Abstract
Diagnosis and treatment of catatonia in the psychiatry consultation service is not infrequent. Usually, the patient either presents to the Emergency Department or develops catatonia on the medical floor. This condition manifests with significant behavioral changes (from mildly decreased speech output to complete mutism) that interfere with the ability to communicate. After structural brain disorders are excluded, one of the diagnoses that always should be considered is catatonia. However, the causes of catatonia are numerous, ranging from psychiatric causes to a plethora of medical illnesses. Therefore, it is not surprising that there are many proposed underlying mechanisms of catatonia and that controversy persists about the etiology of specific cases.
There are only 6 reports of hyponatremia-induced catatonia and psychosis in the literature. Here, we present the case of a 30-year-old woman with catatonia and psychosis induced by hyponatremia, and we use this report to exemplify the multitude of biologic causes of catatonia and to propose a new way to look at the neuroanatomical basis of processing, particularly the vertical processing systems we believe are involved in catatonia.
Introduction
Catatonia is a frequently diagnosed disorder in psychiatry. The following scenarios are the most common: the patient arrives at the Emergency Department with behavioral changes that interfere with communication, or on the medical floor the patient develops significant behavioral changes that interfere with communication. The main features of catatonia are the same, regardless of the cause, and the clinical picture is dominated by three or more of the following symptoms: stupor, cataplexy, waxy flexibility, negativism, mutism, posturing, mannerism, stereotypy, agitation, grimacing, echolalia, and echopraxia.1
The differential diagnostic should include illnesses that mimic catatonia, such as akinetic Parkinson disease, malignant hyperthermia, stiff-person syndrome, conversion disorder, selective mutism (selective mutism is a social anxiety disorder in which people who can speak normally in some situations cannot speak in other situations—especially in performance scenarios), locked-in syndrome, and other hypokinetic and hyperkinetic states.2 Selective mutism, as seen in manifestations of personality disorders, malingering disorder, or factitious disorder, does not share the other features of catatonia and is relatively easily excluded.
After the patient is seen by the neurology and the psychiatry service and structural brain damage (such as stroke, tumor, or abscess) in the dominant hemisphere as well as severe dementia or delirium are excluded, the next line of differential diagnosis will include other medical conditions, including metabolic, neurologic, and substance-induced disorders. According to a review of 261 cases of catatonia, mental illness contributed to only up to 25% of those cases.3
Historically, catatonia is related to schizophrenia and other mental illnesses, such as severe depression, bipolar disorder, and psychosis.4 However, the causes of catatonia are numerous, ranging from psychiatric to medical illnesses. Therefore, it is not surprising that there are several proposed underlying mechanisms of catatonia—including top-down modulation, cholinergic and serotoninergic rebound hyperactivity, sudden and massive blockade of dopamine, and hyperactivity of glutamate.
One theory suggests that catatonia involves a “top-down modulation” in self-related processing of basal ganglia resulting from a deficiency of gamma aminobutyric acid (GABA).5 Top-down modulation is described as a bidirectional process that determines our ability to focus on stimuli relevant to our needs and to ignore background information. Therefore, successful interplay between the enhancement and suppression of the neuronal activity generates the contrast necessary for successful representation of relevant information. Benzodiazepines bind to a specific site on a GABA receptor, making it more efficient. As a result there is an increase in chlorine ions that leads to an increase in polarization of postsynaptic neurons, therefore making them less excitable and more able to filter the relevant stimuli. One report states that malignant catatonia can occur in the setting of benzodiazepine withdrawal.6 Other research suggests that hyperactivity of glutamate can be another underlying chemical dysfunction,7 especially at the decrease in N-methyl d-aspartate receptor.8
Catatonia can also happen in clozapine withdrawal. The proposed mechanism for clozapine-withdrawal-induced catatonia very likely happens owing to cholinergic and serotoninergic rebound hyperactivity.9,10
Significant dopamine blockade can also cause catatonia. England et al11 reported that first-generation antipsychotic medications caused worsening catatonia symptoms. However, second-generation antipsychotic medication on several occasions did treat the symptoms of catatonia.12 In addition, there are many reports that strongly suggest that stimulant medication can treat catatonia symptoms in patients with bipolar disorder13 or depression.14
Imaging results are different in various stages of catatonia. For example, in a patient with very-late-onset schizophrenia, hypoperfusion in the thalamus and striatum and hyperperfusion in the left lateral frontal cortex and left temporal cortex during the catatonia was reported. This scan was compared with the scan after the treatment.15 In another report,16 the imaging results of a patient with schizoaffective disorder in the acute phase of catatonia showed dramatic decrease in perfusion in the left parietal and motor cortex that reversed to normal after resolution of the episode. In addition, in cases of chronic catatonia, functional imaging identified abnormalities that happen bilaterally in the thalamus and frontal lobes.17
It is always interesting to look at the evolutionary perspective—is this part of a survival mechanism, when perceived impending doom leads to catatonia? And do the mechanisms presented above overlap with those circuits leading to what we morphonalytically assess as catatonia?18
Case Presentation
Ms A was a 30-year-old single woman living by herself and working as a factory operator for clean room production. She was born in the Philippines, came to the US as an infant, and was raised in San Diego, CA. She had an intact family and was the second of three children. Her father was in the military and was strict and a disciplinarian. He was occasionally physically abusive with her and her siblings. The patient was very close to her siblings and her mother.
