Abstract
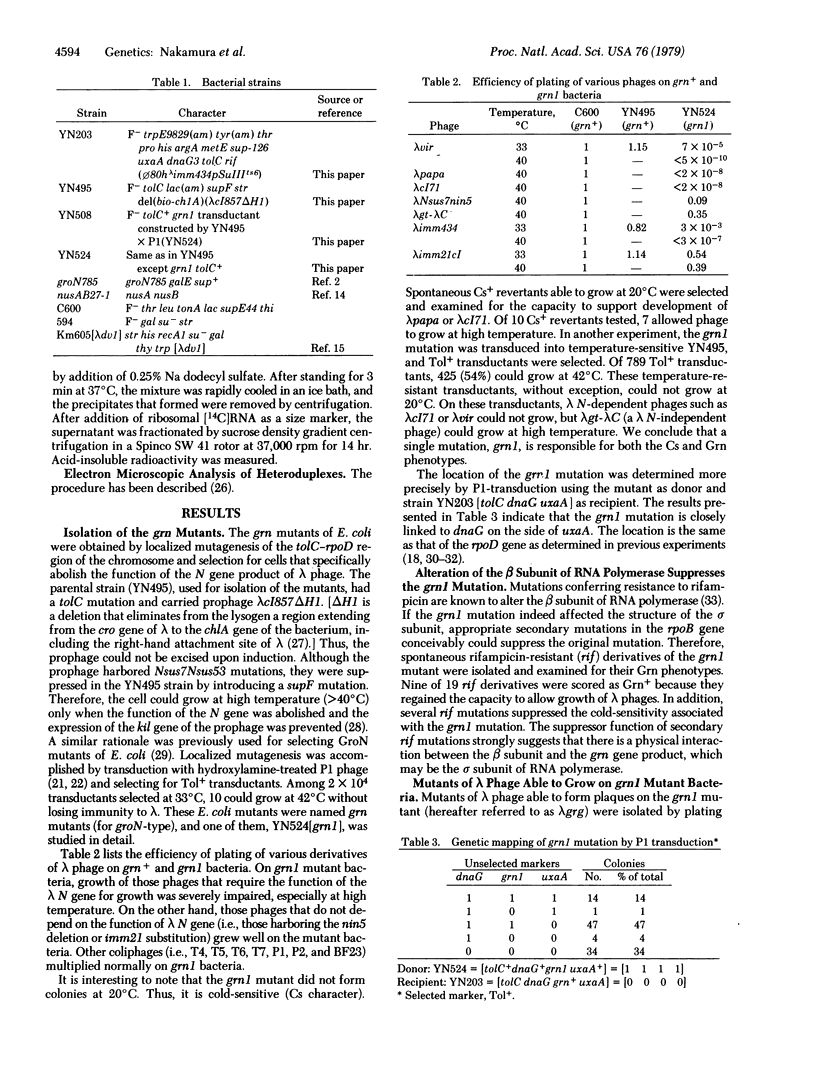
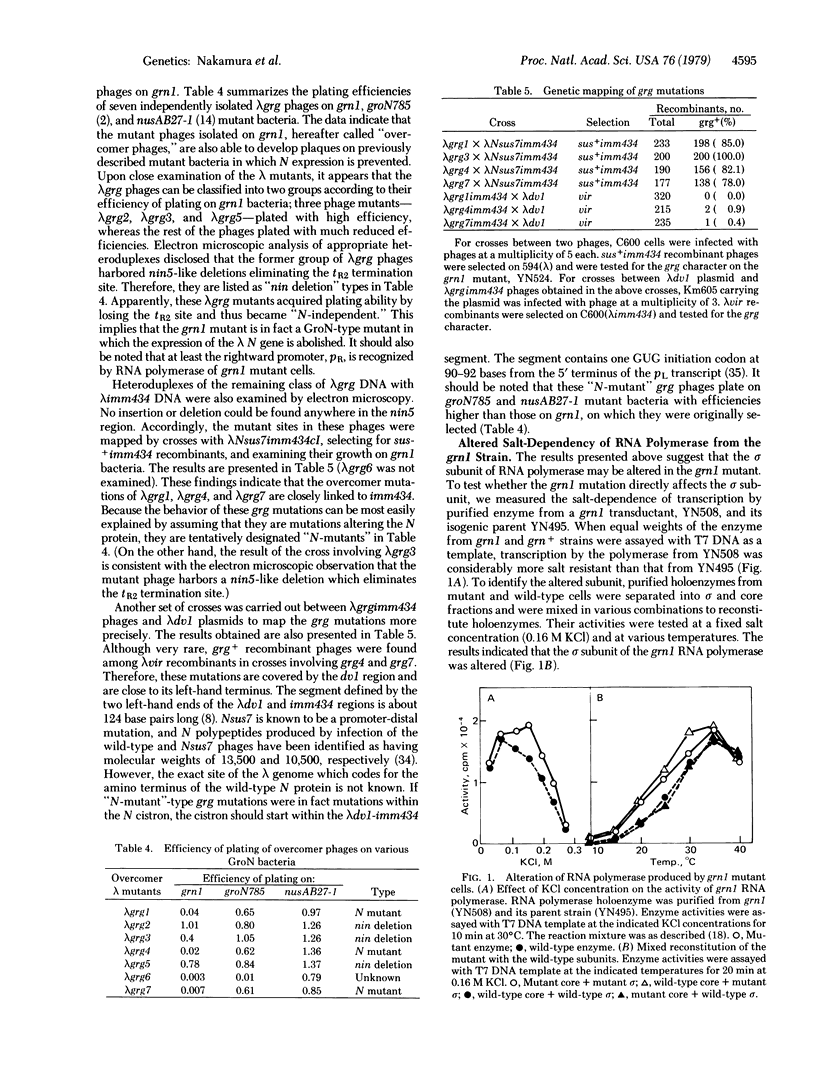
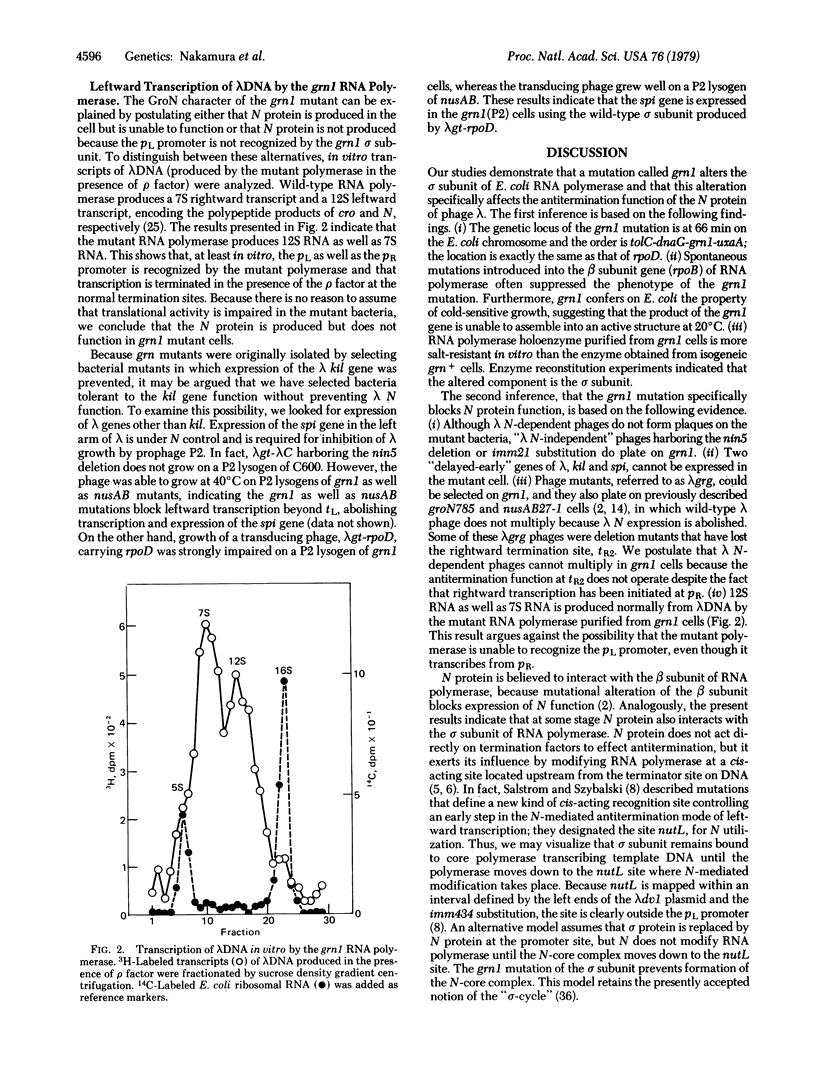
A new class of Escherichia coli mutants, referred to as grn, has been isolated by localized mutagenesis. These mutations affect the sigma subunit of DNA-dependent RNA polymerase (ribonucleoside 5'-triphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) by abolishing the expression of the lambda N gene, and they are closely lniked to dnaG in the order dnaG-grn-uxaA. Detailed study of one such mutant, grn1, yielded the following results: (i) grn1 is a single mutation and the mutant cell shows cold-sensitivity in growth; (ii) the Grn phenotype of the mutant can easily be suppressed by secondary mutations in the beta subunit gene of RNA polymerase; (iii) purified holoenzyme of RNA polymerase isolated from the mutant showed an altered salt-dependency in vitro, and the mixed reconstitution of the mutant with the wild-type subunits showed that the sigma subunit of the grn1 mutant is altered; (iv) lambda phage mutants (lambda grg), which overcome the grn mutation, can be classified into two groups, the "nin-deletion" and the "N-mutant" groups (both of these are also able to grow on the previously described groN mutant of Georgopoulos and nusAB of Friedman); (iv) the mutant polymerase transcribed 12S as well as 7S RNA from lambda DNA in the presence of the rho factor in vitro. These results indicate that the grn mutation alters the sigma subunit of RNA polymerase and that the sigma subunit participates in activating the N-mediated antitermination mode of lambda phage transcription.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Brachet P., Eisen H. Isolation and characterization of deletions in bacteriophage lambda residing as prophage in E. coli K 12. Mol Gen Genet. 1972;117(3):211–218. doi: 10.1007/BF00271648. