Abstract
Research on human pluripotent stem cells (hPSCs) has expanded rapidly over the last two decades, owing to the promises of hPSCs for applications in regenerative medicine, disease modeling, and developmental biology studies. While most studies of hPSCs have so far focused on identifying extrinsic soluble factors, intracellular signaling pathways, and transcriptional networks that are involved in regulating hPSC self-renewal and differentiation, a few promising studies have emerged in recent years to reveal some unique mechanosensitive and -responsive properties of hPSCs and the effect of the physical aspects of the local cellular microenvironment on regulating hPSC behaviors. This Frontier Review is to highlight these recent studies of mechanobiology in hPSCs and to discuss the impact of advancing our understanding of mechanoregulation of hPSC behaviors on improving survival, self-renewal and differentiation of hPSCs using well-controlled synthetic micro/nanoscale cell culture tools.
Introduction
Since the derivation of the first human embryonic stem cell (hESC) line in 19981 and the discovery of human induced pluripotent stem cells (hiPSCs) in 20072, the research of human pluripotent stem cells (hPSCs) has become an exciting and rapidly expanding area. The promising applications of hPSCs include modelling developmental and disease processes (especially with patient-specific hiPSCs), drug and toxicity screening, and cell-based regenerative medicine3. Most studies of hPSCs have so far focused on illustrating different biochemical factors, signalling pathways, and transcriptional networks that are involved in regulating hPSC self-renewal and differentiation4, revealing that, for example, soluble growth factors, such as those in the TGF-β superfamily and FGF, WNT, and Hedgehog families, are important in regulating self-renewal and differentiation of hPSCs in cell culture through their effects on a core network of transcription factors including OCT3/4, NANOG, and SOX2, which function in concert to regulate target genes necessary for pluripotency maintenance and lineage specification of hPSCs.
Although clinical trials using hPSCs to treat degenerative diseases have reported positive preliminary results5, large-scale preclinical and clinical applications of hPSCs remain elusive owing to a few major technical hurdles in hPSC culture (Fig. 1). Firstly, the most robust method to maintain and expand hPSCs in culture is not completely chemically defined and still requires animal-derived materials, which limits the ultimate clinical applications of hPSCs. Secondly, the most popular method for differentiation of hPSCs relies on the process of culture and spontaneous differentiation of hPSCs in three-dimensional aggregates known as embryonic bodies (EBs). However, guided differentiation using EB-based hPSC culture is difficult if not impossible, and due to the heterogeneity of hPSC differentiation in EBs, to obtain a pure population of the desired cell lineage, extensive cell purification is required. Thirdly, the survival rate and cloning efficiency of fully disassociated single hPSCs during enzymatic passaging is extremely low (< 1%), as single hPSCs tend to undergo massive cell death (apoptosis) upon complete dissociation into single cells. Pharmacological drugs such as Y27632 (a chemical inhibitor of Rho-associated kinase (ROCK)) are currently used to enhance survival and cloning efficiency of fully disassociated single hPSCs. However, long-term effects of these drugs on hPSCs are unclear, and these drugs have been associated with aneuploidy, which is implicated in cell transformation6. An alternative method for passaging hPSCs is performed by mechanically fragmenting hPSC colonies into small clusters or clumps and subsequently transferring these cell clusters or clumps to a new tissue culture plate - a tedious, inefficient and difficult process with limited reproducibility and automation possibility. Together, the unique sensitivity of hPSCs to their culture condition has made it difficult in the culture to maintain and expand hPSCs and to efficiently direct their lineage specification. These technical challenges in hPSC culture have prevented the establishment of controllable, reproducible, and scalable fully-defined synthetic culture system for hPSC self-renewal and differentiation, a critical requirement for large-scale applications of hPSCs.
Fig. 1.
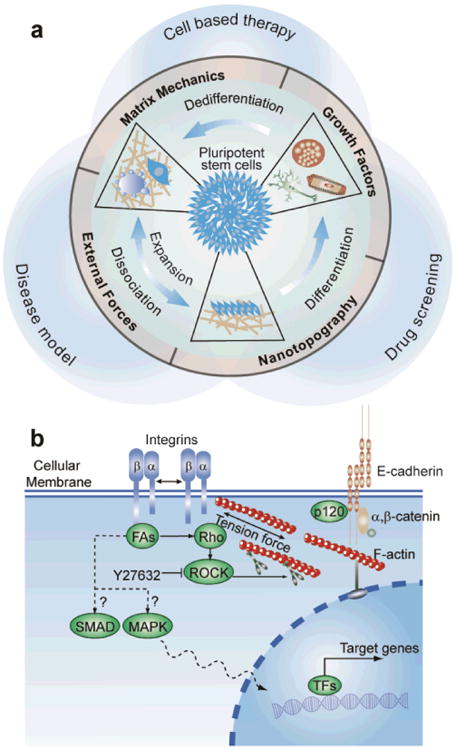
(a) High-throughput micromechanical tools for precise control and measurements of mechanical stimuli and response to improve hPSC culture. (b) Integrin-mediated cell-ECM and E-cadherin-based cell-cell interactions in regulating mechanoresponsive hPSC functions.
