Abstract
Thoracic endometriosis syndrome is the presence of endometrial tissue in or around the lung. Thoracic endometriosis syndrome consists of four distinct clinical entities: catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules. Thoracic endometriosis syndrome is a rare and complex condition, and diagnosis is often delayed or missed by clinicians, which can result in recurrent hospitalizations and other complications. Current treatments include hormone therapy and, where warranted, surgical intervention. We report the case of a 48-year-old woman with endometriosis causing bowel obstruction and concurrent catamenial pneumothorax.
Case Report
A 48-year-old woman was sent to the Emergency Department for further evaluation after being examined in the after-hours medical clinic. She had presented with one week of left-sided cramping abdominal pain with nonbloody, nonbilious emesis, and then developed diarrhea alternating with constipation. She denied fevers, chills, shortness of breath, chest pain, or urinary symptoms, and she reported regular menstrual cycles. She denied using cigarettes, alcohol, or recreational drugs and had no prior abdominal surgeries. Her family history was unremarkable. On examination she had tachycardia with diffuse abdominal tenderness and distention. Seven months before her presentation she was evaluated for right upper quadrant abdominal pain. An abdominal ultrasound at that time was normal, and the pain eventually resolved spontaneously.
In the Emergency Department, her temperature was 37.1°C, blood pressure was 147/108 mm Hg, pulse was 97 beats/min, respiratory rate was 17 breaths/min, and oxygen saturation was 99% on ambient air. She was alert and oriented and was in no distress. Her physical examination was notable for a mildly distended abdomen with high-pitched bowel sounds. She had equal breath sounds bilaterally. Her complete blood count was unremarkable other than a mild elevation in her platelet count of 573K/μL. Her chemistry panel demonstrated an increased anion gap metabolic acidosis (measured CO2, 19 mEq/L; anion gap, 19). Her lactic acid was normal. Renal and liver function tests were normal. A computed tomography (CT) scan of the abdomen and pelvis demonstrated small bowel and colonic dilation, with concern for an obstructing lesion at the distal sigmoid colon (Figure 1A). A small right pneumothorax (Figure 1B) was also identified. A nasogastric tube was placed for decompression. She underwent a colonoscopy to evaluate the etiology of her bowel obstruction, which revealed extrinsic compression causing sigmoid narrowing. The patient subsequently underwent a diagnostic laparoscopy and right chest tube placement. Laparoscopy revealed extensive peritoneal masses with concentric narrowing at the rectosigmoid junction with adhesions in the pelvis. Visualization was limited owing to the dilated bowel, so the decision was made to do exploratory laparotomy. She underwent rectosigmoid resection, appendectomy, diverting loop ileostomy, and total abdominal hysterectomy with bilateral salpingooophorectomy. Pathology results were consistent with implantation of endometrial tissue (Figures 2A and 2B). Given the extensive endometriosis, it was presumed that the pneumothorax was likely catamenial in nature. The chest tube was removed on postoperative day 2. Her diet was slowly advanced and she was discharged in good condition 6 days after her surgery.
Figure 1A.
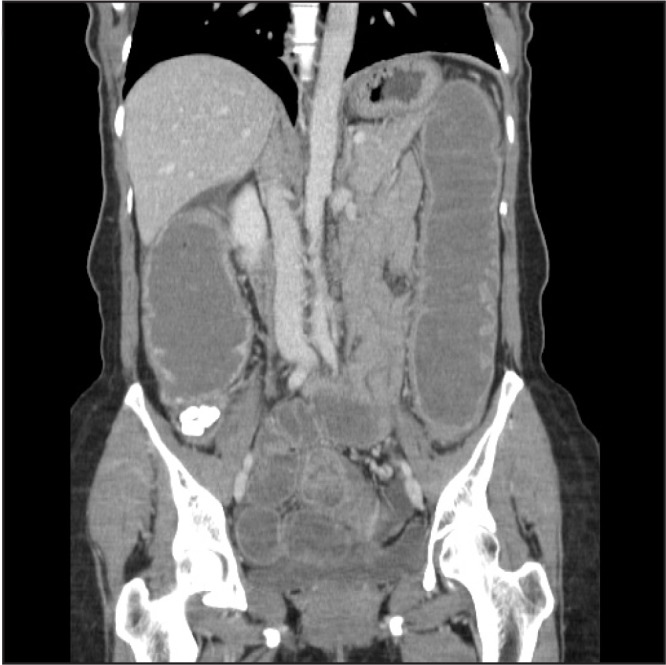
Computed tomography scan of the abdomen demonstrating small bowel and colonic dilation.