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Blattner F. R. Sequence of the promoter-operator proximal region of the major leftward RNA of bacteriophage lambda. Nucleic Acids Res. 1975 Sep;2(9):1441–1458. doi: 10.1093/nar/2.9.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin N. C. Altered reading of genetic signals fused to the N operon of bacteriophage lambda: genetic evidence for modification of polymerase by the protein product of the N gene. J Mol Biol. 1974 Oct 15;89(1):33–48. doi: 10.1016/0022-2836(74)90161-2. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Baumann M., Baron L. S. Cooperative effects of bacterial mutations affecting lambda N gene expression. I. Isolation and characterization of a nusB mutant. Virology. 1976 Aug;73(1):119–127. doi: 10.1016/0042-6822(76)90066-0. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Jolly C. T., Mural R. J. Interference with the expression of the N gene function of phage lambda in a mutant of Escherichia coli. Virology. 1973 Jan;51(1):216–226. doi: 10.1016/0042-6822(73)90381-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Ponce-Campos R. Differential effect of phage regulator functions on transcription from various promoters: evidence that the P22 gene 24 and the lambda gene N products distinguish three classes of promoters. J Mol Biol. 1975 Nov 5;98(3):537–549. doi: 10.1016/s0022-2836(75)80085-4. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Greer H. The kil gene of bacteriophage lambda. Virology. 1975 Aug;66(2):589–604. doi: 10.1016/0042-6822(75)90231-7. [DOI] [PubMed] [Google Scholar]
- Gross C., Engbaek F., Flammang T., Burgess R. Rapid micromethod for the purification of Escherichia coli ribonucleic acid polymerase and the preparation of bacterial extracts active in ribonucleic acid synthesis. J Bacteriol. 1976 Oct;128(1):382–389. doi: 10.1128/jb.128.1.382-389.