Abstract
The California five-spined ips, Ips paraconfusus Lanier, produces the myrcene-derived acyclic monoterpene alcohols ipsenol (2-methyl-6-methylene-7-octen-4-ol) and ipsdienol (2-methyl-6-methylene-2,7-octadien-4-ol) as components of its aggregation pheromone. The pine engraver beetle, Ips pini (Say), produces only ipsdienol. Previous studies have shown that myrcene, a monoterpene in the pines colonized by these beetles, is a direct precursor to these pheromone components. In vivo radiolabeling studies reported here showed that male I. paraconfusus incorporated [1-14C]acetate into ipsenol, ipsdienol, and amitinol (trans-2-methyl-6-methylene-3,7-octadien-2-ol), while male I. pini incorporated [1-14C]acetate into ipsdienol and amitinol. Females of these species produced neither labeled nor unlabeled pheromone components. The purified radiolabeled monoterpene alcohols from-males were identified by comparison of their HPLC and GC retention times with those of unlabeled standards. HPLC-purified fractions containing the individual radiolabeled components were analyzed by GC-MS and were shown to include only the pure alcohols. To further confirm that ipsdienol and ipsenol were radiolabeled, diastereomeric ester derivatives of the isolated alcohols were synthesized and analyzed by HPLC and GC-MS. After derivatization of the radiolabeled alcohols, the HPLC analysis demonstrated expected shifts in retention times with conservation of naturally occurring stereochemistry. The results provide direct evidence for de novo biosynthesis of ipsenol, ipsdienol, and amitinol by bark beetles.
Full text
PDF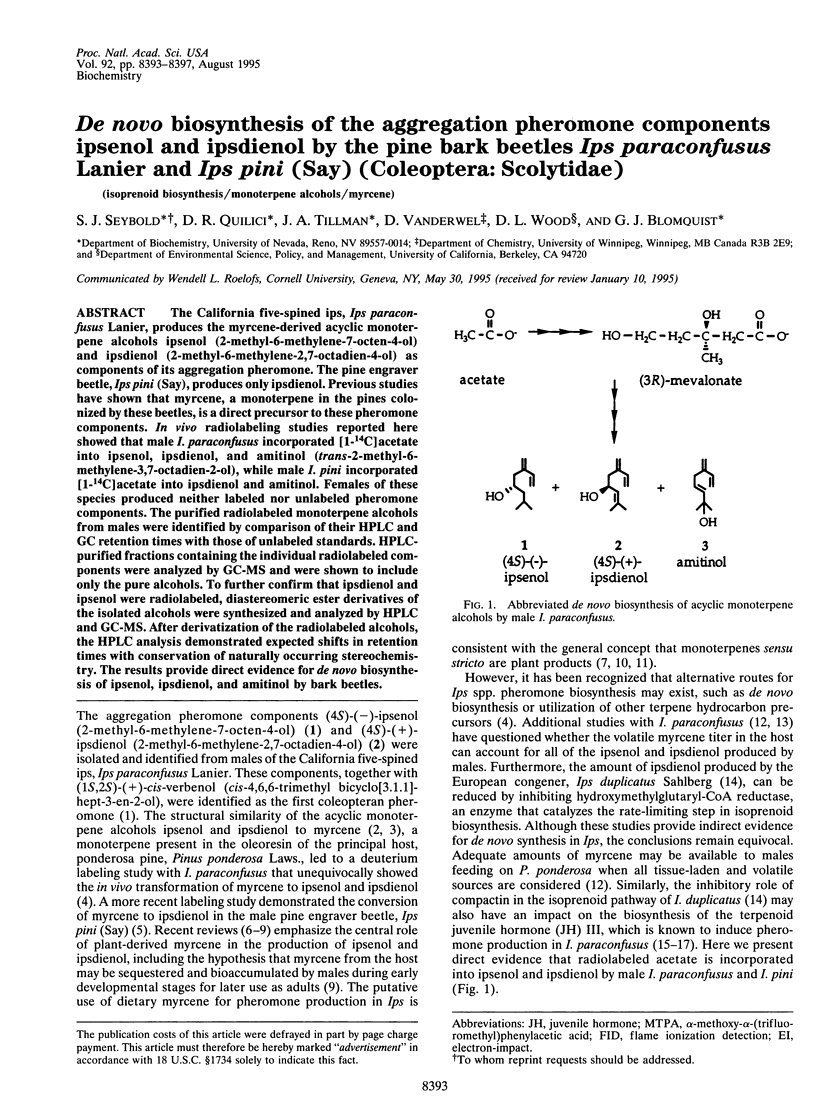


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank A. J., Wilson R. F., Kubo S. H., Holte J. E., Dresing T. J., Wang H. Direct effects of smooth muscle relaxation and contraction on in vivo human brachial artery elastic properties. Circ Res. 1995 Nov;77(5):1008–1016. doi: 10.1161/01.res.77.5.1008. [DOI] [PubMed] [Google Scholar]
- Boriden J. H., Nair K. K., Slater C. E. Synthetic Juvenile Hormone: Induction of Sex Pheromone Production in Ips confusus. Science. 1969 Dec 26;166(3913):1626–1627. doi: 10.1126/science.166.3913.1626. [DOI] [PubMed] [Google Scholar]
- HAPP G. M., MEINWALD J. BIOSYNTHESIS OF ARTHROPOD SECRETIONS. I. MONOTERPENE SYNTHESIS IN AN ANT (ACANTHOMYOPS CLAVIGER). J Am Chem Soc. 1965 Jun 5;87:2507–2508. doi: 10.1021/ja01089a046. [DOI] [PubMed] [Google Scholar]
- Meinwald J., Happ G. M., Labows J., Eisner T. Cyclopentanoid terpene biosynthesis in a phasmid insect and in catmint. Science. 1966 Jan 7;151(3706):79–80. doi: 10.1126/science.151.3706.79. [DOI] [PubMed] [Google Scholar]
- Wood D. L., Stark R. W., Silverstein R. M., Rodin J. O. Unique synergistic effects produced by the principal sex attractant compounds of Ips confusus (LeConte) (Coleoptera: Scolytidae). Nature. 1967 Jul 8;215(5097):206–206. doi: 10.1038/215206a0. [DOI] [PubMed] [Google Scholar]



