Abstract
More than 100 million Americans have prediabetes or diabetes. Prediabetes is a condition in which individuals have blood glucose levels higher than normal but not high enough to be classified as diabetes. People with prediabetes have an increased risk of Type 2 diabetes. An estimated 34% of adults have prediabetes. Prediabetes is now recognized as a reversible condition that increases an individual’s risk for development of diabetes. Lifestyle risk factors for prediabetes include overweight and physical inactivity.
Increasing awareness and risk stratification of individuals with prediabetes may help physicians understand potential interventions that may help decrease the percentage of patients in their panels in whom diabetes develops. If untreated, 37% of the individuals with prediabetes may have diabetes in 4 years. Lifestyle intervention may decrease the percentage of prediabetic patients in whom diabetes develops to 20%.
Long-term data also suggest that lifestyle intervention may decrease the risk of prediabetes progressing to diabetes for as long as 10 years. To prevent 1 case of diabetes during a 3-year period, 6.9 persons would have to participate in the lifestyle intervention program. In addition, recent data suggest that the difference in direct and indirect costs to care for a patient with prediabetes vs a patient with diabetes may be as much as $7000 per year. Investment in a diabetes prevention program now may have a substantial return on investment in the future and help prevent a preventable disease.
Introduction
In the US, 79 million adults have prediabetes,1 a prevalence approximately 3 times that of diabetes.1 Prediabetes is defined as a condition in which people have higher than normal blood glucose levels but not high enough for a diagnosis of diabetes.1 According to the American Diabetes Association, the diagnostic criteria for prediabetes is an elevated fasting plasma glucose level (100 mg/dL–125 mg/ dL), a glycated hemoglobin (HbA1c) value of 5.7% to 6.4%, or an elevated plasma glucose level after an oral glucose tolerance test (140–199 mg/dL).2 The diagnostic criteria for prediabetes and diabetes are shown in Table 1.
Table 1.
American Diabetic Association diagnostic criteria for normal glucose, prediabetes, and diabetes2
| Diabetes test | Normal | Prediabetes | Diabetes |
|---|---|---|---|
| Hemoglobin A1c, % | < 5.7 | 5.7–6.4 | ≥ 6.5 |
| Fasting blood glucose, mg/dL | < 100 | 100–125 | > 125 |
| Oral glucose tolerance, mg/dL | < 140 | 140–199 | > 199 |
Findings of the population-based US National Health and Nutrition Examination Survey suggest that 35% of US adults older than 20 years and 50% of those older than age 65 years have prediabetes.1 Around 5% to 10% of people with prediabetes become diabetic every year.3
The American Diabetes Association recommends that diabetes testing start at age 45 years for all adults who are overweight (body mass index [BMI] ≥ 25 kg/m2) and have any of the following additional risk factors4:
physical inactivity
hypertension or history of cardiovascular disease
low levels of high-density lipoprotein cholesterol and high triglycerides
first-degree relative with diabetes
history of previous elevated blood glucose level or HbA1c measurement
women with polycystic ovarian syndrome
history of gestational diabetes or giving birth to a baby weighing more than 4.082 kg (9 lb)
member of an ethnic or minority racial group.
Kaiser Permanente (KP), with approximately 8.5 million members, may be a microcosmic representation of the national data. It is estimated that KP has 852,031 patients who currently meet the criteria for prediabetes by laboratory testing (Table 2). Not all patients with prediabetes are overweight or obese. Data in Table 3 show that 81% of these patients in the Antelope Valley and Kern Service Areas are overweight or obese (BMI ≥ 25) and 19% are normal weight (BMI < 25).
Table 2.
Estimate of active members in Kaiser Permanente who screened “positive” for prediabetes in 2012a
| Regionb | Active members in diabetes risk cohortc | Percentage of membership age 10 to 75 years |
|---|---|---|
| Colorado | 32,973 | 7 |
| Georgia | 11,234 | 5 |
| Hawaii | 34,997 | 18 |
| Northern California | 327,845 | 12 |
| Northwest | 54,466 | 13 |
| Ohio | 4969 | 7 |
| Southern California | 385,547 | 13 |
| Kaiser Permanente | 852,031 | 12 |
Data specifications reflect an interregional diabetes risk cohort to be used for measurement purposes only and is not intended to inform clinical practice guidelines. Measurement period for membership data was January 1, 2012, through September 30, 2012. Measurement time for diagnoses and laboratory test results was January 1, 2006, through September 30, 2012. Data sources were Geographically Enriched Member Sociodemographics (GEMS) for Kaiser Permanente (KP) members and Clarity for diagnoses and laboratory test results.