In grammar school, she was a very good student. As a teen, she became more rebellious. She started college but was unable to finish. She dated, and her last relationship ended abruptly six months before she came in to the Emergency Department. Ms A was depressed as a result of that loss and received therapy through church counseling. Her depression improved without medications.
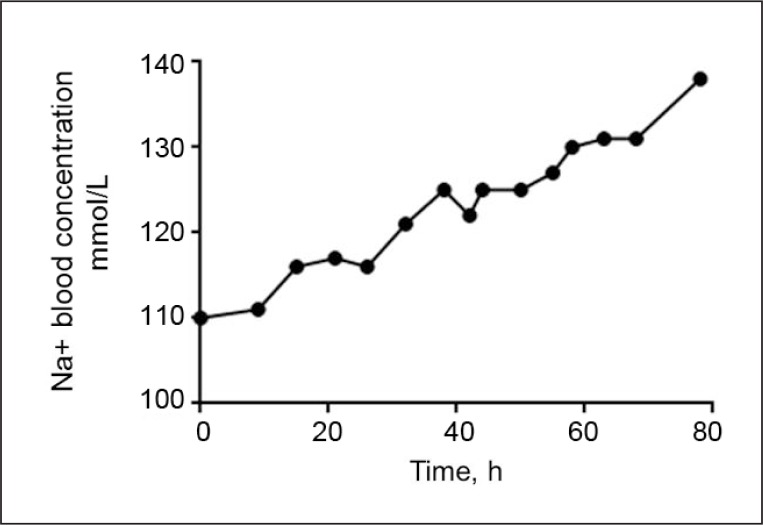
Her medical history was significant for papillary thyroid cancer, which was diagnosed 14 years before this incident and was successfully treated but had resurfaced recently. A few months after her recurrence of cancer, she received iodine-131 therapy for metastatic papillary thyroid cancer, and one day later she became confused and was found to have severe hyponatremia (Figure 1). At the same time she was taking the following medications: levothyroxine 125 mcg every morning, enoxaparin 40 mg subcutaneous every 24 hours, atenolol 50 mg daily, and docusate sodium 100 mg twice a day. On the second day of hospitalization, her confusion worsened, and the next day she developed auditory hallucinations, hearing Britney Spears sing. The patient was dancing: taking small steps, rotating 180 degrees, and repeating this about a dozen times. Then, she would touch the perfusion pole, approximately in the same place, and would repeat the sequence over a period of several hours. She would answer with one or two words to selective questions. When asked to hold a paper, she did and continued to hold it for the next 10 minutes. The psychiatrist ordered clonazepam 0.5 mg orally three times a day and risperidone 0.5 mg orally three times a day.
Figure 1.
Patient’s Na serum concentration reported during her hospitalization.
h = hours; mmol/L = millimoles per liter; Na = sodium.
The following day she was able to sit in a chair, stereotypic activity resolved, and she was able to recognize the psychiatrist. She answered most questions with complete sentences. When asked what happened the day before, she replied, “I had a mild concussion … which is severe … because it is a concussion.” In the following days she recovered entirely. Medications were stopped 3 months after her full recovery. There have been no similar psychiatric symptoms 18 months after her recovery.
