Abstract
Objective:
To assess the feasibility and acceptability of family-based group pediatric obesity treatment in a primary care setting, to obtain an estimate of its effectiveness, and to describe participating parents’ experiences of social support for healthy lifestyle changes.
Methods:
We adapted an evidence-based intervention to a group format and completed six 12- to 16-week groups over 3 years. We assessed program attendance and completion, changes in child and parent body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and changes in child quality of life in a single-arm before-and-after trial. Qualitative interviews explored social support for implementing healthy lifestyle changes.
Results:
Thirty-eight parent-child pairs enrolled (28% of the 134 pairs invited). Of those, 24 (63%) completed the program and another 6 (16%) attended at least 4 sessions but did not complete the program. Children who completed the program achieved a mean change in BMI Z-scores (Z-BMI) of −0.1 (0.1) (p < 0.001) and significant improvement in parent-reported child quality of life (mean change = 8.5; p = 0.002). Mean BMI of parents changed by −0.9 (p = 0.003). Parents reported receiving a wide range of social support for healthy lifestyle changes and placed importance on the absence or presence of support.
Conclusions:
A pilot group program for family-based treatment of pediatric obesity is feasible and acceptable in a primary care setting. Change in child and parent BMI outcomes and child quality of life among completers were promising despite the pilot’s low intensity. Parent experiences with lack of social support suggest possible ways to improve retention and adherence.
Introduction
Years of clinical research have demonstrated the efficacy of family-based behavioral pediatric obesity treatment for school-aged children.1 The US Preventive Service Task Force (USPSTF) recently issued a recommendation to screen children aged six years and older for obesity and refer overweight children to intensive behavioral treatment.2 In contrast to information-focused weight management programs, effective behavioral interventions teach parents and children behavioral skills such as self-monitoring and goal setting to create and sustain lifestyle changes.3–5
Despite evidence for the efficacy of behavioral pediatric obesity treatment, few models exist for their implementation in health care settings.6,7 Few health care systems offer this type of treatment because delivery of behavioral obesity treatment requires a behavioral skill set not typically found among most physicians, nurses, nutritionists, or other primary care personnel, outside of mental health providers. Furthermore, behavioral obesity treatment requires frequent (usually weekly) contacts over time,8 a departure from the health care visit cadence for children this age. The considerable barriers to recruitment and retention encountered in clinical trials pose challenges in health care settings as well.8,9 Parents of obese children are often reluctant to commit to treatment because they minimize the short- and long-term consequences of obesity for their child, are reluctant to embark on family lifestyle changes, or simply lack readiness to change at any particular time.10 Families who do enroll in treatment universally experience some difficulties in adopting and adhering to lifestyle changes, which often disrupt family dynamics. Consequently, faced with the stress of making changes in the absence of social support for change, many families fail to complete treatment.9 Effective strategies for implementing family-based behavioral pediatric obesity treatment in real-world settings are needed.8,10,11
We developed the Family Wellness Program (FWP) in response to a growing demand for pediatric weight management among clinicians at Group Health (GH), where behavioral treatment for children was not available at the time of this pilot. We adapted the FWP intervention from a previous randomized controlled trial of individualized family-based behavioral pediatric obesity treatment (FOCUS, NIH grant R21-054871, Clinical Trial Identifier NCT00746629).12 The FWP differed from FOCUS in two important ways: The FWP relied on a group format rather than individual contacts, and it delivered fewer contact hours. These adaptations were designed to minimize resource demand and participant burden in order to improve the feasibility and acceptability of family-based behavioral pediatric obesity treatment in a primary care setting. Growing evidence suggests that behavioral obesity interventions can be effectively delivered in groups.1,13 As a proof-of-concept, the FWP relied on masters-level research interventionists to deliver treatment, bypassing the barriers related to staffing behavioral interventions in primary care. We conducted a single-arm before-and-after feasibility pilot of the FWP with two principal aims:
to assess feasibility and acceptability of delivering group pediatric behavioral obesity treatment in a primary care setting, and
to estimate the effectiveness of the group program by exploring pre- and posttreatment differences in behavioral skills use, child and parent body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and child quality of life.
As a secondary aim, we used qualitative interviews of a subset of FWP participants to describe parents’ experiences of social support for making healthy lifestyle changes in their families, because these could inform future implementation efforts.
Methods
Design
We conducted a single-arm before-and-after trial of a group adaptation of family-based pediatric obesity treatment in a primary care setting.