Figure 1B.
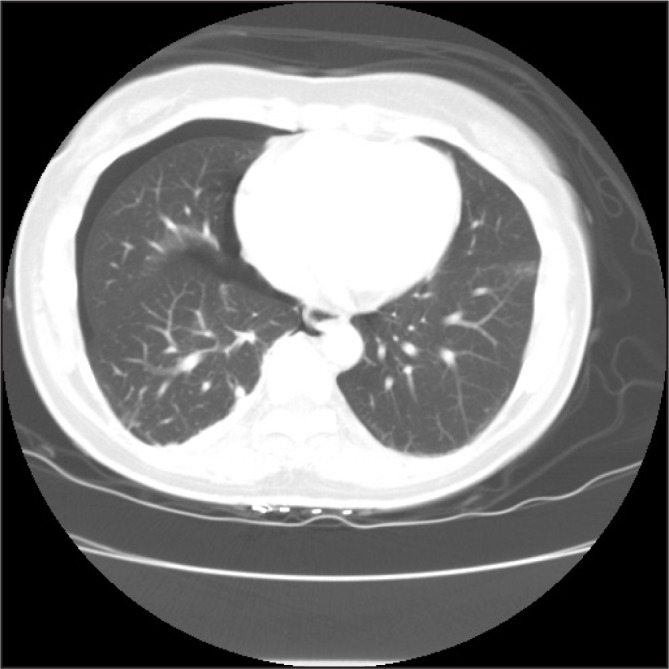
Computed tomography scan of the chest demonstrating a small, right-sided pneumothorax.
Figure 2A.
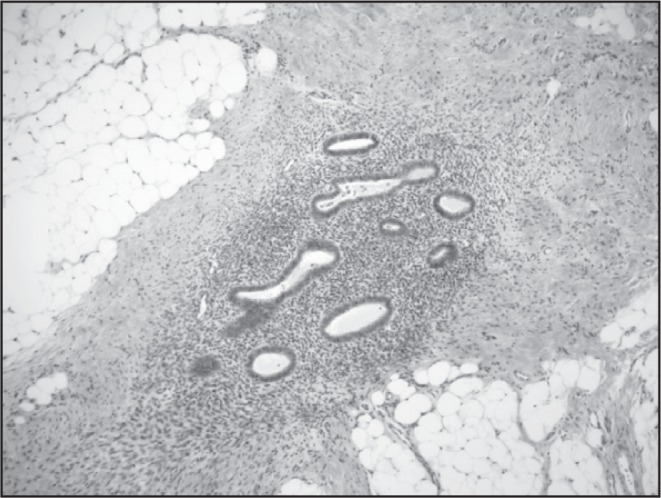
High-power photomicrograph of bowel endometriosis (haematoxylin and eosin stained; original magnification 40×).
Printed with permission from Danielle MP Cronin, MD.
Figure 2B.
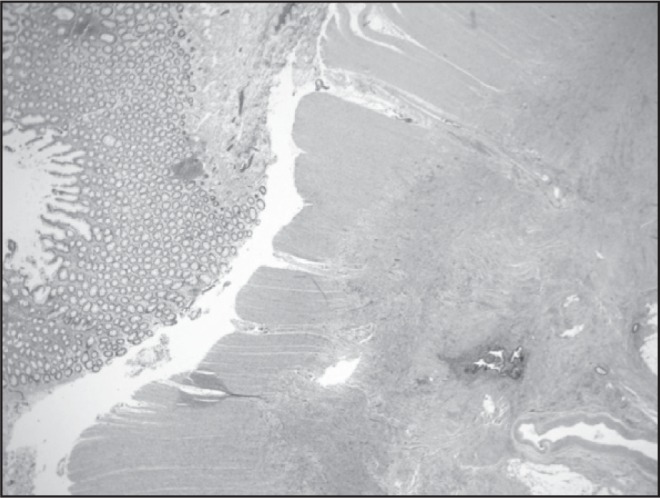
Low-power photomicrograph of bowel endometriosis (haematoxylin and eosin stained; original magnification 20×).
Printed with permission from Danielle MP Cronin, MD.