The unique sensitivity of hPSCs to their culture conditions stems mainly from the poorly understood cell-extracellular matrix (ECM) and cell-cell physical interactions of hPSCs with their local cellular microenvironment. While researchers are still striving to identify the optimal soluble chemical environment for hPSC culture, the insoluble “solid-state” and physical aspect of the local cellular microenvironment of hPSCs should also be taken into consideration7. Importantly, all those three aforementioned major obstacles in hPSC culture are related, to a greater or lesser extent, to dynamic cell-ECM and cell-cell interactions of hPSCs during their self-renewal and differentiation processes. During enzymatic passaging of hPSCs, for instance, E-cadherin-mediated cell-cell contacts in hPSC colonies are disrupted. It has been suggested by several recent studies that dissociation-induced apoptosis of single hPSCs is likely attributable to hyper-activation of myosin-based cytoskeleton tension that is triggered by disruption of cell-cell contacts of hPSCs, and this hyperactive cytoskeleton tension is the upstream regulator and direct cause of hPSC apoptosis8.
Understanding dynamic cell-ECM and cell-cell interactions and their functional cross-talk in regulating diverse functions of adherent cells is a long-term direction for the mechanobiology research. Indeed, mechanoresponsive behaviors of human adult stem cells including hematopoietic, mesenchymal, neural and skeletal muscle stem cells have been well documented recently9. These studies have unambiguously confirmed the potent regulatory roles played by the dynamic biophysical signals in the local cellular microenvironment, such as cell shape and geometry, matrix mechanics, external mechanical forces, and nanotopographical features of the ECM, in regulating the spatiotemporal adhesion-mediated signaling and downstream stem cell self-renewal and differentiation.
Thus, in our view, it is critically important to start taking into consideration and understand the unique mechano-sensitive and -responsive properties of hPSCs to fully appreciate their unique susceptibility to the culture environment, so that future efforts can be directed to unravel integrated cellular responses of hPSCs to complex biomechanical stimuli and to design dynamic, synthetic fully-defined cell culture tools to improve hPSC culture. A major goal of this Frontier Review is therefore to offer a perspective on this new trend of investigating mechano-sensitive and -responsive behaviors of hPSCs and the promise of mechanobiology to improve hPSC culture. We will first provide a concise review of integrin- and cadherin-based cell adhesion molecules (CAMs) of hPSCs that transduce biophysical signals in the cellular microenvironment through cell-ECM and cell-cell interactions into intracellular biochemical and cellular functional responses (i.e., mechanotransduction). We will then highlight some illustrative examples of using innovative approaches to characterize and understand the mechano-sensitive and -responsive properties of hPSCs, particularly those recent studies demonstrating effects of matrix rigidity, external stretching force and enzymatic dissociation on cell-ECM and cell-cell interactions of hPSCs and thus their self-renewal and differentiation. Finally, we offer some speculations about future research studying mechanobiology in hPSCs and how the knowledge gained from such studies can be translated to improvements of future large-scale productions of clinical grade hPSCs and their derivatives.
Cell adhesion molecules (CAMs) of hPSCs
Cell adhesion molecules, or CAMs, are the proteins on the surface of mammalian cells that contribute to juxtacrine cell-cell binding or cell-ECM adhesion. Key members of CAMs expressed on hPSCs include the integrins, cadherins, immunoglobulin (Ig) superfamily, and proteoglycans. Readers interested in further discussions of the CAMs in hPSCs are referred to some excellent reviews published elsewhere10. Here we provide a brief review of CAMs on hPSCs, as CAMs provide two-way communication links from hPSCs to their surrounding microenvironment through which the biophysical signals in the local cellular microenvironment are transmitted across the cell membrane to regulate intracellular signaling activities.
