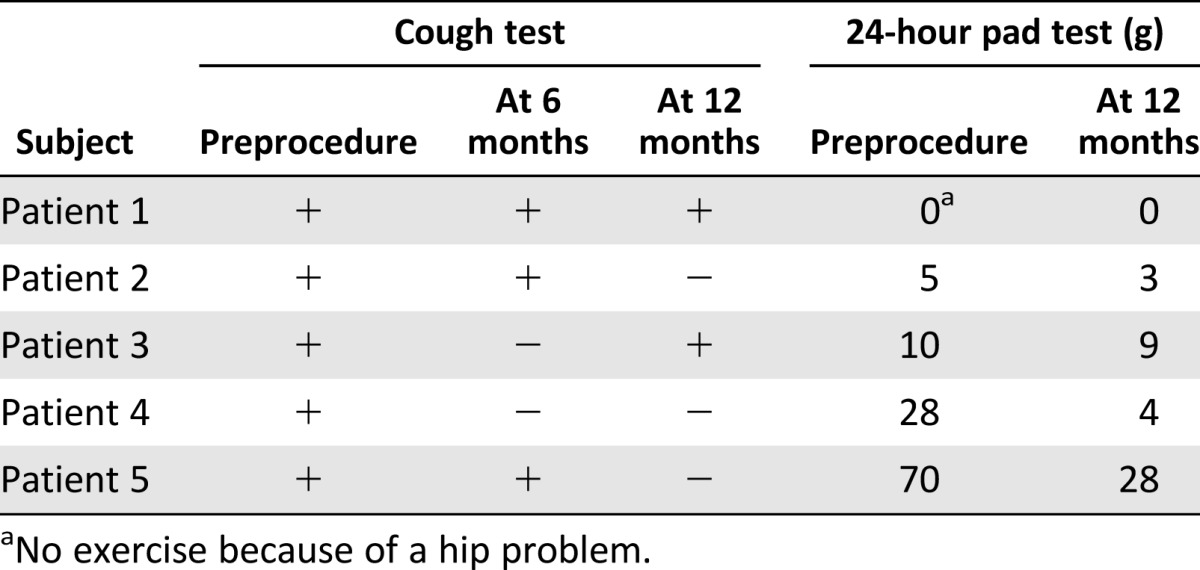
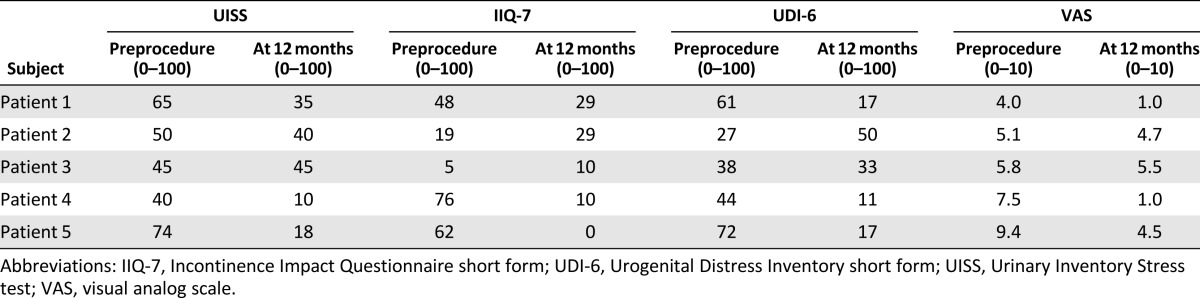
There was subjective improvement with all five treated patients according to the UISS, IIQ-7, UDI-6, and VAS (Table 3); however, the first three patients did not indicate improvement on all of the questionnaires. The two patients who benefitted from the ASC treatment were consistent in their answers and were subjectively cured or improved according to the UISS, IIQ-7, UDI-6, and VAS questionnaires. Three of the patients have been operated on after the 1-year follow-up.
Table 3.
Quality of life/subjective findings of patients treated with transurethral injections of adipose stem cells + Contigen according to UISS, IIQ-7, UDI-6, and VAS
With the exception of small hematomas, there were no adverse events from the adipose tissue collection. There were no major complications (urinary retention, hematuria, or urinary tract infection) after the transurethral injections. One patient displayed mild pollacis and dysuria that resolved spontaneously within a week.