Setting
The study was conducted in 2 GH clinics near Seattle, WA. GH is a consumer-governed, nonprofit health delivery system located in the Pacific Northwest. Member demographic characteristics are representative of the region’s population.14 As of May 2012, GH membership was 5.9% black or African American, 2.1% American Indian/Alaska Native, 10.6% Asian, 1.5% Native Hawaiian or other Pacific Islander, and 79.9% white; 5.5% were Hispanic. Similar to the US population, 15% of GH children are obese and 15% are overweight. GH maintains an evidence-based clinical guideline to aid clinicians in the diagnosis and management of overweight and obesity, but had no weight management program for obese and overweight children at the time of this study.
Recruitment
Eligible families were identified via electronic medical record as having a child age 6 to 12 years with a BMI at the 85th percentile or higher and at least one parent with a BMI of 25 or higher.15 Before the start of each of 6 groups, pediatricians and family medicine physicians reviewed a list of eligible patients and approved families for study contact. Study staff mailed invitation letters to families on behalf of their physician, and then followed-up with select families by phone. Families with children in the highest BMI percentile were prioritized for phone invitation until the upcoming group was filled. Eligible families who did not enroll and did not refuse study contact continued to receive invitations for upcoming groups. Family-based pediatric behavioral obesity treatment targets the parent as the primary change agent for the child’s eating and activity behaviors.16 For each participating child, we also enrolled 1 parent who was expected to attend sessions and was held accountable for implementing changes in the home.
Intervention
The FWP was delivered to six groups from May 2009 to December 2011. Group sessions were facilitated by Health Coaches with masters-level training (MSW, MEd) and eight to ten years’ experience delivering health behavior change interventions. The FWP was delivered weekly in a group format, incorporating only a small number of brief, ad hoc individual contacts as needed (e-mail or face-to-face). Each week, parents and children met together briefly (five to ten minutes) to review weekly goals jointly, after which participants met in separate, simultaneous hour-long parent and child groups.
To minimize participant burden and maximize potential acceptability of the FWP, the intervention was first delivered as 13 contact hours over 12 weeks (groups 1 to 4; years 2009–2010). On the basis of preliminary assessment of the feasibility and acceptability of this duration, in 2011, we increased the FWP to 20 contact hours over 16 weeks (groups 5 and 6). This decision was also informed by the USPSTF 2010 systematic review that concluded moderate-to high-intensity behavioral treatment (> 25 contact hours) is effective whereas lower-intensity treatment is not.8 Resource constraints precluded delivering a full 25 hours of treatment in the FWP.
The FWP intervention materials, protocol, and training manual were adapted from the FOCUS trial.12 Both interventions emphasized basic nutrition and physical activity education as well as behavioral monitoring, goal setting, contingency management, environmental control, and relapse prevention. The FWP Health Coach prescribed standardized weekly goals during the first 4 weeks of treatment to help families learn and apply the behavioral skills. For the remainder of the intervention, families were encouraged to set their own weekly goals and implement the skills that best fit the family’s needs and situations (as if choosing from tools in a toolbox). Content of the 12- and 16-week versions of the FWP did not differ. The additional contact hours reinforced previous content and largely focused on implementing behavior change strategies in families’ day-to-day lives. One author (PL) reviewed audio tapes of group sessions and qualitatively assessed them for treatment fidelity. The intervention team met weekly for supervision and discussion.
Measures
Parent and child BMI and child quality-of-life data were collected at baseline and at program completion. Children and parents were weighed weekly using standard anthropometric procedures in light clothing (no shoes) using a digital scale (Scaletronix; Wheaton, IL) with 0.1-kg accuracy.17 At baseline and after treatment, study staff weighed participants at least 3 times until agreement within 0.1 lb. Height was also measured at these same time points for children and once at baseline for adults using a Holtain stadiometer (Holtain; Crosswell, Wales) with 0.5 cm accuracy. Child BMI Z-scores (Z-BMI) were calculated using Centers for Disease Control and Prevention growth charts.18,19
Parents were asked to complete a self-administered survey that included child quality of life and parent/child use of behavioral skills. Child quality of life was measured by parent proxy-report using the Pediatric Quality of Life Inventory, version 4.0.20,21 The instrument is responsive and distinguishes healthy children from ill children. The Pediatric Quality of Life Inventory meets reliability criteria for group and individual comparisons (self-report Cronbach α = 0.88; proxy-report Cronbach α = 0.90).20
Parents were asked to rate the frequency of their use of behavioral skills in the past 3 weeks on a 5-point Likert scale (1 = never; 5 = very often) at baseline and at program completion. Skills included: 1) setting and reviewing goals for child’s eating and physical activity, 2) monitoring child’s eating and physical activity behaviors, 3) praising child for healthy eating and physical activity, and 4) improving parent’s own health behaviors (ie, modeling healthy diet and physical activity). These items were used in the FOCUS trial; validation studies are underway (Brian Saelens, PhD, personal communication, June 30, 2013).a
Parent and child demographic characteristics were assessed at baseline by parent report (Table 1). Health Coaches recorded attendance at weekly sessions. Program completion was defined post hoc as attending either 1) at least 75% of sessions or 2) more than 50% including the last session.