Discussion
There have been some reports of hyponatremia following radio-contrast iodine therapy.19 The etiology is thought to be caused by a low-iodine diet and withdrawal of thyroxine therapy, which leads to a hypothyroid state.20,21 In this case, the iodine-131 protocol was followed, and she had no other changes in her medications or diet shortly before admission. In the absence of other identified confounding factors, we are suggesting that hyponatremia was caused by the radio-contrast iodine therapy.
Hyponatremia is defined as a decrease in serum sodium concentration below 135 mmol/L, and it can occur with high, normal, or low plasma tonicity.22 In hypervolemic hyponatremia, the body has too much water; this is generally caused by kidney, heart, or liver failure. Euvolemic hyponatremia (normovolemic state) is commonly caused by chronic health conditions including cancer (as in this case) or certain medications; it is often seen in syndrome of inappropriate antidiuretic hormone but also with primary polydipsia and low dietary solute intake. In hypovolemic hyponatremia, there is too little water; this can happen in certain conditions such as strenuous exercise in hot conditions, blood loss, gastrointestinal losses (vomiting or diarrhea), or renal losses (most often thiazide diuretics).
Hypotonicity carries a risk of inducing cerebral edema. The most common causes of hyponatremia are iatrogenic acquisition, cerebral salt wasting, and inappropriate secretion of antidiuretic hormone.23 The severity of symptoms is proportional to the severity of hyponatremia (usually more pronounced at concentrations less than 120 mmol/L) and the rate of sodium decline in serum.24 In general, hyponatremia is treated with 3% saline, or more rapidly with 120 mmol/L if the patient is symptomatic, and then the dosage is corrected more slowly, a maximum of 10 mmol to 12 mmol per day and/or 18 mmol/L for any 48 hours,25 slower if sodium has been chronically low. Each correction would need to be individualized on the basis of specifics of the patient course.26 Most common initial symptoms of symptomatic hyponatremia are a combination of confusion, anorexia, nausea and vomiting, muscle cramps, and aches. Catatonia symptoms may develop in rare situations, such as in this case. Neurologic examination can reveal cognitive changes and occasionally decreased deep tendon reflexes. Left untreated, patients can develop rapid complications from cerebral edema such as generalized tonic-clonic seizures, coma, and death as a result of brain herniation.
Catatonia has a clinical presentation that is similar regardless of the precipitating or causing factors. When a clinical presentation can be the result of various etiologies, it is also expected to have a variety of neurochemical dysfunctions identified as the underlying mechanisms. Knowing the specific dysfunction involved becomes paramount; we chose the medications to treat on the basis of the particular clinical presentation. In this case, by the time of the psychiatrist’s evaluation, the hyponatremia was resolved, but the psychosis and catatonia were getting worse. The prompt response can be explained, in our opinion, as follows: catatonia responded immediately and to a significant though incomplete degree to benzodiazepines; the second-generation antipsychotic medication helped with the residual catatonic symptoms and resolved the emerged psychosis over the next eight days.
There has been much progress in understanding catatonia since it was first described by Kahlbaum27 as an illness characterized by mood syndromes as primary features and by motor disturbances as characteristic features. Processing seems to be integrally involved in catatonia. A comprehensive discussion of the neuroanatomical basis of processing is beyond the scope of this article and has been addressed elsewhere.28
The neuroanatomic basis of processing includes vertical and horizontal processing systems and neuroplasticity. Neuroplasticity29 includes:
dendrite rebranching and its participation in formation of new associations
hippocampal learning with formation of new neurons in the hippocampus
synaptic processes that include a large array of long-term-memory-related mechanisms.
Horizontal processing systems include:
interhemispheric processing, which addresses hemispheric synchrony and is essential in processing30
intrahemispheric processing, which assures the basis for ipsilateral coordination between different brain structures.31
Vertical processing systems include:
self-related processing and subcortical and midline structures that assure integrative bodily functions and basic emotional systems32
re-entrance circuits that are essential in bidirectional mirroring between subcortical and cortical neural activity33–35
prefrontal-subcortical circuitry, which has a major role in mood regulation.36,37
We suggest that self-related processing is involved in catatonia. In addition, it is possible that the neurochemical dysfunction potentiates the observed response, such as the observed limited processing of the information from the outside world and behavioral abnormalities.