Cohort excludes Mid-Atlantic data.
Inclusion criteria were active KP members age 10 through 75 years, including members with a diagnosis of gestational diabetes but no diagnosis of diabetes who had laboratory values in the prediabetes range: 2 laboratory values for fasting plasma glucose of 100 to 125 mg/dL or 1 laboratory value for hemoglobin A1C of 5.7% to 6.4%. Exclusion criteria were deceased members, non-KP members, and members with a diagnosis of diabetes (Type 1 or 2) or laboratory values in the range for diabetes.
Table 3.
Distribution of members with prediabetes by body mass index for Kaiser Permanente Southern California Antelope Valley and Kern Service Areas
| Body mass index, kg/m2 | Antelope Valley, no. (%) | Kern, no. (%) |
|---|---|---|
| ≥ 30 | 4403 (54) | 3534 (51) |
| 25–29 | 2192 (27) | 2098 (30) |
| < 25 | 1510 (19) | 1335 (19) |
| Total | 8105 (100) | 6967 (100) |
| ≥ 25 | 6595 (81) | 5632 (81) |
A systematic review of prospective studies confirms a strong, continuous association between HbA1c level and subsequent diabetes risk.5 Persons with an HbA1c value of 6.0% or above had a high risk for development of clinically defined diabetes. The 5-year risk of diabetes if the baseline HbA1c value was at least 6% ranged from 25% to 50%. The relative risk of diabetes was 20 times higher if the HbA1c was greater than or equal to 6% compared with an HbA1c of 5% or less. Persons with an HbA1c value between 5.5% and 6.0% also had a substantially increased risk of diabetes, with 5-year incidences ranging from 9% to 25%. The authors conclude that the level of HbA1c appears to have a continuous association with diabetes risk. According to an American Diabetes Association expert panel, up to 70% of individuals with prediabetes will eventually have diabetes. If current trends continue, 1 in 3 adults will have diabetes by 2050.6
The total estimated cost of diagnosed diabetes in 2012 is $245 billion. This included $176 billion in direct medical costs and $69 billion in indirect costs. The largest components of direct medical costs were hospital inpatient care and prescription medications. People with diagnosed diabetes incur average medical expenditures of about $13,700 per year, of which approximately $7900 is attributed to diabetes. The largest component of indirect costs was increased absenteeism and reduced productivity while at work.7
National annual medical costs of prediabetes in 2007 exceeded $25 billion, or an additional $443 for each adult with prediabetes.8 Several studies suggest that the long-term damage of end-organs associated with diabetes may start in prediabetes.9–11 In 2012, United Healthcare reported in Health Affairs that adults with diabetes have an average annual health care expenditure of $11,700 per person, whereas adults without diabetes have an average annual expenditure per person of $4400.12
Treatment
Lifestyle Intervention
Treatment of diabetes prevents some of its devastating complications but does not usually restore normal blood glucose levels.13,14 The diagnosis of diabetes is often delayed until complications are present.15 Because current methods of treating diabetes do not prevent all the complications associated with the condition, prevention of diabetes and even prediabetes is preferable. The Diabetes Prevention Program Research Group has published several studies showing that Type 2 diabetes may be preventable by diet and exercise.16–18
In 2002, Knowler et al16 hypothesized that lifestyle intervention would prevent or delay the development of diabetes. The researchers randomly assigned patients with prediabetes to receive a placebo or a lifestyle modification program with the goals of at least a 7% weight loss and at least 150 minutes of physical activity per week. The mean age of the participants was 51 years, and the BMI was 34.0 kg/m2. The average follow-up was 2.8 years. The incidence of diabetes was 11.0 and 4.8 cases per 100 person-years in the placebo and lifestyle groups, respectively. The lifestyle intervention reduced the incidence by 58% compared with placebo. Participants assigned to the lifestyle intervention had more weight loss and greater increase in physical activity than did participants in the placebo group.