In Vitro Results
After ASC isolation, the cells proliferated rapidly in autologous serum containing medium. The live/dead analysis prior to injection therapy confirmed the viability of ASCs in the Contigen: the majority of cells were viable, and only a few dead cells were detected (Fig. 1).
Figure 1.
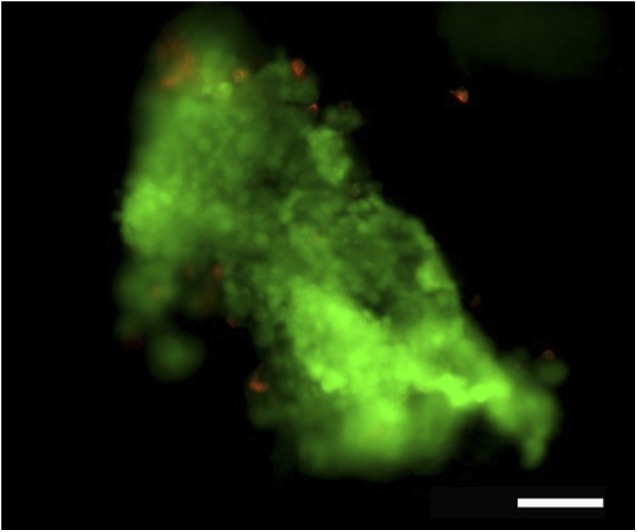
Representative image of viable (green) and dead (red) adipose stem cells mixed with Contigen. Scale bar = 100 μm.
The surface marker expression analysis of the ASCs showed a positive expression for adhesion molecules CD49d, CD73, and CD105; extracellular matrix protein CD90; and MHC class I isotype HLA-ABC. Minor or moderate expression of markers CD14, CD19, CD34, CD45, and MHC class II isotype HLA-DR were detected, which suggested a low number of cells of hematopoietic and angiogenic lineages (Table 4).
Table 4.
Surface marker expression of undifferentiated adipose stem cells analyzed by flow cytometry
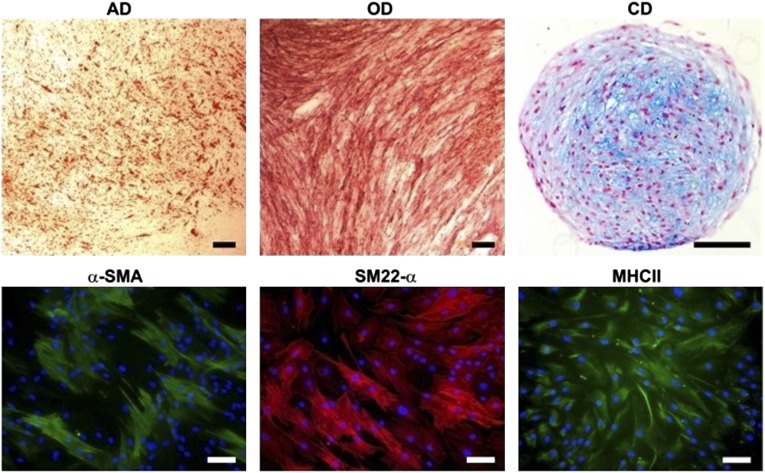
The multidifferentiation potential of ASCs was demonstrated by their capability to differentiate toward myogenic, adipogenic, osteogenic, and chondrogenic cell lineages (Fig. 2). After 14 days of exposing the ASCs to adipogenic medium, the lipid droplets were evident, which confirmed the ASCs potential to differentiate toward adipocytes. The positive ALP staining verified the osteogenic potential of ASCs. The chondrogenic differentiation was detected with Alcian blue staining for cartilage-specific glycosaminoglycans. The ASCs showed positive expression for all the myogenic markers used: SM22-α, α-SMA, and MHCII, thus verifying the myogenic potential of these cells.
Figure 2.