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Hoffman J., Ward C., Hager D., Burdick G., Berger H., Burgess R. Mutation affecting thermostability of sigma subunit of Escherichia coli RNA polymerase lies near the dnaG locus at about 66 min on the E. coli genetic map. Proc Natl Acad Sci U S A. 1978 Jan;75(1):427–431. doi: 10.1073/pnas.75.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Heilig J. S., Martinez I. I., Calendar R., Isaksson L. A. Temperature-sensitive Escherichia coli mutant producing a temperature-sensitive sigma subunit of DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6177–6181. doi: 10.1073/pnas.75.12.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. D., Martinez I. I., Calendar R. A gene from Escherichia coli affecting the sigma subunit of RNA polymerase. Proc Natl Acad Sci U S A. 1977 May;74(5):1836–1840. doi: 10.1073/pnas.74.5.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F., Georgopoulos C. P., Eisen H. Host interference with expression of the lambda N gene product. Biochimie. 1974;56(11-12):1505–1509. doi: 10.1016/s0300-9084(75)80273-2. [DOI] [PubMed] [Google Scholar]
- Matsubara K. M. Interference in phage growth by a resident plasmid lambda dv. I. The mode of interference. Virology. 1972 Dec;50(3):713–726. doi: 10.1016/0042-6822(72)90425-4. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Osawa T., Yura T. Chromosomal location of a structural gene for the RNA polymerase sigma factor in Escherichia coli. Proc Natl Acad Sci U S A. 1977 May;74(5):1831–1835. doi: 10.1073/pnas.74.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. RNA polymerase mutant with altered sigma factor in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):1–6. doi: 10.1007/BF00270369. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Hyperproduction of the sigma subunit of RNA polymerase in a mutant of Escherichia coli. Mol Gen Genet. 1975 Nov 24;141(2):97–111. doi: 10.1007/BF00267677. [DOI] [PubMed] [Google Scholar]