The average weight loss was 0.1 kg and 5.6 kg in the placebo and lifestyle intervention groups, respectively (p < 0.001). Further analysis of the study showed that if patients with prediabetes received no intervention, diabetes would develop in approximately 37% in 4 years. The lifestyle modification program decreased the percentage of persons with prediabetes in whom diabetes developed in 4 years to approximately 20% (Figure 1).
Figure 1.
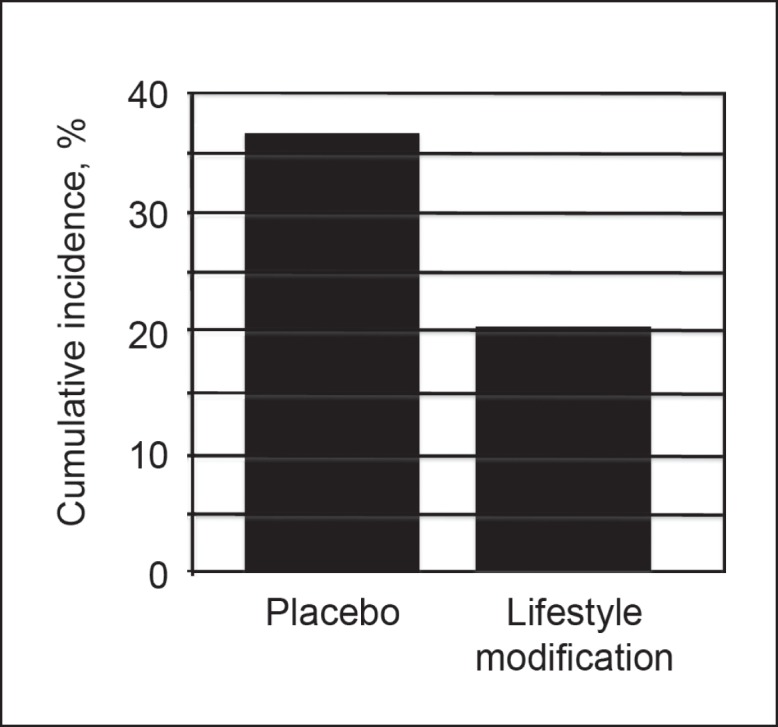
Cumulative incidence of diabetes during a four-year period in patients with prediabetes who engaged in a lifestyle modification program versus no lifestyle modification program (placebo).16
Unless people with prediabetes change their lifestyle, most will have Type 2 diabetes within the next 10 years, according to the National Institute of Diabetes and Digestive and Kidney Diseases.19 Lifestyle changes such as weight loss (7% of body weight) and moderate physical activity (150 minutes per week) can reduce the risk of diabetes by as much as 58%.20 To prevent 1 case of diabetes during a 3-year period, 6.9 persons would have to participate in the lifestyle intervention program.16
The Finnish Diabetes Prevention Study published in 200321 evaluated the effects of a lifestyle intervention on short-term and long-term changes in diet and exercise behavior, and the effect of the intervention on glucose and lipid metabolism. In the study, 522 middle-aged, overweight subjects with impaired glucose tolerance were randomly assigned to receive either usual care or an intensive lifestyle intervention. The control group received general advice about diet and exercise at baseline and had an annual physician’s examination. The subjects in the intervention group received additional individualized dietary counseling from a nutritionist. They were also offered circuit-type resistance training sessions and advised to increase overall physical activity. The intervention was the most intensive during the first year, followed by a maintenance period. The intervention goals were to reduce body weight, reduce intake of dietary and saturated fat, and increase physical activity and dietary fiber intake. The authors found that intensive lifestyle intervention produced long-term beneficial changes in diet, physical activity, and clinical and biochemical parameters and reduced diabetes risk. The incidence of diabetes was related to weight loss. The incidence of converting from prediabetes to diabetes was approximately 2% for subjects who lost at least 5% of their body weight vs about 8% for subjects who gained more than 2.5% of their body weight (p < 0.002).