Multipotency of adipose stem cells. AD was detected using Oil Red O staining. The alkaline phosphatase staining ensured the OD, and the Alcian blue staining confirmed the CD. Myogenesis was verified using three immunostaining markers: α-SMA, SM22-α, and MHCII. Black scale bars = 150 μm; white scale bars = 100 μm. Abbreviations: AD, adipogenic differentiation; CD, chondrogenic differentiation; MHCII, myosin heavy chain II; OD, osteogenic differentiation; SM22-α, smooth muscle protein 22-α; α-SMA, α smooth muscle actin.
Discussion
The purpose of this study was to find out whether transurethral injections of autologous ASCs with collagen could be safe and effective for SUI or MUI treatment. In this study, two of five patients had recurrent SUI, whereas two patients had mixed urinary incontinence.
Two patients had low MUCP, and the BMI of all the patients in the study was ≥25. All of these factors (overweight, mixed incontinence, previous continence surgery, and intrinsic sphincter deficiency) are significant independent predictors for midurethral sling failure [14]. Recurrent incontinence is not an ideal target for any method of treatment. The first three patients did not benefit from the cellular therapy, which may be at least partly due to the introduction of a new method.
Prior to the injection therapy, the adipose tissue was readily collected under local anesthesia. The tissue collection caused some discomfort for the patients, and with the patient who was not eventually treated because of bacterial contamination in repeated samples, there was postoperative hemorrhage that was managed conservatively. The transurethral injections were relatively easily implemented, and the patients were able to return to their everyday activities within the same day. The injections under local anesthesia were well tolerated, and there were no severe complications.
To the best of our knowledge, ASCs have not been previously used clinically for female SUI treatments. However, nowadays stem cell therapies have been under extensive research [15], and prior to this study, some clinical studies on cell-based injection therapies have been published. Mitterberger et al. [16, 17] demonstrated an efficacy of cell-based injection therapy using myoblasts and fibroblasts mixed with Contigen to treat female SUI. However, the primary cells are not the optimal choice because of the difficulties in high-yield expansion of cells. In addition to primary cells, autologous muscle-derived stem cells and allogenic umbilical cord blood stems cells (CBSCs) have both been used in a few clinical trials to treat female SUI patients with promising results [8, 15]. Carr et al. [18] used autologous MDSCs to treat 8 female SUI patients. They demonstrated an improvement of SUI symptoms in 5 patients at the 1-year follow-up, although only 1 patient was totally continent. Furthermore, Sèbe et al. [19] treated 12 female patients with autologous MDSCs, and at 1-year follow-up, 3 patients were dry, and improvement of SUI was detected in 7 patients. Additionally to MDSCs, Lee et al. [20] used allogenic CBSCs in one clinical study to treat 39 female SUI patients. They demonstrated a 36.1% cure rate at the 1-year follow-up. These treatments were demonstrated to be a safe method, and no severe adverse effects were detected. When compared with the aforementioned cell sources, the main advantage of ASCs is that the yield of stem cells from adipose tissue is much higher, and the isolation and expansion of ASCs is also relatively easy [15, 21]. Furthermore, the injection therapy with ASCs was detected as a potential method to treat male urinary incontinence [22], and therefore we wanted to study the efficacy of ASCs for female urinary incontinence. Our approach was to use multipotent autologous ASCs with the bulking agent Contigen for transurethral injection therapy.
The mesenchymal origin was confirmed prior to the clinical injections using fluorescence-activated cell sorting analysis after the subsequent cells expansion of ASCs in laboratory facilities. The fact that the analyzed ASCs were at higher passages compared with the ASCs used for injection therapies could have slightly affected the expression results; however, the expression results were concordant with the previous results for ASCs [9, 13, 23]. Compared with the previous studies, the ASCs in our study expressed the hematopoietic markers CD14, CD34, and CD45 slightly more. This difference in expression results could be explained with the patient variation, which is typically high in ASCs [21, 23]. In addition to adipogenic, chondrogenic, and osteogenic differentiation, several studies have exhibited the ASCs’ capability to undergo myogenic differentiation [24, 25]. The ASCs used in this study were verified to be multipotent, because they also had a myogenic capacity. Compared with other cell sources, the main advantage of ASCs is that the yield of stem cells from adipose tissue is much higher [15].