- Nashimoto H., Uchida H. Late steps in the assembly of 30 S ribosomal proteins in vivo in a spectinomycin-resistant mutant of Escherichia coli. J Mol Biol. 1975 Aug 15;96(3):443–453. doi: 10.1016/0022-2836(75)90171-0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Nakamura Y., Uchida H. A transducing lambda phage carrying grpE, a bacterial gene necessary for lambda DNA replication, and two ribosomal protein genes, rpsP (S16) and rplS (L19). Mol Gen Genet. 1978 Oct 24;165(3):247–256. doi: 10.1007/BF00332523. [DOI] [PubMed] [Google Scholar]
- Saito H., Uchida H. Initiation of the DNA replication of bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1977 Jun 15;113(1):1–25. doi: 10.1016/0022-2836(77)90038-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Uchida H. Organization and expression of the dnaJ and dnaK genes of Escherichia coli K12. Mol Gen Genet. 1978 Aug 4;164(1):1–8. doi: 10.1007/BF00267592. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Szybalski W. Coliphage lambdanutL-: a unique class of mutants defective in the site of gene N product utilization for antitermination of leftward transcription. J Mol Biol. 1978 Sep 5;124(1):195–221. doi: 10.1016/0022-2836(78)90156-0. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Jones B. B., Pearson M. L. Identification of the N gene protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1978 May;75(5):2225–2229. doi: 10.1073/pnas.75.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Buckland R., Goman M., Le Grice S. S., Scaife J. G. A mutation affecting the sigma subunit of RNA polymerase changes transcriptional specificity. Nature. 1978 Jun 1;273(5661):354–358. doi: 10.1038/273354a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969 May 10;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