Table 1.
Demographic characteristics of enrolled Family Wellness Program (FWP) participants by subgroup
| Participant characteristics | All enrolled families n = 38a (%) | Families who completeda,b n = 24 (%) | Families sampled for interviewc n = 16a (%) |
|---|---|---|---|
| Children | |||
| Female | 25 (66) | 15 (63) | 11 (69) |
| Race/ethnicity | |||
| Non-Hispanic | |||
| White | 19 (50) | 13 (54) | 6 (38) |
| All other races | 6 (16) | 4 (17) | 2 (13) |
| More than one race | 7 (18) | 3 (13) | 4 (25) |
| Hispanic | |||
| White | 3 (8) | 2 (8) | 2 (13) |
| More than one race | 3 (8) | 2 (8) | 2 (13) |
| Age, years | |||
| 6–7 | 6 (16) | 4 (17) | 1 (6) |
| 8–9 | 7 (18) | 4 (17) | 4 (25) |
| 10–13 | 24 (63) | 16 (67) | 11 (69) |
| Parents | |||
| Female | 36 (95) | 23 (96) | 14 (88) |
| Married or partnered | 25 (66) | 16 (67) | 10 (63) |
| Education | |||
| < High school | 4 (11) | 4 (17) | 2 (13) |
| High school or GED | 6 (16) | 3 (13) | 1 (6) |
| Some college | 6 (16) | 2 (8) | 3 (19) |
| College or higher | 19 (50) | 14 (58) | 9 (56) |
| Annual household income | |||
| < $25,000 | 5 (13) | 3 (13) | 1 (6) |
| $25,000 – $49,999 | 7 (18) | 4 (17) | 3 (19) |
| ≥ $50,000 | 24 (63) | 17 (71) | 11 (69) |
Data were missing for child age (1 family), parental education (1 family) and household income (2 families).
Families were considered to have completed the FWP if they attended at least 75% of the intervention, or more than 50% including the last session.
Sixteen families were chosen by purposive sampling and invited to participate in qualitative interviews. This sample included families who did and did not complete the FWP.
GED = general educational development.
Quantitative Analyses
Descriptive statistics were used to characterize the sample and summarize results. Engagement differences between the 12- and 16-week programs were examined using Welsch’s independent samples t test. Pre- and posttreatment differences in BMI outcomes and quality of life among those who completed the program were explored using Wilcoxon matched-pairs signed-rank tests. We also estimated mean differences using paired t tests to facilitate comparison with the extant literature.22 We were not able to collect outcome data on individuals who did not complete the program, so an intent-to-treat analysis of before-and-after data was not possible. Demographic and engagement characteristics of the interview sample were compared with all enrolled FWP participants using descriptive statistics and the Fisher exact test. Quantitative analyses were conducted using Stata, version 12.1 (StataCorp, College Station, TX).
Social Support Interviews and Qualitative Analyses
We used purposive sampling to identify 16 parents for qualitative interviews, including some who did and some who did not complete the FWP. Two research assistants conducted 60- to 90-minute face-to-face qualitative interviews within 2 months after treatment. They followed a semistructured interview guide, using an open-ended interviewing style that allowed the interviewer to elicit the participant’s narrative.
Interviews focused on participants’ perceptions, experiences, and opinions related to presence or absence of social support for making family-based changes to diet and physical activity and implementing the program’s behavioral skills. Examples of questions are “What does it mean to you to say that a relationship is supportive?”; “If you decided to make changes to support your child’s healthy eating, how could a friend or family member help support you in that?”; and “Do you feel that participating in the Family Wellness Program affected any of your relationships in any way, positive or negative?”
Each interview was audio-recorded and transcribed for qualitative analysis. One author (KR) used an a priori manual to code each transcript for types of positive and negative support. Two authors (KR and PL) met weekly to review codes and discuss issues of discordance. After all support codes were assigned, these two researchers examined quotations to explore the relationship between social support and making healthy lifestyle changes. They continued meeting weekly to discuss and refine the results.
Human Subjects
At baseline, parents provided informed consent; children provided informed assent before participation. In the first 4 FWP groups, families received a $20 incentive for completing the baseline and follow-up assessments. In the last 2 groups, parent feedback led to replacing the monetary incentives with a weekly prize drawing for children who were present and had met weekly goals (valued at $10 to $40). All study activities were approved by the GH institutional review board.
