Abstract
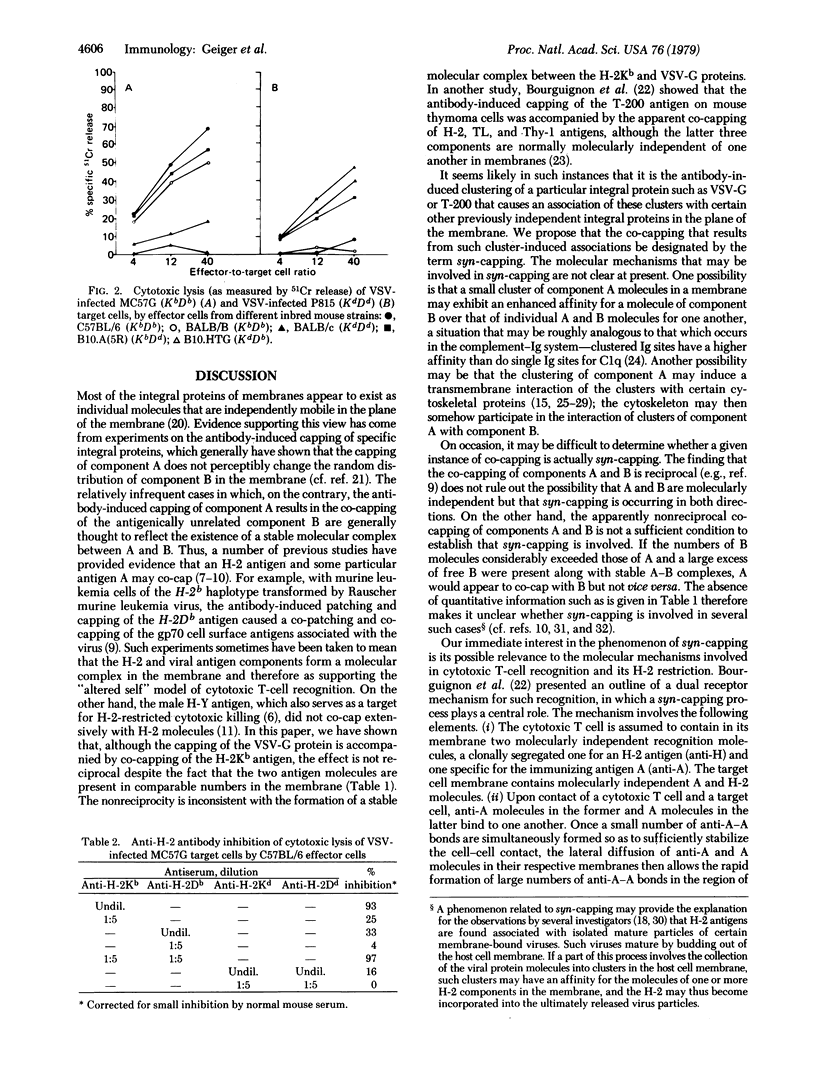
We have studied the co-redistribution of vesicular stomatitis virus (VSV) antigen and of individual H-2 antigens on the surfaces of mouse cells, and in parallel we have also used these VSV-infected cells as targets in cytotoxic T-cell killing experiments. Antibody-induced patching and capping of the VSV antigen caused an extensive co-patching and co-capping of the H-2Kb antigen but not of the H-2Db antigen. In reciprocal experiments, the antibody-induced patching of the H-2Kb or H-2Db antigen did not result in a co-patching of the VSV antigen. Radioimmunoassays showed that the relative numbers of H-2Kb, H-2Db, and VSV antigens on the surfaces of the cells exhibiting such nonreciprocal co-redistributions were closely similar. Furthermore, the H-2 restricted cytotoxic T-cell lysis of these target cells showed a marked preference for H-2Kb compared to H-2Db compatibility. We propose that the VSV and H-2 antigens are molecularly independent entities in the unpreturbed target cell membrane but that the antibody-induced clustering of the VSV antigen causes a selective and unidirectional co-redistribution (which we designate as syn-capping) of H-2Kb with the VSV antigen clusters. It is suggested that such a T-cell-induced syn-capping process involving an antigen and an H-2 molecule on the target cell may play a critical role in the mechanism of cytotoxic T-cell killing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Unanue E. R. Interrelationships of surface immunoglobulin and Fc receptors on mouse B lymphocytes. J Immunol. 1975 Dec;115(6):1665–1671. [PubMed] [Google Scholar]
- Ash J. F., Louvard D., Singer S. J. Antibody-induced linkages of plasma membrane proteins to intracellular actomyosin-containing filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5584–5588. doi: 10.1073/pnas.74.12.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J. F., Singer S. J. Concanavalin-A-induced transmembrane linkage of concanavalin A surface receptors to intracellular myosin-containing filaments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4575–4579. doi: 10.1073/pnas.73.12.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank K. J., Lilly F. Evidence for an H-2/viral protein complex on the cell surface as the basis for the H-2 restriction of cytotoxicity. Nature. 1977 Oct 27;269(5631):808–809. doi: 10.1038/269808a0. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubbers J. E., Chen S., Lilly F. Nonrandom inclusion of H-2K and H-2D antigens in Friend virus particles from mice of various strains. J Exp Med. 1978 Feb 1;147(2):340–351. doi: 10.1084/jem.147.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Geib R., Goldberg E. H., Klein J. Membrane-bound H-2 and H-y antigens move independently of each other. Nature. 1977 Nov 24;270(5635):352–354. doi: 10.1038/270352a0. [DOI] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Gordon R. D., Simpson E., Samelson L. E. In vitro cell-mediated immune responses to the male specific(H-Y) antigen in mice. J Exp Med. 1975 Nov 1;142(5):1108–1120. doi: 10.1084/jem.142.