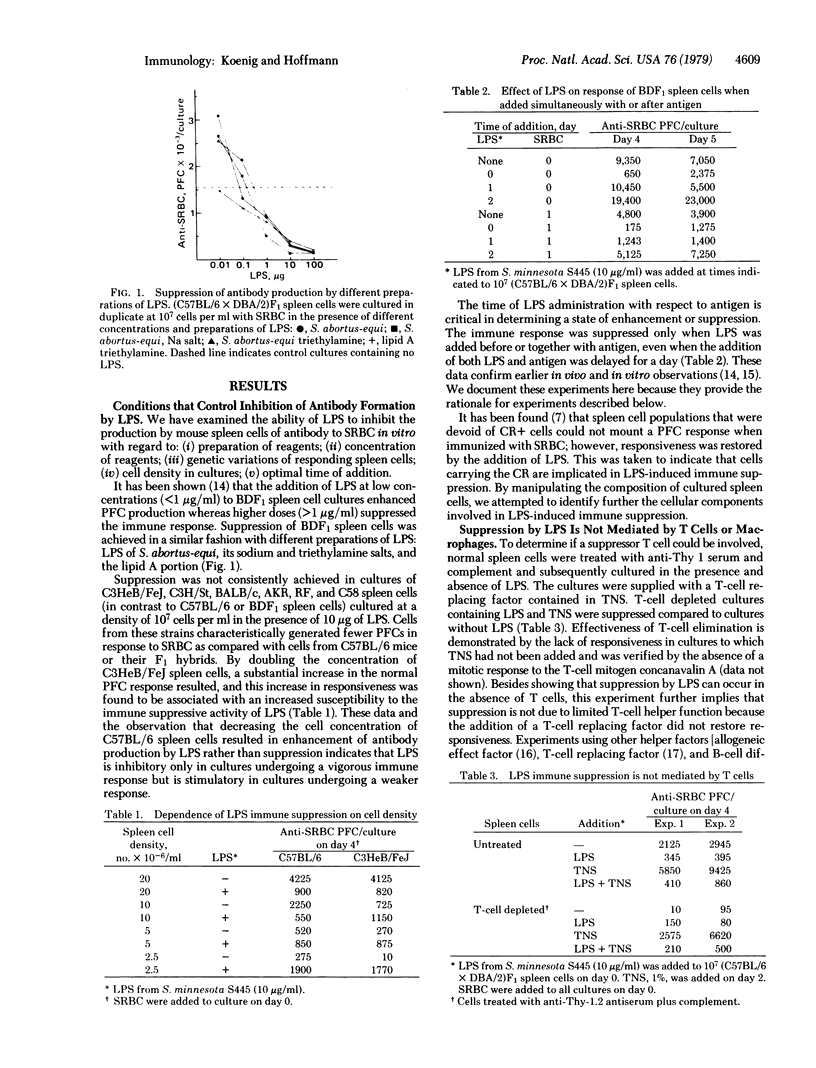
Abstract
Lipopolysaccharide (LPS) extracted from the outer cell wall of Gram-negative bacteria modulates the immune response in vivo and in vitro. Depending on the experimental conditions, it may enhance or inhibit the production of humoral antibody. The pathway by which LPS suppresses antibody production is examined in this study. C57BL/6 spleen cells incubated with LPS (greater than 10 micrograms/ml) not only fail to produce antibody to sheep erythrocytes in vitro but also, when transferred 24 hr after stimulation with LPS, inhibit antibody production in spleen cells that were not treated with LPS. This observation suggested that LPS activates suppressor cells. We have identified a suppressor B cell as mediator of LPS-induced immune suppression and determined its cell surface antigen phenotype as Ig+, Ia+, CR+, Ly-B-2+,PC1-.LPS does not induce suppressor macrophages or suppressor T cells, nor are macrophages or T cells required for the generation of suppressor B cells by LPS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. II. Biological and biochemical properties of an allogeneic effect factor (AEF) active in triggering specific B lymphocytes. J Exp Med. 1974 Jul 1;140(1):19–37. doi: 10.1084/jem.140.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. Restoration of antibody-forming capacity in cultures of nonadherent spleen cells by mercaptoethanol. Science. 1972 Apr 7;176(4030):60–61. doi: 10.1126/science.176.4030.60. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. Most IgM-producing cells in the mouse secrete auto-antibodies (rheumatoid factor). Nature. 1978 Aug 3;274(5670):480–483. doi: 10.1038/274480a0. [DOI] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. II. Evidence for separate stimulatory and inhibitory cells. J Exp Med. 1973 Dec 1;138(6):1496–1505. doi: 10.1084/jem.138.6.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzl R. E., McMaster P. D. The primary immune response in mice. I. The enhancement and suppression of hemolysin production by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1087–1107. doi: 10.1084/jem.127.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerling U., Chin A. F., Abbott J., Scheid M. P. The ontogeny of murine B lymphocytes. I. Induction of phenotypic conversion of Ia-to Ia+ lymphocytes. J Immunol. 1975 Nov;115(5):1425–1431. [PubMed] [Google Scholar]