Integrin expressions in hPSCs have been characterized in detail recently11. It has been shown that hESCs express a broad range of integrins including α1, α2, α3, α5, α6, α7, α11, αV, αE, and β1, β2, β3, β5, β6 integrins10-11. However, among them, only α6, β1, α2β1, and αVβ3 are thought to be critical for hESC adhesion to Matrigel11a, αVβ5 for hESC adhesion to vitronectin12, and α6β1 for hESC adhesion to laminin13. Integrin expressions in hiPSCs are similar to those in hESCs. It has been shown that α5, α6, αV, β1, and β5 integrins are prominently expressed in hiPSCs, and different hiPSC lines have variable integrin expressions. Integrin-mediated adhesion signaling can regulate important intracellular signal transduction pathways that control pluripotency and differentiation of hPSCs, such as the PI3K and FGF/MAPK pathways14. The direct regulatory role of integrin-mediated adhesion signaling in controlling hPSC fate is supported by recent findings that vitronectin and laminin promote the long-term self-renewal of hPSCs in the monolayer culture system12-13. Our recent research has also demonstrated that morphology and subcellular distribution of integrin-mediated adhesion structures (e.g., focal adhesions) in hPSCs change drastically after lose of pluripotency15, again suggesting the involvement of integrin-mediated adhesion in regulating pluripotency and self-renewal of hPSCs.
The classic cadherins include E-cadherin, N-cadherin, and VE-cadherin. E-cadherin is widely expressed in hPSCs when the cells are in close contact with each other in an undifferentiated colony, while N-cadherin and VE-cadherin are not expressed in undifferentiated hPSCs10. E-cadherin is considered as a key molecule to maintain the pluripotency of hPSCs. E-cadherin has an extracellular domain that establishes the calcium-dependent, homophilic interactions with neighbour cells and an intracellular domain that binds to p120 catenin and β-catenin that connect the junctions to actin filaments of the cytoskeleton. Interestingly, β-catenin not only mediates cytoskeletal attachment of adherent junctions but can also translocate to the nucleus and alter gene transcription in the canonical WNT signalling pathway, which has been shown important for regulation of the pluripotency of hPSCs16. The requirement of E-cadherin in the maintenance of pluripotency of hPSCs is strongly supported by the evidence that inhibition of E-cadherin by antibodies or siRNA leads to loss of pluripotency of hPSCs17 and over-expression of E-cadherin promotes hPSC self-renewal18. However, a detailed molecular picture of how E-cadherin is functionally connected to the OCT4-SOX2-NANOG transcription network that regulates the pluripotency of hPSCs is still largely elusive.
Another important class of CAMs is polysaccharides including glycosaminoglycans (GAGs), which are highly expressed on the surface of hPSCs and can control activation of many important growth factor-initiated signalling transduction pathways by serving as co-receptors19. Using a peptide (GKKQRFRHRNRKG) derived from vitronectin that binds to heparin, a highly negatively charged type of GAGs on the surface of hPSCs, Klim et al. have developed a fully-defined cell culture substrate to support the long-term self-renewal of hESCs20. Their findings suggest that adhesion of hPSCs to Matrigel or vitronectin, which has been shown previously to support the growth of undifferentiated hPSCs, may be regulated by GAG-mediated adhesive interactions. Heparan sulfate, another type of GAGs similar to heparin in structure, has also been found essential for the growth and pluripotency maintenance of hESCs21. Moreover, three dimensional hyaluronic acid (a non-sulfated GAG) gel has also been shown supportive for the self-renewal of hESCs in the presence of conditioned media from mouse embryonic fibroblast feeder cells22. Together, these studies suggest that in addition to integrin-mediated cell-ECM adhesions, GAGs may also play significant roles in the adhesive interactions of hPSCs with their surrounding ECM to work synergistically with soluble growth factors to support the long-term growth of undifferentiated hPSCs.
Effect of matrix rigidity on hPSC behaviours
The mechano-sensitive and -responsive properties of hPSCs have not been examined explicitly until recently. Different groups including our own have lately started to investigate whether the survival, self-renewal and differentiation of hESCs can be regulated by substrate rigidity at the single-cell level15, 23. Unlike mouse ESCs (mESCs), single hPSCs tend to differentiate spontaneously in the cell culture. Further, the survival rate and cloning efficiency of single hPSCs is extremely low as they tend to undergo apoptosis upon complete dissociation into single cells. Interestingly, a recent study has shown that the self-renewal and clonal growth of mESCs can be promoted by soft matrix24. hPSCs are intrinsically different from mESCs, in regard to the required growth factors and dominant signal pathways that regulate their pluripotency. Thus, there is still a critical knowledge gap in understanding the mechano-sensitive and -responsive properties of hPSCs.