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale A. H., Witte O. N., Baltimore D., Eisen H. N. Vesicular stomatitis virus glycoprotein is necessary for H-2-restricted lysis of infected cells by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):970–974. doi: 10.1073/pnas.75.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Interactions of vesicular stomatitis virus with murine cell surface antigens. J Virol. 1976 Sep;19(3):833–845. doi: 10.1128/jvi.19.3.833-845.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Ash J. F. Use of the avidin-biotin complex for the localization of actin and myosin with fluorescence microscopy. J Cell Biol. 1977 Jun;73(3):783–788. doi: 10.1083/jcb.73.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The function and interrelationships of T-cell receptors, Ir genes and other histocompatibility gene products. Transplant Rev. 1975;22:175–195. doi: 10.1111/j.1600-065x.1975.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Loor F., Block N., Little R. J. Dynamics of the TL antigens on thymus and leukemia cells. Cell Immunol. 1975 Jun;17(2):351–365. doi: 10.1016/s0008-8749(75)80039-6. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. The molecular basis of the biological activities of complement. Harvey Lect. 1971;66:75–104. [PubMed] [Google Scholar]
- Parish C. R., Hayward J. A. The lymphocyte surface. I. Relation between Fc receptors, C'3 receptors and surface immunoglobulin. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):47–63. doi: 10.1098/rspb.1974.0060. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Senik A., Neauport-Sautes C. Association between H-2 and vaccinia virus-induced antigens on the surface of infected cells. J Immunol. 1979 Apr;122(4):1461–1467. [PubMed] [Google Scholar]
- Shearer G. M. Cell-mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974 Aug;4(8):527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Hard C. C. Actin co-caps with concanavalin A receptors. Nature. 1977 Oct 20;269(5630):695–697. doi: 10.1038/269695a0. [DOI] [PubMed] [Google Scholar]
- Zaleska-Rutczynska Z., Klein J. Histocompatibility-2 system in wild mice. V. Serologic analysis of sixteen B10.W congenic lines. J Immunol. 1977 Dec;119(6):1903–1911. [PubMed] [Google Scholar]
- Zarling D. A., Keshet I., Watson A., Bach F. H. Association of mouse major histocompatibility and Rauscher murine leukaemia virus envelope glycoprotein antigens on leukaemia cells and their recognition by syngeneic virus-immune-cytotoxic T-lymphocytes. Scand J Immunol. 1978;8(6):497–508. doi: 10.1111/j.1365-3083.1978.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Adler B., Holland J. J. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol. 1978;46(1-2):53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Holland J. Target antigens for H-2-restricted vesicular stomatitis virus-specific cytotoxic T cells. J Immunol. 1978 Aug;121(2):744–748. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]