In 2009, Knowler et al and the Diabetes Prevention Program Research Group17 investigated the persistence of the effects in the long term on the patients described in the study published by the same group in 2002. In this follow-up study, 88% of patients enrolled for a median additional follow-up of 5.7 years. On the basis of benefits from the intensive lifestyle intervention in the first study, all 3 groups were offered group-implemented lifestyle intervention. During the 10-year follow-up since randomization, the original lifestyle group lost, then partly regained weight. Diabetes incidence rates in this follow-up study were similar between treatment groups: 5.9 per 100 person-years for lifestyle intervention and 5.6 for placebo. Diabetes incidence in the 10 years since randomization was reduced by 34% in the lifestyle group compared with the placebo group. The authors concluded that prevention or delay of diabetes with lifestyle intervention could persist for at least 10 years.
In 2012, Perreault et al18 reported that patients with prediabetes that did not progress to diabetes after they completed an intensive lifestyle intervention were still at high risk for the development of diabetes. They also discovered that reversion to normal glucose levels, even transiently, was associated with a 56% reduced risk of future diabetes.
In 2013, Schellenberg et al20 compared the effectiveness of lifestyle interventions to standard care on minimizing progression of prediabetes to diabetes or reducing all-cause mortality in diabetes. This meta-analysis study identified 9 randomized, controlled trials with prediabetic patients who were at risk of diabetes and 11 randomized, controlled trials with patients who had diabetes. Seven of the 9 studies looking at patients who were at risk of diabetes reported that lifestyle interventions decreased the risk of diabetes up to 10 years after a lifestyle intervention.
Results of multiple trials support a long-term reduction in diabetes risk or a delay in onset of the disease as a result of lifestyle and drug-based intervention.22–24 In the 20-year follow-up of the Da Qing Diabetes Prevention Study, those receiving a lifestyle intervention had a 51% lower incidence of diabetes.24 Group-based lifestyle interventions over 6 years can prevent or delay diabetes for up to 14 years after the active intervention.
In 2011, Gong et al25 reported a study started in 1986 when 577 adults with impaired glucose tolerance from Da Qing, China, were randomly assigned by clinic to a control group or 1 of 3 lifestyle intervention groups (diet, exercise, and diet plus exercise). In 2006, the researchers conducted a 20-year follow-up study of the original participants to compare the incidence of retinopathy, nephropathy, and neuropathy in the intervention group vs the control group. Follow-up information was obtained on 542 (94%) of the original participants. After adjusting for clinic and age, the incidence of severe retinopathy was 47% lower in the intervention group than the control group. No significant differences were found in the incidence of severe nephropathy or in the prevalence of neuropathy.25
Pharmacologic and Surgical Intervention
Evidence of potential benefits from pharmacotherapy to prevent diabetes in patients with prediabetes was reported by Knowler et al16 in 2002. Biguanides, such as metformin, were shown by the investigators to decrease the incidence of diabetes but not as much as lifestyle interventions. Metformin, which has a good safety profile, has beneficial effects on BMI and lipid concentrations.26 In 2010, Lilly and Godwin27 concluded after a systematic review of the literature and meta-analysis that metformin lowers risk of Type 2 diabetes by 45%. The number needed to treat was between 7 and 14.27 The beneficial effects of metformin were greater in people who were prediabetic with a higher baseline BMI than in the individuals with a lower BMI.14
Thiazolidinediones (troglitazone, rosiglitazone, and pioglitazone) have been shown to reduce the incidence of diabetes in patients at risk of diabetes. However, risks of this medication, which may include hepatoxicity, weight gain, edema, and heart failure, outweigh the benefit in preventing prediabetes from progressing to diabetes.28–30 For example, troglitazone was withdrawn from the market in 2000 because of serious idiosyncratic hepatotoxicity.31
Although inhibitors of the reninangiotensin-aldosterone system may have a beneficial effect on reducing complications of prediabetes and diabetes, there is no evidence that they help in preventing prediabetes from progressing to diabetes. In morbidly obese people, bariatric surgery is associated with sustained weight loss and a substantial reduction in the two-year and ten-year incidence of diabetes.32,33
Discussion
By screening and risk-stratifying individuals as prediabetic, we may be able to develop a strategy to prevent prediabetes from progressing to diabetes. Clinical evidence suggests that we should not accept a prediabetic state but should actually try to convert prediabetes to a normal glucose state. Lifestyle and pharmacologic interventions by themselves may not help prevent long-term microvascular or macrovascular complications of prediabetes. Only by achieving a normal glucose state can we prevent complications of prediabetes and diabetes.