General Overview
Endometriosis is the condition wherein endometrial tissue is present outside of the uterine cavity. It is encountered most commonly in pelvic structures such as the ovary, uterine ligaments, pelvic peritoneum, cervix, labia, and vagina.1 Thoracic endometriosis syndrome (TES) is the presence of endometrial tissue in or around the lung. TES consists of 4 distinct clinical entities: catamenial pneumothorax (CP), catamenial hemothorax (CHx), hemoptysis, and pulmonary nodules. Although endometriosis in general can affect up to 15% of women in their reproductive years, TES remains an exceedingly rare condition.2,3
Described as early as 1912 by Hart,4 endometriosis is documented as causing pulmonary lesions consisting of endometrial glands and stroma.5 In 1938, Schwartz6 described a woman with inguinal node endometriosis with hemoptysis with a “lung tumor” that bled with every menstrual cycle. Since then, endometriosis has been documented in the lung, bronchi, pleura, and diaphragm. In the past 100 years, there has been improved understanding of the prevalence, clinical manifestations, diagnosis, and treatment of TES. The two largest retrospective studies in this period noted the peak incidence of TES occurs between ages 30 to 35 years, with CP the most common presentation of TES.7,8
It remains unclear how endometrial tissue migrates to the thoracic cavity. In 1927, the concept of retrograde menstruation, or the reflux of endometrial tissue from the uterus to the peritoneum via the fallopian tubes, was introduced as the etiology of peritoneal endometrial implants.9 Once in the peritoneal cavity, endometrial tissue was thought to travel to the thorax via diaphragmatic fenestrations.2,3,10,11 These fenestrations were believed to be either congenital or the result of direct erosion by endometrial implants12 and could be as large as four inches in diameter11 (Figure 3). Alternative theories for the presence of endometrial tissue in the lungs have been presented, including coelomic metaplasia, or the transformation of peritoneal/pleural epithelium into endometrial tissue under the influence of physiologic stimuli.1,3 This theory explains the presence of endometrial tissue in patients without a uterus including men on prolonged estrogen therapy.13 However, it fails to explain the right-sided thorax predominance seen in most TES cases. An additional theory is that endometrial transplantation occurs through lymphatic/vascular microembolization, explaining the presence of both intrapulmonary and other extra-uterine sites of implantation.3
Figure 3.
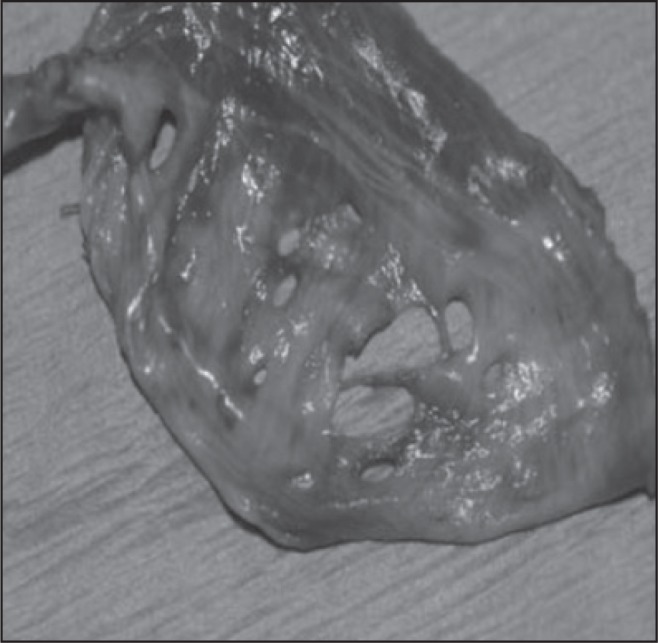
An example (not from our patient) of large diaphragmatic fenestrations.
Reprinted from the Journal of Emergency Medicine, 43(1), Makhija Z, Marrinan M, A case of catamenial pneumothorax with diaphragmatic fenestrations, e1–3, 2012, with permission from Elsevier.