To examine how matrix rigidity affects the survival and self-renewal of hPSCs, we have recently utilized a library of microengineered elastomeric micropost arrays with the same surface geometry but different post heights to modulate substrate rigidity9b. The top surface of the micropost array was coated with vitronectin. Using the micropost simultaneously as a live-cell cytoskeleton tension force sensor, we have shown that hESCs are mechanosensitive, and they increase cytoskeleton tension with matrix rigidity. Interestingly, matrix rigidity appears to play a significant role in regulating pluripotency of single hESCs, as a significantly higher percentage of single hESCs after culture on the rigid micropost array for 24 hrs remains as undifferentiated OCT4-positive cells as compared to the ones on soft PDMS micropost arrays. For small clusters of hESCs, E-cadherin expression was shown positively correlated with both cytoskeleton tension and OCT4 expression, and hESCs had a greater tendency to differentiate on soft micropost arrays while maintaining their OCT4 expressions on rigid ones. To examine specifically the functional role of E-cadherin in mediating rigidity-dependent self-renewal of hESCs, we have performed Ecadherin inhibition assays and shown that matrix mechanics-mediated cytoskeleton tension is functionally connected with Ecadherin expressions at cell-cell contacts and thus involved in fate decisions of small clumps of hESCs. Our results, combined with the evidence that inhibition of E-cadherin leads to loss of hESC pluripotency17 and over-expression of E-cadherin promotes hESC self-renewal18, strongly suggest that for hESC colonies where E-cadherin-mediated cell-cell contacts have been established, matrix mechanics-mediated cytoskeleton tension may help stabilize the OCT4-SOX2-NANOG circuitry, possibly by regulating the E-cadherin-mediated intercellular adhesion.
Keung et al. have recently studied responses of small clusters of hESCs and hiPSCs to bulk rigidity changes in Matrigel coated polyacrylamide (PA) gels23a. Their results have shown that substrate rigidity does not affect proliferation or expression of pluripotent markers including NANOG, OCT4 and SSEA-4 for hPSCs after culturing the cell clusters for 3 days under a self-renewal medium condition. A more recent study by Musah et al. have refined the PA gel system by functionalizing the hydrogel with an adhesive peptide (GKKQRFRHRNRKG) derived from vitronectin23a, which has been shown in their previous work to bind to cell surface GAGs and support the long-term self-renewal of hESCs20. Interestingly, Musah et al. have found that only rigid PA gels functionalized with the adhesive peptide can maintain hESC proliferation and pluripotency, consistent with our finding that rigid micropost arrays coated with vitronectin support maintenance of pluripotency of hESCs23b. All together, our work and the studies by Keung et al. and Musah et al. all point to the conclusion that unlike mESCs24, soft microenvironment does not promote the survival and self-renewal of hPSCs. The differences between the observations by our group and Musah et al. and the results reported by Keung et al. suggest the possibility that rigidity sensing of hPSCs may critically depend on the specific CAMs employed by hPSCs to bind to the surrounding ECM. The differences between these studies also underscore the importance in recognizing the differences in molecular-scale material properties (such as porosity, surface chemistry, molecular backbone flexibility and binding properties of immobilized adhesive ligands) when comparing different hydrogel systems. Recent studies using synthetic hydrogels for hPSCs and human adult stem cells have strongly indicated that these molecular-scale changes in material properties can have profound effects on stem cell function25
Mechanics and forces are known to play important roles in embryogenesis. Given that hPSC differentiation follows developmental principles, it is not at all surprising that mechanical cues can regulate lineage commitment and differentiation of hPSCs. Zoldan et al. have cultured EBs in three dimensional scaffolds with their bulk rigidity engineered to model that found in specific germlayers in vivo. Differentiation of hESCs in the EBs to endoderm, mesoderm, and ectoderm germlayers was shown to be promoted by a different rigidity threshold of the scaffold26. Specifically, their results have demonstrated that high, intermediate, and low elastic moduli of the scaffolds promote mesodermal, endodermal and ectodermal differentiation, respectively, as evidenced by upregulated expressions of genes representative of the three germ layers. A more definitive study on the mechanoresponsive hPSC differentiation has been demonstrated recently by Keung et al., where the authors have investigated the effect of substrate mechanics on neural induction of hESCs and hiPSCs. Matrigel coated PA gels with the bulk rigidity in the range of 100 Pa - 700 Pa were shown to promote neural induction under a culture condition for dual inhibitions of TGF-β and BMP4 signalling, a well established neural induction culture for the monolayer culture system27. Specifically, soft PA gels were shown to upregulate expressions of multiple neuroepithelial markers such as Pax6, Sox1 and even some markers of the later stage neurogenic differentiation