Self-related processing belongs to the vertical processing systems. It is attributed to a set of midline structures that start in the brain stem, in the reticular activating system, and are interconnected with higher brain structures in the subcortical and cortical areas, referred to as the subcortical-cortical mid-line system. They accomplish the integrative bodily functions and the convergence of basic emotional systems to form the proposed “bodily self or proto-self.”38–46 These regions are hierarchically organized and functionally connected. Of particular importance is the ascendant reticular activating system, which receives various direct and indirect collaterals with ultimate function of influencing various aspects of consciousness and wakefulness.47
Conclusion
Catatonia is a condition frequently identified in medical settings and is often induced by organic reasons. Even though the treatment for catatonia is essentially the same, as most of the patients respond well to benzodiazepines and electroconvulsive therapy, identifying the specific organic cause is as important as addressing the underlying condition.
The above-suggested involvement of self-related processing in the development of catatonia provides a unitary explanation for a multitude of described causes, and we believe that further research to elucidate the neuroanatomical pathways of this condition is warranted.
Acknowledgments
Leslie Parker, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
Dr Daniela Bota is on the Advisory Board of Genentec; is on the Advisory Board, the Scholar’s Bureau, and is a Consultant for NovoTTF; and is a Senior Scientific Advisor for ERC Belgium. The author(s) have no other potential conflicts of interest to disclose.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Levenson JL. Medical aspects of catatonia. Prim Psychiatry. 2009 Mar 1;16(3):23–6. [Google Scholar]
- 3.Thorpe LU, Keegan DL, Veeman GA. Conversion mutism: case report and discussion. Can J Psychiatry. 1985 Feb;30(1):71–3. doi: 10.1177/070674378403000114. [DOI] [PubMed] [Google Scholar]
- 4.Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010 Mar;36(2):314–20. doi: 10.1093/schbul/sbp059. DOI: http://dx.doi.org/10.1093/schbul/sbp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012 Feb;16(2):129–35. doi: 10.1016/j.tics.2011.11.014. DOI: http://dx.doi.org/10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M, Freeman S. Clonazepam withdrawal-induced catatonia. Psychosomatics. 2009 May-Jun;50(3):289–92. doi: 10.1176/appi.psy.50.3.289. DOI: http://dx.doi.org/10.1176/appi.psy.50.3.289. [DOI] [PubMed] [Google Scholar]
- 7.Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997 Apr;62(4):404–6. doi: 10.1136/jnnp.62.4.404. DOI: http://dx.doi.org/10.1136/jnnp.62.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000 Jul;5(7):26–33. doi: 10.1017/s1092852900013365. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Sur S, Singh A. Catatonia following abrupt stoppage of clozapine. Aust N Z J Psychiatry. 2011 Jun;45(6):499. doi: 10.3109/00048674.2011.564135. DOI: http://dx.doi.org/10.3109/00048674.2011.564135. [DOI] [PubMed] [Google Scholar]
- 10.Yeh AW, Lee JW, Cheng TC, Wen JK, Chen WH. Clozapine withdrawal catatonia associated with cholinergic and serotonergic rebound hyperactivity: a case report. Clin Neuropharmacol. 2004 Sep-Oct;27(5):216–8. doi: 10.1097/01.wnf.0000145506.99636.1b. DOI: http://dx.doi.org/10.1097/01.wnf.0000145506.99636.1b. [DOI] [PubMed] [Google Scholar]