Results
Participants
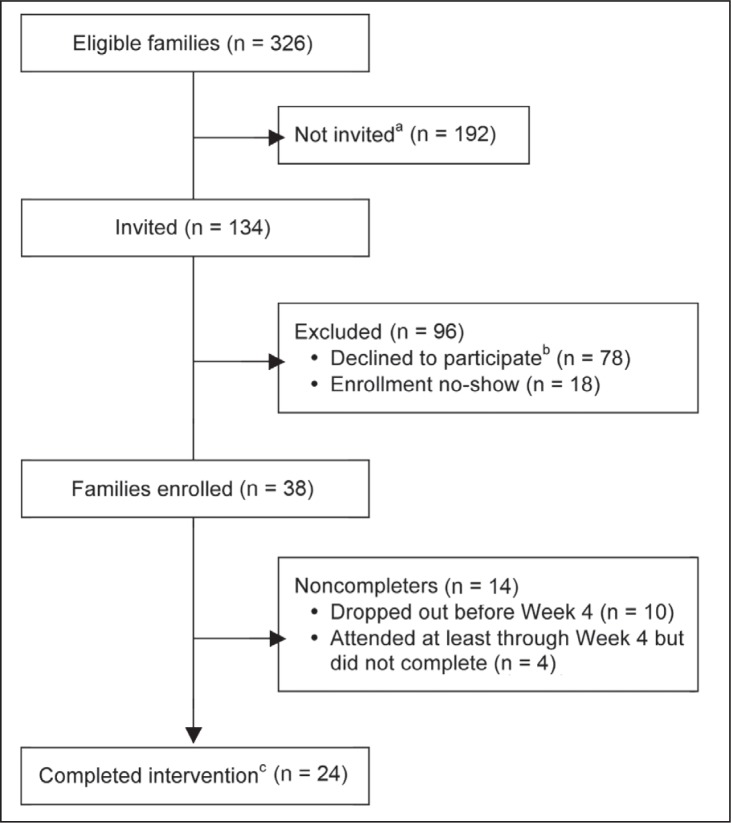
Thirty-eight families enrolled in the FWP (28% of the 134 pairs invited; see Figure 1). Demographic characteristics are reported in Table 1. The mean age (standard deviation [SD]) of enrolled children was 10.1 (2.0) years; 34% were boys; and 50% were white, non-Hispanic. Parents were mostly female (95%), 66% were married, 50% had a college degree or higher, and about two-thirds (63%) had an annual household income of $50,000 or more. Characteristics of program completers were similar to those of all enrolled families (Table 1). The subset of participants sampled for qualitative interviews is shown in the rightmost column and discussed separately below.
Figure 1.
Family Wellness Program recruitment and retention.
aPhone invitations were no longer made after groups were filled.
bIncludes families who refused the study invitation and those who were interested but not available.
cFamilies were considered completers if they attended at least 75% of the intervention, or more than 50% including the last session.
Recruitment and Retention
Of 38 enrolled parent-child pairs, 24 (63%) completed the program and another 4 (11%) attended at least 4 sessions but did not complete the program. Ten (26%) dropped out of the program within the first 3 weeks of treatment. Completers attended an average (SD) of 79% (12%) of all sessions. There were no differences between the 12- and 16-week programs in the proportion of sessions attended (p = 0.86) or completed (p = 0.72).
Quantitative Findings
Parent Report of Behavioral Skills Use
Parents who completed the program reported an increase in recent use of each of the key behavioral skills at the end of treatment compared with baseline (Table 2). About 70% of parents reported setting and reviewing goals for their child’s eating or physical activity after treatment, whereas only about half (46%) reported actually monitoring these behaviors. Slightly more than half (54%) reported praising their child for making healthy choices after treatment. Self-reported changes in parental behaviors (ie, modeling) were more common for eating than for physical or sedentary activity (79% and 33% after treatment, respectively).
Table 2.