such as Tuj-1 in hPSCs after culturing the cells for 9 days on the PA gels. Interestingly, Keung et al. have further shown that a short 5-day soft stiffness “pulse” treatment for hPSCs before neural patterning can promote their neural induction to the same extend as a 9-day “pulse”. Keung et al. have also studied whether soft PA gels promote the maturation of dopaminergic neurons. They passaged the neural progenitor cells induced after 9 days of culture on PA gels of different rigidities to glass chamber wells for additional 10 days to allow for neuronal maturation. They observed that while a greater number of dopaminergic neurons were obtained from neural progenitor cells on soft PA gels, the ratio between the dopaminergic neurons and Tuj-1-positive neural cells remained about the same. The results reported by Keung et al. suggest that mechanosensitivity of hPSCs in the initial neural induction phase may not be preserved in the later neuronal differentiation and maturation. We have also recently shown that hESCs cultured on soft micropost arrays are more sensitive to caudalization signals induced by retinoic acid with increased expressions of Sox1 and Islet1/2 (a transcription factor found in motor neurons) in hESCs as compared to the cells cultured on rigid micropost arrays and tissue culture plates28. All together, these results strongly support that matrix rigidity can influence neural differentiation of hPSCs in multiple stages, from initial neural induction to primitive neural progenitor cells to the later stage specification and maturation.
Effect of mechanical forces on hPSC fate
Evidence related to regulation of hPSC fate by mechanical forces in vitro has only recently begun to emerge. Compared to matrix rigidity, external forces are more dynamically controllable and can be applied through a variety of formats, including cyclic membrane stretches, magnetic and optical tweezers, and shear flow assays29. Saha et al. have reported that hESCs cultured on a Matrigel coated stretchable surface under a mouse embryonic fibroblast conditioned medium can maintain expressions of pluripotency markers for 2 weeks when the surface is stretched at a frequency of 10 cycles per min30. Interestingly, the authors have shown that mechanical strains do not inhibit differentiation of hESCs if the cells are cultured in the control unconditioned medium, highlighting the synergy between mechanical stimuli and chemical signals in regulating hPSC behaviors. Saha et al. have further reported that under mechanical strain, Smad2/3 in hESCs is phosphorylated, indicating activation of the TGF-β/Activin/Nodal pathway30. Blocking TGF-β1 receptors abolished the effect of mechanical strain on hESC self-renewal30, suggesting that mechanical strain might promote the self-renewal of hESCs by stimulating the autocrine/paracrine signaling in hESCs via the TGF-β pathway.
Dissociation associated apoptosis of single hPSCs is related to unbalanced intracellular forces
For rapid expansion and genetic manipulation of hPSCs, enzymatic dissociation of hPSCs into single cells is often a required step. However, one of the distinct features of hPSC culture is that hPSCs undergo massive cell death upon complete dissociation into single cells. Therefore, dissociation induced hPSC apoptosis is a fundamental problem in hPSC culture. The study by Watanabe et al. in 2007 has reported that Y27632, a ROCK inhibitor, can drastically rescue the apoptosis and thus improve the survival threshold of single hPSCs31. As a result, ROCK inhibitors began to be routinely used in hPSC culture. Recently, several different groups have attempted to elucidate the molecular mechanism underlying the ability of Y27632 to promote survival of dissociated single hPSCs8, 18. These studies have led to the finding that dissociation-induced apoptosis of single hPSCs is attributable to hyper-activation of myosin II-mediated cytoskeleton tension that is triggered by disruption of E-cadherin based cell-cell contacts of hPSCs, and this hyperactive cytoskeleton tension is the upstream regulator and direct cause of hPSC apoptosis. These studied have also suggested that integrin-mediated cell-ECM adhesions disrupted during enzymatic passaging are not important for dissociation-induced apoptosis of single hPSCs, and E-cadherin-regulated adhesive interactions alone are insufficient to promote survival of hPSCs8a, 18.
One major biochemical pathway mediating cytoskeleton tension is through the RhoA-GTPase/ROCK/myosin-II pathway. Consistent with this notion, these same studies have revealed that RhoA activation, coupled with Rac inhibition, upon disruption of cell-cell contacts in hPSCs8, is a major driver of dissociation-induced hPSC apoptosis via ROCK-mediated myosin light chain (MLC) phosphorylation. A guanine exchange factor of RhoA, Abr, was identified to play an upstream functional role in this signal transduction process8a. An open question remains as to how signals are transduced from E-cadherin to Abr after disruption of E-cadherin based cell-cell contacts of hPSCs. Is there any other biochemical or even direct biomechanical routes to relay signals from E-cadherin mediated cell-cell contacts to RhoA-GTPase? Finding answers for these questions will enable a greater understanding at the molecular level of the unique mechano-sensitive and -responsive properties of hPSCs.