- 11.England ML, Ongür D, Konopaske GT, Karmacharya R. Catatonia in psychotic patients: clinical features and treatment response. J Neuropsychiatry Clin Neurosci. 2011 Spring;23(2):223–6. doi: 10.1176/appi.neuropsych.23.2.223. DOI: http://dx.doi.org/10.1176/appi.neuropsych.23.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den Eede F, Van Hecke J, Van Dalfsen A, Van den Bossche B, Cosyns P, Sabbe BG. The use of atypical antipsychotics in the treatment of catatonia. Eur Psychiatry. 2005 Aug;20(5–6):422–9. doi: 10.1016/j.eurpsy.2005.03.012. DOI: http://dx.doi.org/10.1016/j.eurpsy.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Neuhut R, Levy R, Kondracke A. Resolution of catatonia after treatment with stimulant medication in a patient with bipolar disorder. Psychosomatics. 2012 Sep-Oct;53(5):482–4. doi: 10.1016/j.psym.2011.12.006. DOI: http://dx.doi.org/10.1016/j.psym.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Prowler ML, Weiss D, Caroff SN. Treatment of catatonia with methylphenidate in an elderly patient with depression. Psychosomatics. 2010 Jan-Feb;51(1):74–6. doi: 10.1176/appi.psy.51.1.74. DOI: http://dx.doi.org/10.1016/S0033-3182(10)70662-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsujino N, Nemoto T, Yamaguchi T, et al. Cerebral blood flow changes in very-late-onset schizophrenia-like psychosis with catatonia before and after successful treatment. Psychiatry Clin Neurosci. 2011 Oct;65(6):600–3. doi: 10.1111/j.1440-1819.2011.02257.x. DOI: http://dx.doi.org/10.1111/j.1440-1819.2011.02257.x. [DOI] [PubMed] [Google Scholar]
- 16.Galynker II, Weiss J, Ongseng F, Finestone H. ECT treatment and cerebral perfusion in catatonia. J Nucl Med. 1997 Feb;38(2):251–4. [PubMed] [Google Scholar]
- 17.Lauer M, Schirrmeister H, Gerhard A, et al. Disturbed neural circuits in a subtype of chronic catatonic schizophrenia demonstrated by F-18-FDG-PET and F-18-DOPA-PET. J Neural Transm. 2001;108(6):661–70. doi: 10.1007/s007020170043. DOI: http://dx.doi.org/10.1007/s007020170043. [DOI] [PubMed] [Google Scholar]
- 18.Moskowitz AK. “Scared stiff”: catatonia as an evolutionary-based fear response. Psychol Rev. 2004 Oct;111(4):984–1002. doi: 10.1037/0033-295X.111.4.984. DOI: http://dx.doi.org/10.1037/0033-295X.111.4.984. [DOI] [PubMed] [Google Scholar]
- 19.Kim SK, Yun GY, Kim KH, et al. Severe hyponatremia following radioactive iodine therapy in patients with differentiated thyroid cancer. Thyroid. 2014 Apr;24(4):773–7. doi: 10.1089/thy.2013.0110. DOI: http://dx.doi.org/10.1089/thy.2013.0110. [DOI] [PubMed] [Google Scholar]
- 20.Shakir MK, Krook LS, Schraml FV, Hays JH, Clyde PW. Symptomatic hyponatremia in association with a low-iodine diet and levothyroxine withdrawal prior to I131 in patients with metastatic thyroid carcinoma. Thyroid. 2008 Jul;18(7):787–92. doi: 10.1089/thy.2008.0050. DOI: http://dx.doi.org/10.1089/thy.2008.0050. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy VR, McDougall IR. Severe hyponatremia: a danger of low-iodine diet. Thyroid. 2007 Sep;17(9):889–92. doi: 10.1089/thy.2007.0094. DOI: http://dx.doi.org/10.1089/thy.2007.0094. [DOI] [PubMed] [Google Scholar]
- 22.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000 May 25;342(21):1581–9. doi: 10.1056/NEJM200005253422107. DOI: http://dx.doi.org/10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 23.Rabinstein AA, Wijdicks EF. Hyponatremia in critically ill neurological patients. Neurologist. 2003 Nov;9(6):290–300. doi: 10.1097/01.nrl.0000095258.07720.89. DOI: http://dx.doi.org/10.1097/01.nrl.0000095258.07720.89. [DOI] [PubMed] [Google Scholar]
- 24.Verbalis JG. Adaptation to acute and chronic hyponatremia: implications for symptomatology, diagnosis, and therapy. Semin Nephrol. 1998 Jan;18(1):3–19. [PubMed] [Google Scholar]
- 25.Sood L, Sterns RH, Hix JK, Silver SM, Chen L. Hypertonic saline and desmopressin: a simple strategy for safe correction of severe hyponatremia. Am J Kidney Dis. 2013 Apr;61(4):571–8. doi: 10.1053/j.ajkd.2012.11.032. DOI: http://dx.doi.org/10.1053/j.ajkd.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Mulloy AL, Caruana RJ. Hyponatremic emergencies. Med Clin North Am. 1995 Jan;79(1):155–68. doi: 10.1016/s0025-7125(16)30089-x. [DOI] [PubMed] [Google Scholar]
- 27.Peralta V, Cuesta MJ, Serrano JF, Martinez-Larrea JA. Classification issues in catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(Suppl 1):I14–6. doi: 10.1007/pl00014194. DOI: http://dx.doi.org/10.1007/PL00014194. [DOI] [PubMed] [Google Scholar]