Parent self-reported use of behavioral skills among those who completed the Family Wellness Program (n = 24)
| Skill used often or very often in the past 3 weeksa | Pretreatment n (%) | Posttreatment n (%) |
|---|---|---|
| Set and reviewed child’s goals | ||
| Either eating or physical activity goals | 7 (29.2) | 17 (70.8) |
| Eating goals | 2 (8.3) | 13 (54.2) |
| Physical activity goals | 6 (25.0) | 14 (58.3) |
| Monitored child’s habits | ||
| Either food and drink or physical activity | 2 (8.3) | 11 (45.8) |
| Food and drink | 2 (8.3) | 9 (37.5) |
| Physical activity | 1 (4.4) | 8 (33.3) |
| Praised child | ||
| Either healthy eating or physical activity | 10 (41.7) | 13 (54.2) |
| Healthy eating | 5 (21.7) | 13 (54.2) |
| Physical activity | 9 (37.5) | 7 (29.2) |
| Improved own habits | ||
| Any physical activity improvement | 5 (21.7) | 8 (33.3) |
| Increased own physical activity | 3 (13.0) | 7 (29.2) |
| Decreased own sedentary behavior | 5 (21.7) | 6 (25.0) |
| Any diet improvement | 8 (34.8) | 19 (79.2) |
| Decreased own calories | 4 (17.4) | 15 (62.5) |
| Decreased own unhealthy foods | 5 (21.7) | 15 (62.5) |
| Increased own fruits and vegetables | 6 (26.1) | 16 (66.7) |
5-point Likert scale dichotomized as Often/Very often (4 or more) vs Sometimes/Rarely/Never (3 or less)
Health Outcomes
Child and parent BMI outcomes and child quality of life results among the 24 families who completed the program are shown in Table 3. Among child completers, mean change (SD) in Z-BMI was −0.1 (0.1) (p < 0.001); nearly half (46%) had a Z-BMI reduction of 0.1 or greater after treatment. Mean change in BMI percentile among child completers was −0.8 (p < 0.006). Mean parent BMI change among completers was −0.9 (p = 0.003). Two-thirds (67%) of parent completers had a BMI reduction of 0.1 or greater. Child quality-of-life composite score rose by a mean of 8.5 points from a baseline of 71.2 (p = 0.002) (parent-report). In addition, child quality of life significantly improved from baseline to posttreatment in each separate domain: physical, emotional, social, and school functioning (Table 3). Notably, the proportion of children with meaningfully impaired quality-of-life scores (defined as ≥ 1 SD below the population mean)23 dropped by half from pre- to post-treatment (n = 8 [33%] to n = 4 [16%], respectively). Linear regression models showed that weight status at baseline was not associated with either changes in child or parent weight outcomes or child quality of life.
Table 3.
Pre- and posttreatment results among families who completed the Family Wellness Program (n = 24)
| Outcomes | Median | Wilcoxon signed-rank | Mean (SD) | Paired t-test | |||
|---|---|---|---|---|---|---|---|
| Pre- | Post- | Pa | Pre- | Post- | Δ | Pa | |
| Weight | |||||||
| Child BMI percentile | 98.8 | 98.3 | < 0.001 | 98.2 (1.4) | 97.4 (2.5) | −0.8 (1.4) | 0.006 |
| Child BMI Z-score | 2.2 | 2.1 | < 0.001 | 2.2 (0.4) | 2.1 (0.5) | −0.1 (0.1) | < 0.001 |
| Parent BMI | 34.5 | 33.8 | 0.012 | 36.4 (8.1) | 35.5 (8.2) | −0.9 (1.5) | 0.003 |
| Child quality of lifeb | |||||||
| Overall score | 71.7 | 75.0 | 0.002 | 71.2 (20.4) | 79.7 (16.1) | 8.5 (12.5) | 0.002 |
| Subscales | |||||||
| Psychosocial | 72.9 | 77.1 | 0.003 | 70.3 (20.8) | 80.0 (16.0) | 8.4 (16.9) | 0.002 |
| Emotional | 65.0 | 77.5 | 0.004 | 69.1 (16.0) | 76.5 (16.7) | 7.0 (17.0) | 0.032 |
| Social | 72.5 | 72.5 | 0.019 | 68.8 (28.3) | 77.2 (20.3) | 8.4 (16.9) | 0.011 |
| School | 80.0 | 80.0 | 0.035 | 74.2 (23.7) | 79.1 (20.0) | 6.1 (10.8) | 0.006 |
| Physical | 76.6 | 84.4 | 0.003 | 71.7 (23.5) | 83.9 (16.6) | 12.1 (17.9) | 0.002 |
Qualitative Findings
Social Support Interviews
Demographic characteristics of the interview sample (n = 16) were similar to those of all enrolled families (Table 1); 69% of the interview sample completed the program compared with 63% of all enrolled (p = 0.74). Parents interviewed valued social support for making healthy changes to diet and physical activity. Parents who felt successful in the FWP attributed much of their success to positive support they received from others. At the same time, parents who struggled with making changes to their family’s diet and physical activity ascribed it to negative support or lack of positive support from others. Representative quotations from interviews among parents who did and did not complete the FWP are shown in Sidebar: Selected Quotations from Parent Interviews: Social Support for Making Family-Based Healthy Lifestyle Changes.