TES occurs almost exclusively in the right hemithorax (approximately 95% of cases).7,8 There are several potential reasons for this laterality. Physiologically, peritoneal fluid moves in a clockwise fashion from the pelvis along the right paracolic gutter to the subphrenic space. Endometrial tissue located within the peritoneum likely follows the same directional flow, landing more commonly on the right hemidiaphragm. Once there, the falciform ligament prevents further travel of tissue to the left. Additionally, interperitoneal pressure variation with respiration causes the right hemidiaphragm to contract against the liver, known as the “piston effect,” which potentially allows for endometrial implantation and/or migration across the diaphragm. Finally, although congenital diaphragmatic hernias are far more common on the left side, congenital diaphragmatic defects, particularly fenestrations, are known to occur more commonly on the right, leading to the right-sided predominance of TES.14
Specific Clinical Entities
Catamenial Pneumothorax
CP is responsible for only 2.5% to 5% of cases of women with spontaneous pneumothorax10,15 even though it accounts for 73% of the cases of TES.7 The first case of CP was described by Maurer et al12 in 1958, but the term catamenial pneumothorax was not introduced until 1972.16 CP is typically defined as spontaneous and recurrent pneumothorax occurring within 72 hours from the onset of menstruation.7,10 According to Karpel et al,17 the number of recurrent pneumothoraces can range from 2 to 42 per patient.
Three theories have developed to explain this entity. The first is transdiaphragmatic passage, or movement, of air from the vagina to the peritoneum via the fallopian tubes, and subsequently to the thorax via diaphragmatic fenestrations. This is thought to occur during the menstrual cycle when the cervical mucus plug is absent.3,12,14 The second is air leakage triggered by sloughing of the endometrial implants located on the pleura.3,14 The third proposes a hormonally mediated mechanism in which high levels of sprostaglandin from thoracic endometrial implants cause vascular and bronchiolar vasoconstriction, leading to ischemic injury and ultimately causing alveolar rupture and subsequent air leakage.14 The etiology of CP is likely multifactorial given that none of these theories alone accounts for the varied clinical presentations of CP.
Patients with CP most often report right-sided pleuritic chest pain and dyspnea.2,14 One study found that right scapular pain was highly specific for the diagnosis of CP.18 Accurate diagnosis is enhanced by heightened clinical suspicion regarding women in their reproductive years who present with chest pain or spontaneous pneumothorax.
Catamenial Hemothorax
CHx accounts for approximately 14% of cases of TES3,7 but overall is a very rare cause of pleural effusions. As with CP, the right hemithorax is involved in most cases, although both bilateral19 and left-sided CHx have been described.20 Clinical presentation typically includes acute onset of chest pain and dyspnea. Diagnostic imaging reveals pleural effusions ranging from 200 mL to 2000 mL in size.19 CHx is associated with pelvic/pleural endometrial implants and diaphragmatic defects as seen on thorascopy,7,21 indicating that the pathogenesis likely involves migration of endometrial tissue from the pelvis to the thorax, consistent with the theory of retrograde menstruation.
Catamenial Hemoptysis
Catamenial hemoptysis (CHy) is an uncommon manifestation of TES, accounting for only 7% of cases, and is rarely mentioned in the general differential diagnosis of hemoptysis. Unlike other forms of TES, pleuritic chest pain is not a common clinical presentation. Symptoms may not occur with every menstrual cycle although they can still occur frequently throughout a person’s lifetime. One woman was reported to have hemoptysis with more than 200 menstrual cycles.22 It is presumed to be caused by endobronchial or parenchymal endometrial tissue deposits,23 the presence of which is best explained by the microembolization theory. In a small retrospective study of patients with CHy in South Korea, 16 of 19 study subjects had a history of obstetric or gynecologic procedures before developing hemoptysis.23 Interestingly, this supports the notion that trauma and uterine manipulation are predisposing factors for microembolization.7 Although no deaths from CHy have been reported, it may be life threatening owing to its associated risk of asphyxiation.23
Pulmonary Nodules
Pulmonary nodules are a rare effect of TES, accounting for approximately 6% of cases of TES.7 As such, TES is almost never mentioned as a possible cause of a newly discovered pulmonary nodule. The most common clinical manifestation of TES lung nodules is hemoptysis, because the proliferating endometrial implant(s) may communicate with a nearby bronchus, but the nodules are often asymptomatic.7 Pulmonary nodules appear to occur more frequently in older women (mean age, 38 to 39 years) compared with the other manifestations of TES (mean age, 34 years).5,7,8 The endometrial implants may be surrounded by fibrotic tissue and therefore can present as a mass or infiltration on lung imaging that can be mistaken for malignancy. In one report, a pulmonary nodule appeared as a well-demarcated subpleural ovoid tumor with cavitation.24 Symptoms do not necessarily correlate with menses, which may delay diagnosis.
Diagnosis
Heightened clinical suspicion is the key to diagnosis of TES. A woman in her reproductive years who reports chest pain, dyspnea, or cough around the time of her menstrual cycle should clue the physician to the possibility of TES. Physical examination may reveal diminished or absent breath sounds on the affected side, suggesting pleural effusions and/or pneumothorax. Chest radiography may reveal pneumothorax, pleural effusions, or pulmonary nodules.