The identification and treatment of prediabetic individuals is therefore crucial to our efforts to make health care more affordable, prevent preventable disease, and save lives. Recent evidence presented in this article suggests that prevention of progression of prediabetes to diabetes and conversion of prediabetes to a normal glucose state is possible. On the basis of a literature review and published meta-analyses, we recommend that physicians screen and risk-stratify individuals with prediabetes. By doing so, we may be able to develop interventions that focus on the risk of the patient with prediabetes.
As shown in Table 4, it may be possible to risk-stratify individuals with prediabetes by HbA1c and BMI. All patients with prediabetes should complete the following goals: 1) lifestyle modification training, 2) 150 minutes per week of physical activity, and 3) 7% weight loss if BMI exceeds 25 kg/m2. For high-risk individuals with high BMI, pharmacologic and surgical interventions may be considered if these goals are not achieved. Metformin appears to have a good safety record and may help with weight and lipid management. Other antidiabetes medications may have risks that prohibit their use at this time.34
Table 4.
Proposed risk stratification and treatment strategies for prediabetes
| Risks and treatments | Low risk | Medium risk | High risk |
|---|---|---|---|
| Hemoglobin A1C % | 5.7–5.8 | 5.9–6.1 | 6.2–6.49 |
| Risk stratification | √ | √ | √ |
| Lifestyle modification, 16-week course | √ | √ | √ |
| Physical activity of at least 150 minutes per week | √ | √ | √ |
| Weight loss of 7% of body weight if BMI ≥ 25 kg/m2 | √ | √ | √ |
| Hemoglobin A1C < 5.7% | √ | √ | √ |
| Metformin therapy for patients with BMI ≥ 25 kg/m2 if no weight loss after 16-week lifestyle modification course | √ | ||
| Gastric bypass surgery for patients with BMI > 35 kg/m2 per guidelines for treating obesity and/or if no weight loss after lifestyle modification and/or metformin therapy | √ |
BMI = body mass index; √ = yes.
Economic considerations are important for health care groups because the cost to care for individuals with diabetes far exceeds the cost to care for patients with prediabetes or normal glucose levels. Most studies support the notion that lifestyle change should be the cornerstone for diabetes prevention. On the basis of results published by Knowler et al16 in 2002 and others,35 it may make financial sense to invest in the treatment of prediabetes. Knowler et al16 estimated that 37% of individuals with prediabetes would have diabetes in 4 years without intervention. If individuals with prediabetes complete a lifestyle intervention program, their risk of diabetes developing in 4 years decreases to about 20%. Studies were not performed to determine if interventions to promote healthy eating and active living over many years could help reduce the percentage of people whose prediabetes progresses to diabetes to less than 20% (Figure 1). The difference in direct and indirect costs to treat a patient with prediabetes vs diabetes is estimated to be about $7000 per year.7,12
KP estimates having at least 852,031 patients with prediabetes (Table 2). If we do nothing, diabetes may develop in 4 years in approximately one-third, or 284,010 patients. If we were able to persuade the 852,031 prediabetic members to participate in and complete a lifestyle management program, the number in whom diabetes develops might decrease to approximately 170,406, or 113,604 patients saved from development of diabetes. The estimated cost savings per year would be costs saved from treating thousands of patients with diabetes minus the cost to implement an effective screening and lifestyle management program for all patients with prediabetes.