- 28.Novac A, Bota RG. Neurobiological considerations of psychotherapeutic processing. Mental Illness Journal. 2014 doi: 10.4081/mi.2014.5077. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998 Mar 13;279(5357):1714–8. doi: 10.1126/science.279.5357.1714. DOI: http://dx.doi.org/10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 30.Pennebacker JW. Putting stress into words: health, linguistic, and therapeutic implications. Behav Res Ther. 1993 Jul;31(6):536–48. doi: 10.1016/0005-7967(93)90105-4. DOI: http://dx.doi.org/10.1016/0005-7967(93)90105-4. [DOI] [PubMed] [Google Scholar]
- 31.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003 Sep;126(Pt 9):2093–107. doi: 10.1093/brain/awg203. DOI: http://dx.doi.org/10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 32.Panksepp J, Northoff G. The trans-species core SELF: the emergence of active cultural and neuro-ecological agents through self-related processing within subcortical-cortical midline networks. Conscious Cogn. 2009 Mar;18(1):193–215. doi: 10.1016/j.concog.2008.03.002. DOI: http://dx.doi.org/10.1016/j.concog.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988 Oct;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. DOI: http://dx.doi.org/10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 34.Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003 Oct;160(10):1726–39. doi: 10.1176/appi.ajp.160.10.1726. DOI: http://dx.doi.org/10.1176/appi.ajp.160.10.1726. Erratum in: Am J Psychiatry 2003 Dec;16(12):2258. DOI: http://dx.doi.org/10.1176/appi.ajp.160.12.2258. [DOI] [PubMed] [Google Scholar]
- 35.Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J. Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997 Feb;76(4):957–1006. doi: 10.1016/s0306-4522(96)00405-8. DOI: http://dx.doi.org/10.1016/S0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- 36.Panksepp J. The periconscious substrates of consciousness: affective states and the evolutionary origins of the self. Journal of Consciousness Studies. 1998;5(5–6):566–82. [Google Scholar]
- 37.Watt DF. Consciousness and emotion: review of Jaak Panksepp’s ‘Affective Neuroscience’. Journal of Consciousness Studies. 1999;6(6–7):191–200. [Google Scholar]
- 38.Damasio A. The feeling of what happens: body and emotion in the making of consciousness. New York, NY: Houghton Mifflin Harcourt; 1999. [Google Scholar]
- 39.Strehler BL. Where is the self? A neuroanatomical theory of consciousness. Synapse. 1991 Jan;7(1):44–91. doi: 10.1002/syn.890070105. DOI: http://dx.doi.org/10.1002/syn.890070105. [DOI] [PubMed] [Google Scholar]
- 40.Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001 Apr;79(1–2):135–60. doi: 10.1016/s0010-0277(00)00127-x. DOI: http://dx.doi.org/10.1016/S0010-0277(00)00127-X. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003 Aug;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. DOI: http://dx.doi.org/10.1016/S0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 42.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002 Aug;3(8):655–66. doi: 10.1038/nrn894. DOI: http://dx.doi.org/10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 43.Panksepp J. At the interface of the affective, behavioral, and cognitive neurosciences: decoding the emotional feelings of the brain. Brain Cogn. 2003 Jun;52(1):4–14. doi: 10.1016/s0278-2626(03)00003-4. DOI: http://dx.doi.org/10.1016/S0278-2626(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 44.Panksepp J. On the embodied neural nature of core emotional affects. J Conscious Stud. 2005;12(8–10):158–84. [Google Scholar]
- 45.Denton D. The primordial emotions: the dawning of consciousness. New York, NY: Oxford University Press, USA; 2006. [Google Scholar]
- 46.Panksepp J. Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn. 2005 Mar;14(1):30–80. doi: 10.1016/j.concog.2004.10.004. DOI: http://dx.doi.org/10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Jellinger KA. [Functional pathophysiology of consciousness]. [Article in German] Neuropsychiatr. 2009;23(2):115–33. [PubMed] [Google Scholar]