Both CT and magnetic resonance imaging have been shown to be useful in the diagnosis of TES. CT imaging may reveal diaphragmatic endometrial implants as hypo-attenuating areas or identify single or multiple pulmonary nodules. Unfortunately, findings on CT in patients with hemoptysis are nonspecific but may sometimes be represented as a focal ground-glass opacity or consolidation.25 One study of five women with CHy found CT scans performed during and two weeks after the menstrual cycle allowed for precise perioperative localization of parenchymal endometrial tissue.26 Magnetic resonance imaging may be superior to CT not only because of less radiation exposure but also in differentiating pleural from parenchymal implants.27 Endometrial implants on magnetic resonance imaging, however, will be hyper-intense, not hypoattenuating as seen on CT.25
There is a limited role for bronchoscopy in diagnosis because most pathologic features are located in the peripheral lung. However, Hao-Chen et al28 found that bronchoscopy was useful if performed within 1 to 2 days of the onset of menses. Shiota et al29 were able to make the diagnosis of pulmonary endometriosis by cytologic examination of bronchial washings, although earlier studies indicated that the diagnostic yield of bronchial brushings or washing is very low.7,29 Additionally, bronchoscopy may play a role in the localization of a bleeding lobe or segment of the lung in CHy cases.3
Video-assisted thorascopic surgery allows direct visualization of the lung and diaphragmatic surfaces, and descriptions of the findings include perforations on the surface of the diaphragm, brown and violet endometrial deposits, and larger masses.3,30,31
Treatment
The cornerstone of TES management is the suppression of ovarian estrogen secretion, commonly with oral contraceptives, progesterone agents, danazol, or gonadotropin-releasing hormone (GnRH) agonists. Since 1994, the latter 2 have been used the most frequently. GnRH analogs cause GnRH receptor down-regulation, creating reversible hypogonatrophic hypogonadism, making it the treatment of choice for women wishing to preserve fertility. Although it is conveniently administered via monthly intramuscular depot injections, it is expensive and requires a long duration of therapy to achieve control.14 Danazol directly prevents the midcycle luteinizing hormone surge, resulting in anovulation and thereby decreasing the secretion of estradiol. The optimal duration of both therapies is uncertain.14 Unfortunately, treatment with hormonal therapy alone is associated with a recurrence rate exceeding 50% within 6 months after treatment was stopped.7,8,14,32 Because there has been no demonstrated difference in efficacy between these drugs, the drug cost, patient preference, and side effects such as hot flashes, depression, and osteoporosis may dictate which agent to use.32,33
Hysterectomy with bilateral salpingooophorectomy, although effective, results in permanent infertility and does not address dormant endometrial implants that may become active with administration of exogenous estrogen.7,14,20
Patients for whom hormonal therapy has failed may benefit from surgical treatment. This is best accomplished by video-assisted thorascopic surgery, which allows multiple treatment modalities to be implemented: 1) resection of areas of the lung and diaphragm where endometrial implants are present24,32,34; 2) closure of diaphragmatic fenestrations with mechanical staplers, sutures,24,32 or placement of a diaphragmatic patch21; and 3) abrasive mechanical pleurodesis32,35 and chemical pleurodesis with tetracycline, minocycline, or talc.7,32,34 Ikeda et al36 found that the use of polyglactin mesh on the lung and polyglycolate felt on the diaphragm (causing fibrotic adhesions) was successful in treating 4 patients with CP, all of whom remained symptom free for 5 years after surgery. According to Härkki et al,37 the ideal surgical approach would entail maximizing pleural adhesion to the thoracic wall, removing any potential space for pathologic features. Because of its ability to address most of the intrathoracic pathologic features, a surgical approach has been suggested to be superior to hormonal therapy alone.7,32 However, it has also been shown that surgical treatment followed by hormonal therapy was associated with no recurrence in a follow-up period as long as 45 months,38 and therefore, a combination of surgical and hormonal therapy may be the preferred approach for treatment and prevention.