Discussion
Results from this mixed-method pilot suggest that it may be feasible and acceptable to deliver family-based behavioral pediatric obesity treatment in a group format in a primary care setting. Only about one quarter of eligible families agreed to participate despite outreach and endorsement by primary care clinicians. However, our retention rate (63%) was similar to other group-based clinical behavioral treatment programs.8 Moreover, program duration (13 hours in 12 weeks vs 20 hours in 16 weeks) did not appear to affect retention, which suggests a more intensive group program may also be acceptable. Participating families represented the demographic characteristics of GH’s membership and of the geographic region, although college-educated parents were overrepresented.
Although we were able to assess outcomes only of families who completed the FWP, our as-treated estimates of effect on weight outcomes and quality of life suggest that delivering this group-based behavioral obesity treatment to families in a primary care setting has the potential to result in improved outcomes. Families who completed the program reported an increase in behavioral skills use and experienced significant improvements in all health outcomes. Mean improvement in child Z-BMI was comparable with group family-based treatment interventions in the extant literature13,24–26 and was above the threshold for clinically meaningful improvement.27 In addition, change in child quality of life reflected clinically meaningful improvement. The proportion of children with significantly impaired quality of life23 dropped by half from before to after treatment.
The parents’ experiences regarding social support for weight management suggest opportunities for enhancing this type of treatment. Overall, parents described the lifestyle change process as disruptive and stressful, and they received varying amounts and types of support from friends and family. Parents who had a supportive social network ascribed some measure of their success in the program to the support they received. The universality of this desire for and appreciation of support suggests that attending to the social context of pediatric weight management could help boost program retention, adherence, and outcomes.
The major strength of this study is that it was conducted in a real-world health care setting, in contrast to the many behavioral pediatric weight management trials conducted in research settings. Other strengths include the adaptation of a curriculum that has been evaluated in randomized controlled clinical trials, assessment of BMI outcomes, and the use of a validated quality-of-life measurement tool.
Certain limitations of this pilot study should also be noted. As a pre-post study without a control group and with incomplete follow-up (loss of families who did not complete the FWP), this pilot can provide only limited evidence about the effect of the intervention. The lower number of contact hours—below USPSTF recommendations—is also a limitation but was consistent with our aim of determining the program’s acceptability and feasibility in this setting. Participants are admittedly self-selected, but the motivation required for behavior change programs usually dictates a certain degree of self-selection. Finally, we used a self-report behavioral skills use instrument that is unvalidated, but it has been used in research settings and is currently being validated (Brian Saelens, PhD, personal communication, June 30, 2013).a
To meet USPSTF recommendations for treating overweight and obese children and their families, evidence-based interventions must be adapted to address real-world challenges while maximizing their effective components. On the basis of this pilot study, group family-based treatment interventions based in primary care settings are a promising strategy for meeting this need. Nonetheless, important challenges remain. Recommended next steps are to conduct a randomized trial of this primary care-based group behavioral weight management program of moderate-to-high intensity (≥ 25 contact hours) and to evaluate key program processes and outcome measures, ideally with a 1-year follow-up to establish whether the approach is capable of producing significant, meaningful BMI changes. Future work should also address the feasibility of training primary care staff to deliver this intervention, for better generalizability. Acceptability and effectiveness may be enhanced by improving social support for making healthy lifestyle changes and by integrating the program more fully into the primary care setting through point-of-care enrollment, increased use of electronic health records, and ongoing relapse prevention support from primary care clinicians.
Selected Quotations From Parent Interviews: Social Support for Making Family-Based Healthy Lifestyle Changes
Noncompleters
I do have to say my son’s father was very pessimistic … because I brought home all this paperwork to fill out and he was just being really pessimistic about [child] being in the program. I mean, he didn’t feel like taking him back, and I’m like, “You guys are the main ones causing this issue with food with him, and you don’t want him to be in the program?” He was not supportive at all.
— Parent of 8-year-old child, 0% of intervention attended.
My mom will actually cook two meals. One, which she’ll eat with my dad and, two, whatever my daughter wants. And when I say no, she [child] gets angry, then she’ll go to my mom, and my mom will say yeah. My mom’s yes will override any decision I make.
— Parent of 13-year-old child, 50% of intervention attended.
When I would try to make healthy meals and stuff, my husband would basically say, “Well, I’m not the one that needs to lose weight.” And he would prepare a whole other meal. And of course, maybe his hamburgers looked more fun to eat than maybe a chicken breast. You can’t make kids eat what you cooked.
— Parent of 11-year-old child, 0% of intervention attended.
Completers
Now that my mom’s on board, I think it would be a lot easier to have a family gathering … because she’s gung-ho on this. Interviewer: That was a big transformation for her. Parent: Yeah, that was pretty huge. And honestly, if she hadn’t been on board, I’m not sure that we would have been successful [in the program]. Because we go there every day, you know? And she’s really changed her home environment, so that there’s not a lot of high-calorie foods for [my child] there.