- Hoffmann M. K., Galanos C., Koenig S., Oettgen H. F. B-cell activation by lipopolysaccharide. Distinct pathways for induction of mitosis and antibody production. J Exp Med. 1977 Dec 1;146(6):1640–1647. doi: 10.1084/jem.146.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K., Green S., Old L. J., Oettgen H. F. Serum containing endotoxin-induced tumour necrosis factor substitutes for helper T cells. Nature. 1976 Sep 30;263(5576):416–417. doi: 10.1038/263416a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann M. K., Hämmerling U., Simon M., Oettgen H. F. Macrophage requirements of CR- and CR+ B lymphocytes for antibody production in vitro. J Immunol. 1976 May;116(5):1447–1451. [PubMed] [Google Scholar]
- Hoffmann M. K., Oettgen H. F., Old L. J., Chin A. F., Hammerling U. Endotoxin-induced serum factor controlling differentiation of bone-marrow-derived lymphocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1200–1203. doi: 10.1073/pnas.74.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K., Weiss O., Koenig S., Hirst J. A., Oettgen H. F. Suppression and enhancement of the T cell-dependent production of antibody to SRBC in vitro by bacterial lipopolysaccharide. J Immunol. 1975 Feb;114(2 Pt 2):738–741. [PubMed] [Google Scholar]
- Hämmerling U., Chua R., Hoffmann M. K. Polyclonal activation of CR+ and CR- B lymphocytes: the kinetics of initiation of DNA and immunoglobulin synthesis by lipopolysaccharide. J Immunol. 1978 Mar;120(3):750–753. [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E., Pardon P. Effects of bacterial lipopolysaccharide on the induction and expression of cell-mediated immunity. I. Depression of the afferent arc. J Immunol. 1975 Jan;114(1 Pt 2):442–446. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson U. Lipopolysaccharide-induced suppression of the primary immune response to a thymus-dependent antigen. J Immunol. 1977 Mar;118(3):789–796. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Replacement of T-cell function by a T-cell product. Nat New Biol. 1972 May 3;237(70):15–17. doi: 10.1038/newbio237015a0. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Hämmerling U. Functional analysis of B cell subpopulations: responsiveness of phenotypically characterized CR+ and CR- B cell subsets to a T-dependent antigen in vitro. J Immunol. 1977 Nov;119(5):1700–1705. [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan-Bar I., Vitetta E. S., Assisi F., Strober S. The relationship between surface immunoglobulin isotype and immune function of murine B lymphocytes. III. Expression of a single predominant isotype on primed and unprimed B cells. J Exp Med. 1978 May 1;147(5):1374–1394. doi: 10.1084/jem.147.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L., Noworolski J., Mayhew B. Contact sensitivity to picryl chloride: the occurrence of B suppressor cells in the lymph nodes and spleen of immunized mice. Cell Immunol. 1976 Aug;25(2):266–278. doi: 10.1016/0008-8749(76)90117-9. [DOI] [PubMed] [Google Scholar]