Previously, ASCs have been used in several in vivo studies to treat SUI with potential results. Lin et al. [25] showed that transplantation of ASCs via urethral or intravenous injection was an effective treatment and/or prevention of SUI in a preclinical setting in rats. Fu et al. [24] induced rat ASCs to differentiate into myoblasts in vitro prior to transurethral injection therapy and showed that the myoblasts served an important function in improving the urine controlling ability. Furthermore, Wu et al. [26] showed that ASCs transplanted under the urethral mucosa of pudendal nerve-mutilated rats improved maximum bladder capacity, abdominal leak point pressure, maximum urethral closure pressure, and functional urethral length in urodynamic testing compared with the control group.
Although traditionally the ASCs' regenerative capacity was associated with their plasticity, their therapeutic effects appear to be particularly due to their paracrine function through the secretion of a broad range of bioactive molecules. Their potential mechanism of action might be related to immunomodulation, support of growth and differentiation of local stem and progenitor cells, proangiogenic action, chemoattraction, antiscarring, and antiapoptosis effects [27].
New techniques in many tissue regeneration applications are emerging, but midurethral slings are still the first-line surgical treatments for SUI. Reported cure rates of midurethral sling operations rise even up to 90% [28]. Furthermore, other treatment methods such as bulking agents are an option, especially for patients who are unresponsive to traditional treatments or who are not suitable for surgical operation. However, injections with bulking agents have not been as effective as surgical methods, and the main problem has been the relatively poor sustainability of the bulking effect [29]. The short-term subjective cure rate with plain Contigen has been low in women with severe sphincteric incontinence [30]. Several investigators have produced variable results from 23% to 83% cure rates at 1 and 2 years of follow-up after collagen therapy [31].
In our pilot study, we chose bovine collagen (Contigen) to be the carrier of the autologous ASCs, because it is a widely studied biomaterial in SUI injection therapy. Furthermore, it is the only commercially available collagen gel accepted for clinical use. Additionally, collagen is shown to be biocompatible and biodegradable [32]. Our results also showed that ASCs remain viable when combined with Contigen. However, Contigen was not the ideal carrier for cells because of its poor mechanical properties. When Contigen was combined with ASCs, the collagen gel became more liquid, which may have affected the overall bulking efficacy of the injection therapy. More preclinical studies are needed to develop an ideal carrier material for the stem cells.
Conclusion
In this pilot study, we studied the effect of ASCs in combination with injectable bulking agent to treat female SUI. During the 1-year follow-up period, the treatment with autologous ASCs was shown to be safe and well-tolerated and reasonably effective in two of five patients. Tissue engineering techniques hold the potential to provide an efficient treatment for SUI in the future, but more research is needed to reach this goal. Developing more optimal biomaterial carriers for cells may especially increase the efficiency of the stem cell-based injection therapies for SUI.
Acknowledgments
We thank Anna-Liisa Kuukka for clinical assistance and Miia Juntunen, Sari Kalliokoski, and Anna-Maija Honkala for technical assistance. This research was supported by the competitive research funding of the Pirkanmaa Hospital District. This work was approved by Pirkanmaa Hospital District Ethics Committee (number R09179).