— Parent of 8-year-old child, 92% of intervention attended.
[My husband] sets the pace. If I have something I want to go do, and [the kids] don’t want to come with me, and he’s willing to stay home, they stay home. So, instead of encouraging all of us to go do something, he tends to set a pattern. … [Grandma] likes to come over and hang out with the boys when I work, but she won’t come without cinnamon rolls or doughnuts or things that I’ve asked her numerous times not to. And, you know, [she says] “They’re kids. They’re going to outgrow it, they’ll work this off in a week, don’t worry about it …” So, yeah, she’s not so good on my support level as far as that goes. Emotionally, she’s very supportive of me, but not with what I’m trying to do with the kids.
— Parent of 11-year-old child, 75% of intervention attended.
Schedules are busy and we constantly sort of think, “Gosh, I don’t want to do this [healthy eating or physical activity].” But in our house, it’s just not an option. The one thing that I do feel about our family is that everyone has really come together. [Child] is kind of in the middle, and all the adults around, and even my kids and my niece and nephews, everyone’s been really supportive—that this is really serious for [child]. … I can remember [child’s dad] saying, “Well, you don’t need to go walking today, you can do it tomorrow.” But he’s kind of come around in that he has become very supportive. And he’s always checking in with [child], “How are you doing? Gosh, what should I do, should we go get this or should we go do that?” Or if they have an afternoon where the other two [kids] are off with their friends or doing something else, he’s really good about being like, “Let’s go outside and go for a walk.” Now he has taken on more of that supporter—kind of cheerleader—for [child].
— Parent of 13-year-old child, 83% of intervention attended.
Acknowledgments
We thank all the parents and children who participated in the Family Wellness Program; the research staff and clinic personnel who assisted with group sessions, particularly Pamela Mouser, MD; Jim Rogalla, PT; Allan Kam, PT; and Pam Rock, PT; Brian Saelens, MD, for sharing FOCUS trial protocols; and Group Health Research Institute for supporting this work.
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Professor of Pediatrics, University of Washington, Seattle, WA.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Approaching the Ideal
To approach the ideal, precise scientific knowledge of the body machine must be supplemented with a more empirical attitude in the practice of medicine.
— René Jules Dubos, PhD, 1901–1982, French-born American microbiologist, experimental pathologist, environmentalist, humanist and Pulitzer Prize winner
References
- 1.Ho M, Garnett SP, Baur L, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012 Dec;130(6):e1647–71. doi: 10.1542/peds.2012-1176. DOI: http://dx.doi.org/10.1542/peds.2012-1176. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010 Feb;125(2):361–7. doi: 10.1542/peds.2009-2037. DOI: http://dx.doi.org/10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- 3.Khaylis A, Yiaslas T, Bergstrom J, Gore-Felton C. A review of efficacious technology-based weight-loss interventions: five key components. Telemed J E Health. 2010 Nov;16(9):931–8. doi: 10.1089/tmj.2010.0065. DOI: http://dx.doi.org/10.1089/tmj.2010.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract. 2011 Jan;91(1):1–12. doi: 10.1016/j.diabres.2010.06.030. DOI: http://dx.doi.org/10.1016/j.diabres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Spahn JM, Reeves RS, Keim KS, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc. 2010 Jun;110(6):879–91. doi: 10.1016/j.jada.2010.03.021. DOI: http://dx.doi.org/10.1016/j.jada.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TN. Treating pediatric obesity: generating the evidence. Arch Pediatr Adolesc Med. 2008 Dec;162(12):1191–2. doi: 10.1001/archpedi.162.12.1191. DOI: http://dx.doi.org/10.1001/archpedi.162.12.1191. [DOI] [PubMed] [Google Scholar]
- 7.Klesges LM, Williams NA, Davis KS, Buscemi J, Kitzmann KM. External validity reporting in behavioral treatment of childhood obesity: a systematic review. Am J Prev Med. 2012 Feb;42(2):185–92. doi: 10.1016/j.amepre.2011.10.014. DOI: http://dx.doi.org/10.1016/j.amepre.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010 Feb;125(2):e396–418. doi: 10.1542/peds.2009-1955. DOI: http://dx.doi.org/10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000 Jan;19(1 Suppl):5–16. doi: 10.1037/0278-6133.19.suppl1.5. DOI: http://dx.doi.org/10.1037/0278-6133.19.Suppl1.5. [DOI] [PubMed] [Google Scholar]