In 2012, Health Affairs published an article looking at the economics of screening and treating individuals with prediabetes.36 Using a simulation model, theauthors projected the costs and benefits of a nationwide community-based lifestyle intervention program for preventing Type 2 diabetes. In the hypothetical intervention program, nearly 100 million Americans aged 18 to 84 years would be screened over the next 25 years and nearly 23 million of those would have prediabetes. Another 13 million would be expected to be enrolled in a lifestyle intervention. The researchers projected that over the 25-year simulation period the hypothetical program would prevent or delay 885,000 new cases of Type 2 diabetes and result in a gain of 952,000 life-years and 669,000 quality-adjusted life-years. The researchers projected that the program would result in $29.8 billion in downstream savings for those in whom diabetes and diabetes-related complications may have developed without intervention.36
Of concern are recent data showing that an intensive lifestyle intervention focusing on weight loss did not reduce the rate of cardiovascular events in overweight or obese adults with Type 2 diabetes.37 This finding suggests there is a window of opportunity in patients with prediabetes to save lives that may not occur in patients with diabetes. However, in another study, lifestyle modification was exceptionally effective in preventing diabetes in older individuals, and this finding was largely explained by greater weight loss and an increase in physical activity.38 These data suggest that even older adults may benefit from behavior-change interventions aimed at preventing diabetes and its complications. Recent data suggest that lifestyle interventions to prevent diabetes may overcome the genetic risk of diabetes.39 This information may be helpful to parents of children with a strong family history of diabetes.
Finally, because most of the direct costs for diabetes are associated with treating complications of diabetes,40 a managed care organization like KP, which is not dependent on fee-for-service reimbursement, may be in a unique position to help implement upstream programs in schools and community programs now to prevent prediabetes, diabetes, and complications of diabetes.41
Conclusions
The time is right to develop a proactive approach to prediabetes. This approach may include the following recommendations for practice:
Develop a business case for screening for and for treating prediabetes. This will involve estimating the cost savings resulting from preventing hundreds of thousands of patients from diabetes minus the estimated cost to screen all patients at risk of diabetes and the estimated cost for implementing an effective lifestyle modification program to all patients with prediabetes.
Develop clinical guidelines for physicians on how to identify and manage patients with prediabetes, as outlined in Table 4. The guidelines will help us determine a standardized approach to treating prediabetes and allow us to develop measurable outcomes to determine if all patients with prediabetes are completing a lifestyle modification program and achieving weight loss, HbA1c, and physical activity goals, as shown in Table 4.
Develop a registry of patients who have prediabetes similar to registries we have developed for hypertension, diabetes, and asthma.
Develop a low-cost, easily accessible lifestyle management program that would potentially be available for the hundreds of thousands of patients with prediabetes.
The primary aim of lifestyle interventions is to prevent diabetes and its complications by targeting obesity and physical inactivity. Patients not responding to lifestyle interventions may be considered for pharmacologic interventions or surgery. The goal for prediabetes treatment should be to normalize blood glucose levels. Strategies targeting interventions aimed at the entire population at risk of prediabetes can make health care more affordable, prevent a preventable disease, and save lives.42
Acknowledgments
The author would like to thank Colleen McClurkin-Birge for her advice and help in the preparation of this manuscript.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Think About It
Heart disease and diabetes, which account for more deaths in the US and worldwide than everything else combined, are completely preventable by making comprehensive lifestyle changes. Without drugs or surgery.
— Dean Ornish, MD, b 1953, American physician, president and founder of the Preventive Medicine Research Institute, and a clinical professor of medicine
References