Author Contributions
K.K., R.S., S.H., B.M., E.T., and S.M.: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; K.N.: conception and design, administrative support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2004;93:324–330. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 2.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 3.McGuire EJ, Lytton B, Pepe V, et al. Stress urinary incontinence. Obstet Gynecol. 1976;47:255–264. [PubMed] [Google Scholar]
- 4.Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 5.Delancey JO. Why do women have stress urinary incontinence? Neurourol Urodyn. 2010;29(suppl 1):S13–S17. doi: 10.1002/nau.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rud T, Andersson KE, Asmussen M, et al. Factors maintaining the intraurethral pressure in women. Invest Urol. 1980;17:343–347. [PubMed] [Google Scholar]
- 7.Virkud A. Management of stress urinary incontinence. Best Pract Res Clin Obstet Gynaecol. 2011;25:205–216. doi: 10.1016/j.bpobgyn.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: A critical review. Stem Cells Dev. 2012;21:834–843. doi: 10.1089/scd.2011.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sándor GK, Tuovinen VJ, Wolff J, et al. Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: A case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg. 2013;71:938–950. doi: 10.1016/j.joms.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Corcos J, Beaulieu S, Donovan J, et al. Quality of life assessment in men and women with urinary incontinence. J Urol. 2002;168:896–905. doi: 10.1016/S0022-5347(05)64540-5. [DOI] [PubMed] [Google Scholar]
- 11.Kauppila A, Alavaikko P, Kujansuu E. Detrusor instability score in the evaluation of stress urinary incontinence. Acta Obstet Gynecol Scand. 1982;61:137–141. doi: 10.3109/00016348209156544. [DOI] [PubMed] [Google Scholar]
- 12.Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995;14:131–139. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 13.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–209. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Stav K, Dwyer PL, Rosamilia A, et al. Risk factors of treatment failure of midurethral sling procedures for women with urinary stress incontinence. Int Urogynecol J. 2010;21:149–155. doi: 10.1007/s00192-009-1020-9. [DOI] [PubMed] [Google Scholar]
- 15.Ho CP, Bhatia NN. Development of stem cell therapy for stress urinary incontinence. Curr Opin Obstet Gynecol. 2012;24:311–317. doi: 10.1097/GCO.0b013e328357ae03. [DOI] [PubMed] [Google Scholar]
- 16.Mitterberger M, Marksteiner R, Margreiter E, et al. Autologous myoblasts and fibroblasts for female stress incontinence: A 1-year follow-up in 123 patients. BJU Int. 2007;100:1081–1085. doi: 10.1111/j.1464-410X.2007.07119.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitterberger M, Pinggera GM, Marksteiner R, et al. Adult stem cell therapy of female stress urinary incontinence. Eur Urol. 2008;53:169–175. doi: 10.1016/j.eururo.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Carr LK, Steele D, Steele S, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881–883. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 19.Sèbe P, Doucet C, Cornu JN, et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: A prospective study. Int Urogynecol J. 2011;22:183–189. doi: 10.1007/s00192-010-1255-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee CN, Jang JB, Kim JY, et al. Human cord blood stem cell therapy for treatment of stress urinary incontinence. J Korean Med Sci. 2010;25:813–816. doi: 10.3346/jkms.2010.25.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Gotoh M, Kato M, et al. Periurethral injection of autologous adipose-derived regenerative cells for the treatment of male stress urinary incontinence: Report of three initial cases. Int J Urol. 2012;19:652–659. doi: 10.1111/j.1442-2042.2012.02999.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolff J, Sándor GK, Miettinen A, et al. GMP-level adipose stem cells combined with computer-aided manufacturing to reconstruct mandibular ameloblastoma resection defects: Experience with three cases. Ann Maxillofac Surg. 2013;3:114–125. doi: 10.4103/2231-0746.119216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Q, Song XF, Liao GL, et al. Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology. 2010;75:718–723. doi: 10.1016/j.urology.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Lin G, Wang G, Banie L, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Song Y, Zheng X, et al. Adipose-derived stromal cell transplantation for treatment of stress urinary incontinence. Tissue Cell. 2011;43:246–253. doi: 10.1016/j.tice.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 27.de Girolamo L, Lucarelli E, Alessandri G, et al. Mesenchymal stem/stromal cells: A new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr Pharm Des. 2013;19:2459–2473. doi: 10.2174/1381612811319130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson CG, Palva K, Rezapour M, et al. Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1043–1047. doi: 10.1007/s00192-008-0666-z. [DOI] [PubMed] [Google Scholar]
- 29.Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2012;2:CD003881. doi: 10.1002/14651858.CD003881.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Groutz A, Blaivas JG, Kesler SS, et al. Outcome results of transurethral collagen injection for female stress incontinence: Assessment by urinary incontinence score. J Urol. 2000;164:2006–2009. [PubMed] [Google Scholar]
- 31.Corcos J, Collet JP, Shapiro S, et al. Multicenter randomized clinical trial comparing surgery and collagen injections for treatment of female stress urinary incontinence. Urology. 2005;65:898–904. doi: 10.1016/j.urology.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 32.Chapple CR, Wein AJ, Brubaker L, et al. Stress incontinence injection therapy: What is best for our patients? Eur Urol. 2005;48:552–565. doi: 10.1016/j.eururo.2005.06.012. [DOI] [PubMed] [Google Scholar]