Since blebbing of hPSC surface, which is a direct indication of unregulated hyper-activation of cytoskeleton tension, occurs almost immediately after their dissociation8a, there must be some mechanism to prevent hPSCs from blebbing when they are grown in colonies. It is possible that ROCK may be associated to Ecadherin in hPSCs when they form cell-cell contacts, as reported in some cancer cell lines32, or cytoskeleton tension in hPSCs needs to be balanced mechanically by direct cell-cell contacts. The molecular picture for the existence of such a unique mechanism is still elusive. Ohgushi et al. have speculated that pre-activated ROCK and/or myosin II may exist in hPSCs as quality control machineries to exclude dissociated cells during early embryonic development8a. Another possibility is that cytoskeleton reconfiguration may be required as an essential initiator for key steps of cell differentiation8a.
Recent studies have suggested that myosin II-mediated cytoskeleton tension and E-cadherin expression can form a positive feedback loop to promote maintenance of pluripotency of hPSCs17b. Depletion of myosin-II or treatment with blebbistatin, a small molecule inhibitor for myosin-II, for hPSC colonies reduces E-cadherin accumulation at the cell-cell junction sites and impairs the formation of characteristic hPSC colonies17b, 33. As a result, those hPSC cells show a lower level of alkaline phosphatase activity and reduced expressions of OCT4, SOX2 and NANOG proteins, indicating an impaired status of self-renewal. Our research has also suggested that matrix mechanics-mediated cytoskeleton tension may help stabilize the OCT4-SOX2-NANOG circuitry, possibly by regulating E-cadherin-mediated intercellular adhesion9b. On the other hand, cell-cell adhesion can directly regulate RhoA signaling34. Collectively, these lines of evidence support a possible mechano-sensing and -transduction mechanism based on cell-ECM and cell-cell interactions and involving the RhoA/ROCK/myosin-II signaling axis and its interplay with E-cadherin-mediated cell-cell contacts for regulation of self-renewal and differentiation of hPSCs.
Conclusions and perspective
hPSCs offer great promise for developmental biology studies and cell replacement therapies. However, large-scale preclinical and clinical applications of hPSCs remain elusive owing to their unique sensitivity to the culture environment, which can be largely attributed to the poorly understood dynamic cell-ECM and cell-cell physical interactions of hPSCs with their local cellular microenvironment. In addition, the puzzling mechano-sensitive and -responsive properties of hPSCs have just started to emerge, and our current understanding of the mechanotransductive systems in hPSCs is still very limited. Thus, in our view, advancing understanding of how mechanobiology is involved in regulating hPSC behaviours will contribute significantly to improvements of large scale production of clinical grade hPSCs and their derivatives, a critical requirement for future hPSC applications.
Existing data suggests that the cytoskeleton structure and tension of hPSCs are very sensitive to the environmental cues and may be a critical controller of their survival, self-renewal, and lineage specification. RhoA/ROCK, myosin II, and E-cadherin seem to form a closely interconnected regulatory loop to control adhesion signaling and thus the survival and self-renewal of hPSCs. bFGF/MAPK, TGF-β/Activin/Nodal, BMP4 signalling in hPSCs are also regulated by mechanical stimuli, and they are central pathways controlling the self-renewal and differentiation programmes of hPSCs35. Moreover, YAP/TAZ has been shown to transmit mechanical signals to nucleus through a Hippo independent pathway36, and YAP/TAZ controls many critical downstream signals such as SMAD in hPSCs37. Collectively, discovering the hPSC mechanotransductive systems based on the cell-ECM and cell-cell interactions and their functional cross-talk during the self-renewal and differentiation processes of hPSCs will significantly improve our molecular understanding of the unique sensitivity of hPSCs to the culture environment and the early embryogenesis and tissue development. It remains to be determined the relative importance of endogenous vs. exogenous cellular forces in regulating the mechano-sensitive and -responsive behaviours of hPSCs and how such force-mediated functional regulations for hPSCs are different in two-dimensional vs. three-dimensional culture environments38. These general but significant questions in mechanobiology of hPSCs remain to be answered in future studies.