Of note, once the diagnosis of TES is established, it is prudent to have proper gynecologic follow-up for these patients because TES can be a marker of concurrent severe pelvic endometriosis, which potentially has serious implications on a woman’s fertility and overall health.39
Conclusion
Because TES is a rare and complex condition, diagnosis is often delayed or missed by clinicians. To avoid such issues and implement appropriate treatment, a high index of suspicion is essential in any woman of reproductive age or receiving hormone replacement therapy who is experiencing cyclical chest pain, dyspnea, and/or hemoptysis. Hormone therapy is a suitable first-line treatment because it is less invasive and can preserve fertility. However, surgical intervention is available for women for whom medical therapy fails or who have a high burden of disease.
Acknowledgments
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Journey
From childhood to maturity
And youth to age;
From innocence to knowing;
From foolishness to discretion,
And then, perhaps, to wisdom;
From weakness to strength—
And often back again;
From health to sickness,
And back, we pray to health again …
Birth is a beginning
And death is a destination
And life is a journey.— Jewish prayer as recited in Synogogue
References
- 1.Agarwal N, Subramanian A. Endometriosis— morphology, clinical presentations and molecular pathology. J Lab Physicians. 2010 Jan;2(1):1–9. doi: 10.4103/0974-2727.66699. DOI: http://dx.doi.org/10.4103/0974-2727.66699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinatier D, Orazi G, Cosson M, Dufour P. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001 May;96(1):21–34. doi: 10.1016/s0301-2115(00)00405-x. DOI: http://dx.doi.org/10.1016/S0301-2115(00)00405-X. [DOI] [PubMed] [Google Scholar]
- 3.Alifano M, Trisolini R, Cancellieri A, Regnard JF. Thoracic endometriosis: current knowledge. Ann Thorac Surg. 2006 Feb;81(2):761–9. doi: 10.1016/j.athoracsur.2005.07.044. DOI: http://dx.doi.org/10.1016/j.athoracsur.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Hart C. Histologisch benigne metastasen vom bau eines adenomyoms 22 jahre nach exstirpation eines tumors der genitalien [Article in German] Frankf Z Pathol. 1912;10:78–90. [Google Scholar]
- 5.Di Palo S, Mari G, Castoldi R, Staudacher C, Taccagni G, Di Carlo V. Endometriosis of the lung. Respir Med. 1989 May;83(3):255–8. doi: 10.1016/s0954-6111(89)80044-7. DOI: http://dx.doi.org/10.1016/S0954-6111(89)80044-7. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz OH. Discussion. In: Counsellor VS. Endometriosis, a clinical and surgical review. Am J Obstet Gynecol. 1938;36:887. [Google Scholar]
- 7.Joseph J, Sahn SA. Thoracic endometriosis syndrome: new observations from an analysis of 110 cases. Am J Med. 1996 Feb;100(2):164–70. doi: 10.1016/s0002-9343(97)89454-5. DOI: http://dx.doi.org/10.1016/S0002-9343(97)89454-5. [DOI] [PubMed] [Google Scholar]
- 8.Channabasavaiah AD, Joseph JV. Thoracic endometriosis: revisiting the association between clinical presentation and thoracic pathology based on thoracoscopic findings in 110 patients. Medicine (Baltimore) 2010 May;89(3):183–8. doi: 10.1097/MD.0b013e3181df67d5. DOI: http://dx.doi.org/10.1097/MD.0b013e3181df67d5. [DOI] [PubMed] [Google Scholar]
- 9.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–69. [Google Scholar]
- 10.Alifano M, Roth T, Broët SC, Schussler O, Magdeleinat P, Regnard JF. Catamenial pneumothorax: a prospective study. Chest. 2003 Sep;124(3):1004–8. doi: 10.1378/chest.124.3.1004. DOI: http://dx.doi.org/10.1378/chest.124.3.1004. [DOI] [PubMed] [Google Scholar]