- 1.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011 [Internet] Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [cited 22014 Mar 3]. Available from: www.cdc.gov/diabetes/pubs/pdf/ndfs_2011_pdf. [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011 Jan;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. DOI: http://dx.doi.org/10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of Type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabetes Med. 2007 Feb;24(2):200–7. doi: 10.1111/j.1464-5491.2007.02068.x. DOI: http://dx.doi.org/10.1111/j.1464-5491.2007.02068.x. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Summary of revisions to the 2011 clinical practice recommendations. Diabetes Care. 2011 Jan;34(Suppl 1):S3. doi: 10.2337/dc11-S003. DOI: http://dx.doi.org/10.2337/dc11-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Gregg EW, Williamson DF, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010 Jul;33(7):1665–73. doi: 10.2337/dc09-1939. DOI: http://dx.doi.org/10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heianza Y, Hara S, Arase Y, et al. HbA1C 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011 Jul 9;378(9786):147–55. doi: 10.1016/S0140-6736(11)60472-8. DOI: http://dx.doi.org/10.1016/S0140-6736(11)60472-8. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Economic costs of diabetes in the US in 2012. Diabetes Care. 2013 Apr;36(4):1033–46. doi: 10.2337/dc12-2625. DOI: http://dx.doi.org/10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Dall TM, Chen Y, et al. Medical cost associated with prediabetes. Popul Health Manag. 2009 Jun;12(3):157–63. doi: 10.1089/pop.2009.12302. DOI: http://dx.doi.org/10.1089/pop.2009.12302. [DOI] [PubMed] [Google Scholar]
- 9.Williams DE, Cadwell BL, Cheng YJ, et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics. 2005 Nov;116(5):1122–6. doi: 10.1542/peds.2004-2001. DOI: http://dx.doi.org/10.1542/peds.2004-2001. [DOI] [PubMed] [Google Scholar]
- 10.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007 Jul;116(2):151–7. doi: 10.1161/CIRCULATIONAHA.106.685628. http://dx.doi/org/10.1161/CIRCULATIONAHA.106.685628. PMID 17576864. [DOI] [PubMed] [Google Scholar]
- 11.Plantinga LC, Crews DC, Coresh J, et al. CDC CKD Surveillance Team Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010 Apr;5(4):673–82. doi: 10.2215/CJN.07891109. DOI: http://dx.doi.org/10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vojta D, De Sa J, Prospect T, Stevens S. Effective interventions for stemming the growing crisis of diabetes and prediabetes: a national payer’s perspective. Health Aff (Millwood) 2012 Jan;31(1):20–6. doi: 10.1377/hlthaff.2011.0327. DOI: http://dx.doi.org/10.1377/hlthaff.2011.0327. [DOI] [PubMed] [Google Scholar]
- 13.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–53. DOI: http://dx.doi.org/10.1016/S0140-6736(98)07019-6. Erratum in: Lancet 1999 Aug 14;354(9178):602. DOI: http://dx.doi.org/10.1016/S0140-6736(05)77965-4. [PubMed] [Google Scholar]
- 14.Effect of intensive blood-glucose control with metformin on complications in over-weight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):854–65. DOI: http://dx.doi.org/10.1016/S0140-6736(98)07037-8. Erratum in: Lancet 1998 Nov 7;352(9139):1558. DOI: http://dx.doi.org/10.1016/S0140-6736(05)60381-9. [PubMed] [Google Scholar]
- 15.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000 Jul-Aug;16(4):230–6. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. DOI: http://dx.doi.org/10.1002/1520-7560(2000)9999:9999%3C::AIDDMRR122%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. DOI: http://dx.doi.org/10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009 Nov 14;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. DOI: http://dx.doi.org/10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012 Jun 16;379(9833):2243–51. doi: 10.1016/S0140-6736(12)60525-X. DOI: http://dx.doi.org/10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes prevention program (DPP): Type 2 diabetes and prediabetes [Internet] Bethesda, MD: National Diabetes Information Clearinghouse; 2013. Sep 09, [cited 2014 May 22]. Available from: http://diabetes.niddk.nih.gov/dm/pubs/preventionprogram/ [Google Scholar]
- 20.Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013 Oct 15;159(8):543–51. doi: 10.7326/0003-4819-159-8-201310150-00007. DOI: http://dx.doi.org/10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 21.Lindström J, Louheranta A, Mannelin M, et al. Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003 Dec;26(12):3230–6. doi: 10.2337/diacare.26.12.3230. DOI: http://dx.doi.org/10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 22.Hopper I, Billah B, Skiba M, Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil. 2011 Dec;18(6):813–23. doi: 10.1177/1741826711421687. DOI: http://dx.doi.org/10.1177/1741826711421687. [DOI] [PubMed] [Google Scholar]