- 10.Skelton JA, Beech BM. Attrition in paediatric weight management: a review of the literature and new directions. Obes Rev. 2011 May;12(5):e273–81. doi: 10.1111/j.1467-789X.2010.00803.x. DOI: http://dx.doi.org/10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homer CJ. Responding to the childhood obesity epidemic: from the provider visit to health care policy—steps the health care sector can take. Pediatrics. 2009 Jun;123(Suppl 5):S253–7. doi: 10.1542/peds.2008-2780B. DOI: http://dx.doi.org/10.1542/peds.2008-2780B. [DOI] [PubMed] [Google Scholar]
- 12.Saelens BE, Lozano P, Scholz K. A randomized clinical trial comparing delivery of behavioral pediatric obesity treatment using standard and enhanced motivational approaches. J Pediatr Psychol. 2013 Oct;38(9):954–64. doi: 10.1093/jpepsy/jst054. DOI: http://dx.doi.org/10.1093/jpepsy/jst054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janicke DM, Sallinen BJ, Perri MG, et al. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: outcomes from project STORY. Arch Pediatr Adolesc Med. 2008 Dec;162(12):1119–25. doi: 10.1001/archpedi.162.12.1119. DOI: http://dx.doi.org/10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.State & county quickfacts: King County, Washington [Internet] Washington, DC: US Census Bureau; [updated 2014 Mar 27; cited 2013 Jul 25]. Available from: http://quickfacts.census.gov/qfd/states/53/53033.html. [Google Scholar]
- 15.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007 Jul;26(4):381–91. doi: 10.1037/0278-6133.26.4.381. DOI: http://dx.doi.org/10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilfley DE, Kass AE, Kolko RP. Counseling and behavior change in pediatric obesity. Pediatr Clin North Am. 2011 Dec;58(6):1403–24. doi: 10.1016/j.pcl.2011.09.014. x. DOI: http://dx.doi.org/10.1016/j.pcl.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkner F, Tanner JM, editors. Human growth: a comprehensive treatise Volume 3: methodology, ecological, genetic, and nutritional effects on growth. 2nd ed. New York, NY: Plenum Press; 1986. [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Hyattsville, MD: National Center for Health Statistics; 2000. Dec 4, Advance data from vital and health statistics of the Centers for Disease Control and Prevention/National Center for Health Statistics, 314 [Internet] [cited 2014 Apr 10]. Available from: www.cdc.gov/nchs/data/ad/ad314.pdf. [Google Scholar]
- 19.Growth chart training: a SAS program for the CDC growth charts (ages 0 to <20 y) Atlanta, GA: Centers for Disease Control and Prevention; [Internet] [updated 2014 Mar 26; cited 2012 Oct 1]. Available from: www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 20.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001 Aug;39(8):800–12. doi: 10.1097/00005650-200108000-00006. DOI: http://dx.doi.org/10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the Pediatric Quality of Life inventory. Med Care. 1999 Feb;37(2):126–39. doi: 10.1097/00005650-199902000-00003. DOI: http://dx.doi.org/10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B. Fundamentals of biostatistics. 6th ed. Belmont, CA: Thomson Brooks/Cole; 2006. [Google Scholar]
- 23.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003 Nov-Dec;3(6):329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. DOI: http://dx.doi.org/10.1367/1539-4409(2003)003%3C0329:TPAAPP%3E2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Golan M, Kaufman V, Shahar DR. Childhood obesity treatment: targeting parents exclusively v parents and children. Br J Nutr. 2006 May;95(5):1008–15. doi: 10.1079/bjn20061757. DOI: http://dx.doi.org/10.1079/BJN20061757. [DOI] [PubMed] [Google Scholar]
- 25.Goldfield GS, Epstein LH, Kilanowski CK, Paluch RA, Kogut-Bossler B. Cost-effectiveness of group and mixed family-based treatment for childhood obesity. Int J Obes Relat Metab Disord. 2001 Dec;25(12):1843–9. doi: 10.1038/sj.ijo.0801838. DOI: http://dx.doi.org/10.1038/sj.ijo.0801838. [DOI] [PubMed] [Google Scholar]
- 26.Kalavainen MP, Korppi MO, Nuutinen OM. Clinical efficacy of group-based treatment for childhood obesity compared with routinely given individual counseling. Int J Obes (Lond) 2007 Oct;31(10):1500–8. doi: 10.1038/sj.ijo.0803628. DOI: http://dx.doi.org/10.1038/sj.ijo.0803628. [DOI] [PubMed] [Google Scholar]
- 27.Hunt LP, Ford A, Sabin MA, Crowne EC, Shield JP. Clinical measures of adiposity and percentage fat loss: which measure most accurately reflects fat loss and what should we aim for? Arch Dis Child. 2007 May;92(5):399–403. doi: 10.1136/adc.2006.103986. DOI: http://dx.doi.org/10.1136/adc.2006.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]