- 11.Makhija Z, Marrinan M. A case of catamenial pneumothorax with diaphragmatic fenestrations. J Emerg Med. 2012 Jul;43(1):e1–3. doi: 10.1016/j.jemermed.2009.05.023. DOI: http://dx.doi.org/10.1016/j.jemermed.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Maurer ER, Schaal JA, Mendez FL., Jr Chronic recurring spontaneous pneumothorax due to endometriosis of the diaphragm. J Am Med Assoc. 1958 Dec 13;168(15):2013–4. doi: 10.1001/jama.1958.63000150008012c. DOI: http://dx.doi.org/10.1001/jama.1958.63000150008012c. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga M. Paratesticular endometriosis in a man with a prolonged hormonal therapy for prostatic carcinoma. Pathol Res Pract. 2012 Jan 15;208(1):59–61. doi: 10.1016/j.prp.2011.10.007. DOI: http://dx.doi.org/10.1016/j.prp.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Papafragaki D, Concannon L. Catamenial pneumothorax: a case report and review of the literature. J Womens Health (Larchmt) 2008 Apr;17(3):367–72. doi: 10.1089/jwh.2007.0553. DOI: http://dx.doi.org/10.1089/jwh.2007.0553. [DOI] [PubMed] [Google Scholar]
- 15.Shearin RP, Hepper NG, Payne WS. Recurrent spontaneous pneumothorax concurrent with menses. Mayo Clin Proc. 1974 Feb;49(2):98–101. DOI: http://dx.doi.org/10.1097/00006254-197501000-00015. [PubMed] [Google Scholar]
- 16.Lillington GA, Mitchell SP, Wood GA. Catamenial pneumothorax. JAMA. 1972 Mar 6;219(10):1328–32. DOI: http://dx.doi.org/10.1001/jama.1972.03190360038009. [PubMed] [Google Scholar]
- 17.Karpel JP, Appel D, Merav A. Pulmonary endometriosis. Lung. 1985;163(3):151–9. doi: 10.1007/BF02713817. DOI: http://dx.doi.org/10.1007/BF02713817. [DOI] [PubMed] [Google Scholar]
- 18.Rousset-Jablonski C, Alifano M, Plu-Bureau G, et al. Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum Reprod. 2011 Sep;26(9):2322–9. doi: 10.1093/humrep/der189. DOI: http://dx.doi.org/10.1093/humrep/der189. [DOI] [PubMed] [Google Scholar]
- 19.Ravindran P, Raj RJ, Parameswaran K. Concurrent catamenial hemothorax and hemopneumothorax. Chest. 1993 Feb;103(2):646–8. doi: 10.1378/chest.103.2.646. DOI: http://dx.doi.org/10.1378/chest.103.2.646. [DOI] [PubMed] [Google Scholar]
- 20.Joseph J, Reed CE, Sahn SA. Thoracic endometriosis. Recurrence following hysterectomy with bilateral salpingo-oophorectomy and successful treatment with talc pleurodesis. Chest. 1994 Dec;106(6):1894–6. doi: 10.1378/chest.106.6.1894. DOI: http://dx.doi.org/10.1378/chest.106.6.1894. [DOI] [PubMed] [Google Scholar]
- 21.Nwiloh J. Diaphragmatic patch: a useful adjunct in surgical treatment of recurrent cata-menial hemothorax. Rev Port Pneumol. 2011 Nov-Dec;17(6):278–80. doi: 10.1016/j.rppneu.2011.06.006. DOI: http://dx.doi.org/10.1016/j.rppneu.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Hibbard LT, Schumann WR, Goldstein GE. Thoracic endometriosis: a review and report of two cases. Am J Obstet Gynecol. 1981 May 15;140(2):227–32. doi: 10.1016/0002-9378(81)90112-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim CJ, Nam HS, Lee CY, et al. Catamenial hemoptysis: a nationwide analysis in Korea. Respiration. 2010;79(4):296–301. doi: 10.1159/000228831. DOI: http://dx.doi.org/10.1159/000228831. [DOI] [PubMed] [Google Scholar]
- 24.Lee CH, Huang YC, Huang SF, Wu YK, Kuo KT. Thoracic endometriosis: rare presentation as a solitary pulmonary nodule with eccentric cavitations. Thorax. 2009 Oct;64(10):919–20. doi: 10.1136/thx.2008.111294. DOI: http://dx.doi.org/10.1136/thx.2008.111294. [DOI] [PubMed] [Google Scholar]
- 25.Rousset P, Rousset-Jablonski C, Alifano M, Mansuet-Lupo A, Buy JN, Revel MP. Thoracic endometriosis syndrome: CT and MRI features. Clin Radiol. 2014 Mar;69(3):323–30. doi: 10.1016/j.crad.2013.10.014. DOI: http://dx.doi.org/10.1016/j.crad.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Chung SY, Kim SJ, Kim TH, et al. Computed tomography findings of pathologically confirmed pulmonary parenchymal endometriosis. J Comput Assist Tomogr. 2005 Nov-Dec;29(6):815–8. doi: 10.1097/01.rct.0000176014.37051.c7. DOI: http://dx.doi.org/10.1097/01.rct.0000176014.37051.c7. [DOI] [PubMed] [Google Scholar]