Looking forward, we envision that innovative high-throughput, high-content approaches and tools integrating cellular biotechnology, materials science, microscale technology, mechanobiology, and advanced stem cell biology will be developed or adopted to characterize and understand the mechano-sensing and -transduction mechanisms and their involvements in regulation of hPSC self-renewal and differentiation. Due to the space limit, a detailed discussion of such high-throughput, high-content bioengineering and biointerfacial approaches and tools is not possible. The readers are referred to some excellent reviews elsewhere29a, 39. The ultimate functional goal for such research is to achieve the capability for predicting and ultimately controlling the integrated functional response of hPSCs. In the near future, these innovative tools, which can span different scales, from molecular (or subcellular) to cellular to organ levels, can allow us to generate dynamic and complex synthetic cellular microenvironments, with the molecular, structural, hydrodynamic, and mechanical cues well controlled in conjunction with their spatial and temporal levels and combinations, to regulate cell-ECM and cell-cell interactions of hPSCs. Miniaturized cell array methods based on these innovative techniques should also be developed to minimize reagent requirements. Such integrated cell-based high-throughput assays can be coupled with advanced biological tools to permit rapid, high-content, real-time monitoring of the effects of multiple mechanobiological stimuli on hPSC self-renewal and differentiation, speeding up the capacity for discovery of novel mechano-sensitive and -responsive behaviours of hPSCs.
Acknowledgments
Research in Dr. Fu's group is partially supported by the National Science Foundation (CMMI 1129611 and CBET 1149401), the American Heart Association (Scientist Development Grant, 12SDG12180025), and the department of Mechanical Engineering at the University of Michigan, Ann Arbor.
Biographies

Yubing Sun is a Ph. D. candidate in the Department of Mechanical Engineering at the University of Michigan, Ann Arbor. He received his B. S. from the University of Science and Technology of China in 2006. His current research interests include mechanobiology of stem cells, embryo development, and constructing synthetic ex vivo stem cell microenvironment using micro/nanoengineering tools.

Dr. Jianping Fu has been an assistant professor of Mechanical and Biomedical Engineering at the University of Michigan, Ann Arbor since 2009. Dr. Fu received his Ph. D. degree from the Massachusetts Institute of Technology in 2007. He was an American Heart Association Postdoctoral Fellow at the University of Pennsylvania from 2007 to 2009. Dr. Fu's research focuses on mechanobiology, stem cell biology, and applying microfabrication technology to illuminate biological systems at both the molecular and cellular levels. Dr. Fu is the recipient of the American Heart Association Scientist Development Grant (2012) and the National Science Foundation CAREER Award (2012).
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.(a) Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]; (b) Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotech. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 4.Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, Schultz PG. Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed Engl. 2011;50:200–42. doi: 10.1002/anie.201004284. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012 doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 6.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Zhou JX, Chowdhury F, Cheng JJ, Wang N, Wang F. Role of mechanical factors in fate decisions of stem cells. Regen Med. 2011;6:229–240. doi: 10.2217/rme.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, Ishizaki T, Suemori H, Narumiya S, Niwa H, Sasai Y. Molecular Pathway and Cell State Responsible for Dissociation-Induced Apoptosis in Human Pluripotent Stem Cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]; (b) Chen GK, Hou ZG, Gulbranson DR, Thomson JA. Actin-Myosin Contractility Is Responsible for the Reduced Viability of Dissociated Human Embryonic Stem Cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JE. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotech. 2010;28:1123–8. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]; (b) Fu JP, Wang YK, Yang MT, Desai RA, Yu XA, Liu ZJ, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Meth. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Bennett SAL, Wang LS. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhes Migr. 2012;6:59–70. doi: 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Meng Y, Eshghi S, Li YJ, Schmidt R, Schaffer DV, Healy KE. Characterization of integrin engagement during defined human embryonic stem cell culture. Faseb Journal. 2010;24:1056–1065. doi: 10.1096/fj.08-126821. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rowland TJ, Miller LM, Blaschke AJ, Doss EL, Bonham AJ, Hikita ST, Johnson LV, Clegg DO. Roles of Integrins in Human Induced Pluripotent Stem Cell Growth on Matrigel and Vitronectin. Stem Cells Dev. 2010;19:1231–1240. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 12.Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via αVβ5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 13.Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–5. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 14.(a) Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, Stojkovic M, Lako M. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]; (b) Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mattheyses AL, Sun YH, Menendez L, Kulik M, Dalton S. Signaling Network Crosstalk in Human Pluripotent Cells: A Smad2/3-Regulated Switch that Controls the Balance between Self-Renewal and Differentiation. Cell Stem Cell. 2012;10:312–326. doi: 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun YB, Villa-Diaz LG, Lam RHW, Chen WQ, Krebsbach PH, Fu JP. Mechanics Regulates Fate Decisions of Human Embryonic Stem Cells. Plos One. 2012:7. doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 17.(a) Li L, Wang SA, Jezierski A, Moalim-Nour L, Mohib K, Parks RJ, Retta SF, Wang LS. A Unique Interplay Between Rap1 and E-Cadherin in the Endocytic Pathway Regulates Self-Renewal of Human Embryonic Stem Cells. Stem Cells. 2010;28:247–257. doi: 10.1002/stem.289. [DOI] [PubMed] [Google Scholar]; (b) Li D, Zhou J, Wang L, Shin ME, Su P, Lei X, Kuang H, Guo W, Yang H, Cheng L, Tanaka TS, Leckband DE, Reynolds AB, Duan E, Wang F. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol. 2010;191:631–44. doi: 10.1083/jcb.201006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–34. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jastrebova N, Vanwildemeersch M, Lindahl U, Spillmann D. Heparan Sulfate Domain Organization and Sulfation Modulate FGF-induced Cell Signaling. Journal of Biological Chemistry. 2010;285:26842–26851. doi: 10.1074/jbc.M109.093542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klim JR, Li LY, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Meth. 2010;7:989–U72. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stelling MP, Lages YMV, Tovar AMF, Mouräo PAS, Rehen SK. Matrix Bound Heparan Sulfate Is Essential for the Growth and Pluripotency of Human Embryonic Stem Cells. Glycobiology. 2012 doi: 10.1093/glycob/cws133. [DOI] [PubMed] [Google Scholar]
- 22.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Keung AJ, Asuri P, Kumar S, Schaffer DV. Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells. Integrative Biology. 2012;4:1049–1058. doi: 10.1039/c2ib20083j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal. ACS Nano. 2012 doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. Plos One. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]; (b) Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, Cho SW, Mitalipova M, Pyzocha N, Rojas F, Van Vliet KJ, Davies MC, Alexander MR, Langer R, Jaenisch R, Anderson DG. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoldan J, Karagiannis ED, Lee CY, Anderson DG, Langer R, Levenberg S. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials. 2011;32:9612–21. doi: 10.1016/j.biomaterials.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotech. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Villa-Diaz L, Lam HWR, Chen W, Krebsbach P, Fu J. Proceedings of 16th International Conference on Miniaturized Systems for Chemistry and Life Sciences (μTAS 2012) Okinawa, Japan: 2012. In press. [Google Scholar]
- 29.(a) Lele TP, Sero JE, Matthews BD, Kumar S, Xia S, MontoyaZavala M, Polte T, Overby D, Wang N, Ingber DE. In: Methods in Cell Biology. YuLi W, Dennis ED, editors. Vol. 83. Academic Press; 2007. pp. 441pp. 443–472. [DOI] [PubMed] [Google Scholar]; (b) Kuo SC, Sheetz MP. Optical tweezers in cell biology. Trends Cell Biol. 1992;2:116–118. doi: 10.1016/0962-8924(92)90016-g. [DOI] [PubMed] [Google Scholar]; (c) Sniadecki NJ. A tiny touch: activation of cell signaling pathways with magnetic nanoparticles. Endocrinology. 2010;151:451–7. doi: 10.1210/en.2009-0932. [DOI] [PubMed] [Google Scholar]
- 30.(a) Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]; (b) Saha S, Ji L, de Pablo JJ, Palecek SP. TGF β/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa Si, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotech. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 32.Smith AL, Dohn MR, Brown MV, Reynolds AB. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012;23:99–110. doi: 10.1091/mbc.E11-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harb N, Archer TK, Sato N. The Rho-Rock-Myosin Signaling Axis Determines Cell-Cell Integrity of Self-Renewing Pluripotent Stem Cells. Plos One. 2008:3. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.(a) Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Meth. 2005;2:185–90. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]; (b) James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 36.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 37.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 38.Kraehenbuehl TP, Langer R, Ferreira LS. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods. 2011;8:731–6. doi: 10.1038/nmeth.1671. [DOI] [PubMed] [Google Scholar]
- 39.(a) Kshitiz, Kim DH, Beebe DJ, Levchenko A. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol. 2011;29:399–408. doi: 10.1016/j.tibtech.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim DH, Wong PK, Park J, Levchenko A, Sun Y. Microengineered platforms for cell mechanobiology. Annu Rev Biomed Eng. 2009;11:203–33. doi: 10.1146/annurev-bioeng-061008-124915. [DOI] [PubMed] [Google Scholar]