- 23.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Finnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006 Nov 11;368(9548):1673–9. doi: 10.1016/S0140-6736(06)69701-8. DOI: http://dx.doi.org/10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008 May 24;371(9626):1783–9. doi: 10.1016/S0140-6736(08)60766-7. DOI: http://dx.doi.org/10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 25.Gong Q, Gregg EW, Wang J, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011 Feb;54(2):300–7. doi: 10.1007/s00125-010-1948-9. DOI: http://dx.doi.org/10.1007/s00125-010-1948-9. [DOI] [PubMed] [Google Scholar]
- 26.Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008 Feb;121(2):149–57. doi: 10.1016/j.amjmed.2007.09.016. e2. DOI: http://dx.doi.org/10.1016/j.amjmed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Lily M, Godwin M. Treating prediabetes with metformin: systematic review and meta-analysis. Can Fam Physician. 2009 Apr;55(4):363–9. [PMC free article] [PubMed] [Google Scholar]
- 28.DREAM On (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Ongoing Follow-up) Investigators. Gerstein HC, Mohan V, Avezum A, et al. Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia. 2011 Mar;54(3):487–95. doi: 10.1007/s00125-010-1985-4. DOI: http://dx.doi.org/10.1007/s00125-010-1985-4. [DOI] [PubMed] [Google Scholar]
- 29.DREAM Trial Investigators. Dagenais GR, Gerstein HC, Holman R, et al. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes Reduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 2008 May;31(5):1007–14. doi: 10.2337/dc07-1868. DOI: http://dx.doi.org/10.2337/dc07-1868. [DOI] [PubMed] [Google Scholar]
- 30.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet. 2010 Jul 10;376(9735):103–11. doi: 10.1016/S0140-6736(10)60746-5. DOI: http://dx.doi.org/10.1016/S0140-6736(10)60746-5. [DOI] [PubMed] [Google Scholar]
- 31.Yokoi T. Troglitazone. Handb Exp Pharmacol. 2010;(196):419–35. doi: 10.1007/978-3-642-00663-0_14. http://dx.doi.org:10.1007/978-3-642-00663-0_14.) [DOI] [PubMed] [Google Scholar]
- 32.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004 Dec 23;351(26):2683–93. doi: 10.1056/NEJMoa035622. DOI: http://dx.doi.org/10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 33.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012 Jan 4;307(1):56–65. doi: 10.1001/jama.2011.1914. DOI: http://dx.doi.org/10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 34.Glauber H, Karnieli E. Preventing type 2 diabetes mellitus: a call for personalized intervention. Perm J. 2013 Summer;17(3):74–9. doi: 10.7812/TPP/12-143. DOI: http://dx.doi.org/10.7812/TPP/12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee R, Narayan KM, Lipscomb J, Phillips LS. Screening Adults for prediabetes and diabetes may be cost-saving. Diabetes Care. 2010 Jul;33(7):1484–90. doi: 10.2337/dc10-0054. DOI: http://dx.doi.org/10.2337/dc10-0054PMCID:PMC2890345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuo X, Zhang P, Gregg EW, et al. A nationwide community-based lifestyle program could delay or prevent type 2 diabetes cases and save $5.7 billion in 25 years. Health Aff (Millwood) 2012 Jan;31(1):50–60. doi: 10.1377/hlthaff.2011.1115. DOI: http://dx.doi.org/10.1377/hlthaff.2011.1115. [DOI] [PubMed] [Google Scholar]
- 37.Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013 Jul 11;369(2):145–54. doi: 10.1056/NEJMoa1212914. DOI: http://dx.doi.org/10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diabetes Prevention Program Research Group. Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006 Oct;61(10):1075–81. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florez JC. Genetic susceptibility to type 2 diabetes and implications for therapy. J Diabetes Sci Technol. 2009 Jul 1;3(4):690–6. doi: 10.1177/193229680900300413. DOI: http://dx.doi.org/10.1177/193229680900300413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997 Sep;20(9):1396–402. doi: 10.2337/diacare.20.9.1396. DOI: http://dx.doi.org/10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 41.Kim C, Williamson DF, Mangione CM, et al. Translating Research Into Action for Diabetes (TRIAD) Study Managed care organization and the quality of diabetes care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2004 Jul;27(7):1529–34. doi: 10.2337/diacare.27.7.1529. DOI: http://dx.doi.org/10.2337/diacare.27.7.1529. [DOI] [PubMed] [Google Scholar]
- 42.Diabetes: successes and opportunities for population-based prevention and control: at a glance 2011 [Internet] Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. Aug 1, [cited 2014 Mar 4]. Available from: www.cdc.gov/chronicdisease/resources/publications/aag/ddt.htm. [Google Scholar]