- 27.Cassina PC, Hauser M, Kacl G, Imthurn B, Schröder S, Weder W. Catamenial hemoptysis. Diagnosis with MRI. Chest. 1997 May;111(5):1447–50. doi: 10.1378/chest.111.5.1447. DOI: http://dx.doi.org/10.1378/chest.111.5.1447. [DOI] [PubMed] [Google Scholar]
- 28.Wang HC, Kuo PH, Kuo SH, Luh KT. Catamenial hemoptysis from tracheobronchial endometriosis: reappraisal of diagnostic value of bronchoscopy and bronchial brush cytology. Chest. 2000 Oct;118(4):1205–8. doi: 10.1378/chest.118.4.1205. DOI: http://dx.doi.org/10.1378/chest.118.4.1205. [DOI] [PubMed] [Google Scholar]
- 29.Shiota Y, Umemura S, Arikita H, et al. A case of parenchymal pulmonary endometriosis, diagnosed by cytologic examination of bronchial washing. Respiration. 2001;68(4):439. doi: 10.1159/000050545. DOI: http://dx.doi.org/10.1159/000050545. [DOI] [PubMed] [Google Scholar]
- 30.Alifano M, Vénissac N, Mouroux J. Recurrent pneumothorax associated with thoracic endometriosis. Surg Endosc. 2000 Jul;14(7):680. doi: 10.1007/s004640000110. [DOI] [PubMed] [Google Scholar]
- 31.Byanyima RK. Menstruation in an unusual place: a case of thoracic endometriosis in Kampala, Uganda. Afr Health Sci. 2001 Dec;1(2):97–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall MB, Ahmed Z, Kucharczuk JC, Kaiser LR, Shrager JB. Catamenial pneumothorax: optimal hormonal and surgical management. Eur J Cardiothorac Surg. 2005 Apr;27(4):662–6. doi: 10.1016/j.ejcts.2004.12.047. DOI: http://dx.doi.org/10.1016/j.ejcts.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 33.Koizumi T, Inagaki H, Takabayashi Y, Kubo K. Successful use of gonadotropin-releasing hormone agonist in a patient with pulmonary endometriosis. Respiration. 1999 Nov-Dec;66(6):544–6. doi: 10.1159/000029433. DOI: http://dx.doi.org/10.1159/000029433. [DOI] [PubMed] [Google Scholar]
- 34.Tsunezuka Y, Oda M, Moriyama H, Ohshima M, Kurumaya H. Thoracoscopic findings and surgical management of catamenial hemopneumothorax. Ann Thorac Cardiovasc Surg. 2006 Jun;12(3):197–9. [PubMed] [Google Scholar]
- 35.Pappalardo E, Laungani A, Durieux R, Dekoster G, Limet R. Catamenial pneumothorax: a case report and review of the literature. Acta Chir Belg. 2007 Nov-Dec;107(6):695–6. doi: 10.1080/00015458.2007.11680150. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda T, Sasaki M, Sakon K, Koshiji T. An effective method of pleurodesis involving absorbable mesh for repetitive catamenial pneumothorax. Eur J Cardiothorac Surg. 2012 Aug;42(2):370–2. doi: 10.1093/ejcts/ezs101. DOI: http://dx.doi.org/10.1093/ejcts/ezs101. [DOI] [PubMed] [Google Scholar]
- 37.Härkki P, Jokinen JJ, Salo JA, Sihvo E. Menstruation-related spontaneous pneumothorax and diaphragmatic endometriosis. Acta Obstet Gynecol Scand. 2010 Sep;89(9):1192–6. doi: 10.3109/00016349.2010.493194. DOI: http://dx.doi.org/10.3109/00016349.2010.493194. [DOI] [PubMed] [Google Scholar]
- 38.Leong AC, Coonar AS, Lang-Lazdunski L. Cata-menial pneumothorax: surgical repair of the diaphragm and hormone treatment. Ann R Coll Surg Engl. 2006 Oct;88(6):547–9. doi: 10.1308/003588406X130732. DOI: http://dx.doi.org/10.1308/003588406X130732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriano D, Schonman R, Gat I, et al. Thoracic endometriosis syndrome is strongly associated with severe pelvic endometriosis and infertility. J Minim Invasive Gynecol. 2012 Nov-Dec;19(6):742–8. doi: 10.1016/j.jmig.2012.08.773. DOI: http://dx.doi.org/10.1016/j.jmig.2012.08.773. [DOI] [PubMed] [Google Scholar]


