Abstract
Propionibacterium acnes induction of inflammatory responses is a major etiologic factor contributing to the pathogenesis of acne vulgaris. In particular, the IL-1 family of cytokines plays a critical role in both initiation of acne lesions and in the inflammatory response in acne. In this study, we demonstrated that human monocytes respond to P. acnes and secrete mature IL-1β partially via NLRP3 mediated pathway. When monocytes were stimulated with live P. acnes, caspase-1 and caspase-5 gene expression was upregulated; however, IL-1β secretion required only caspase-1 activity. P. acnes induced key inflammasome genes including NLRP1 and NLPR3. Moreover, silencing of NLRP3, but not NLRP1, expression by siRNA attenuated P. acnes-induced IL-1β secretion. The mechanism of P. acnes-induced NLRP3 activation and subsequent IL-1β secretion was found to involve potassium efflux. Finally, in acne lesions, mature caspase-1 and NLRP3 were detected around the pilosebaceous follicles and co-localized with tissue macrophages. Taken together, our results indicate that P. acnes triggers a key inflammatory mediator, IL-1β, via NLRP3 and caspase-1 activation, suggesting a role for inflammasome-mediated inflammation in acne pathogenesis.
Introduction
Acne vulgaris is the most common skin disease; yet the pathogenesis is not fully understood due to its complexity. Acne affects the majority of adolescent and young adult populations worldwide. Although acne is a non-lethal and self-limiting disease, its chronicity and effect on appearance and self-esteem can impose significant emotional morbidity.
Propionibacterium acnes which resides in pilosebaceous follicles in both acne and non-acne subjects, plays a key role in eliciting host inflammatory responses that are thought to be essential for the pathogenesis and responsible for the clinical manifestation of acne vulgaris (Bojar and Holland, 2004). P. acnes contributes to the inflammatory nature of acne by inducing innate immune cells to secrete pro-inflammatory cytokines including TNF-α, IL-6, IL-8 and IL-12 (Kim et al., 2002). The IL-1 family of cytokines has been implicated as an initiator and a key player in the pathogenesis of acne (Ingham et al., 1992). IL-1β has been shown to be a potent inducer of proinflammatory cytokines IL-6 and IL-8 in sebocytes suggesting a potential role in diseases of the pilosebaceous unit such as acne (Mastrofrancesco et al., 2010). Our laboratory and others have previously demonstrated that P. acnes induces inflammatory cytokines and metalloproteinases (MMPs) in part through Toll-like receptor (TLR)-2 (Jalian et al., 2008; Kim et al., 2002).
Nucleotide Oligomerization Domain (NOD) like receptors (NLRs), are an important class of cytosolic pattern recognition receptors sensing microbial molecules and danger signals and triggering inflammation and anti-microbial responses (Martinon and Tschopp, 2005). NLRs are a part of inflammasome complexes, which are a central component for regulation of IL-1β maturation and secretion (Martinon et al., 2002). Involvement of inflammasome complexes in inducing inflammatory responses in skin diseases, including psoriasis and Staphylococcus infection, has been recently demonstrated (Dombrowski et al., 2011; Miller et al., 2007; Murphy et al., 2000). However, the involvement of inflammasome activation in P. acnes-induced inflammation remains elusive. Therefore, we have investigated the role of inflammasome activation in IL-1β regulation in human monocytes in response to P. acnes.
Results
P. acnes triggers IL-1β secretion in human monocytes
Our previous studies showed that P. acnes induces the inflammatory cytokines IL-8 and IL-12 in human monocytes (Kim et al., 2002). In order to determine whether P. acnes induces IL-1β in primary human monocytes, we obtained monocytes from normal subjects and exposed them to live P. acnes at various MOIs (0.1, 0.5 and 1.0) for 24 hours and the expression of IL-1β mRNA was determined by qRT-PCR. P. acnes induced IL-1β gene expression by 100-200 fold in comparison to media control over a range of MOI (p<0.01, Fig. 1a). The induction of pro-IL-1p protein was determined by western blot analysis of cell lysates, confirming the upregulation of pro-IL-1β (31kD) when cells were stimulated with live P. acnes (Fig. 1b). In addition, secretion of mature IL-1β following stimulation with P. acnes was significantly induced (4,000-5,000 pg/ml) over a range of MOI (p<0.01) (Fig. 1c).
Fig 1. P. acnes induction of IL-1β in human monocytes.
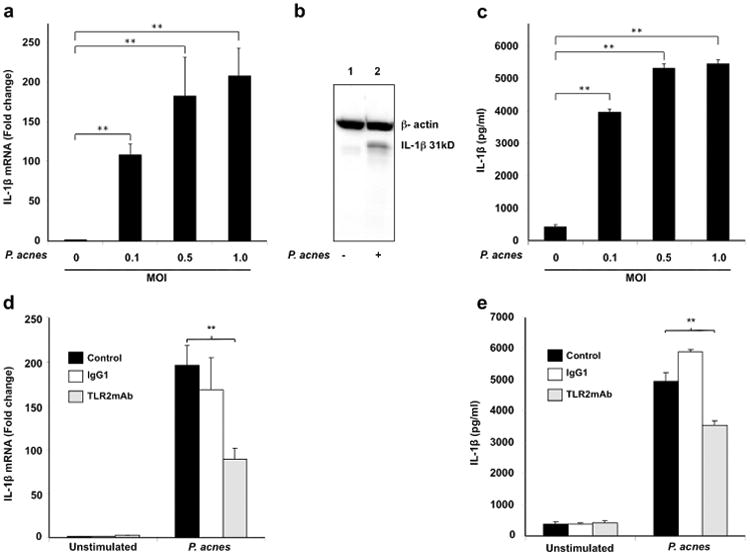
Primary human monocytes from normal donors were stimulated in the presence or absence of various concentrations of live P. acnes (MOI of 0.1, 0.5 and 1.0) for 24 hours. a) IL-1β gene expression was assessed by qRT-PCR. Data represents 3 donors; b) Pro-IL-1β protein (31kD band) in the cell lysates was determined by western blot; and c) Mature IL-1β secretion into culture supernatant was assessed by ELISA. Monocytes were pretreated anti-TLR2, or isotype control mAbs 30 minutes prior to stimulation with P. acnes (MOI 0.5) and the induction of IL-1β at mRNA and supernatant protein levels was determined by qPCR (d) and ELISA (e) respectively. Data represent mean ± SD (n=3; *p ≤ 0.05, **p ≤ 0.01).
Role of TLR2 in P. acnes induction of IL-1β in human monocytes
It has been shown that several bacterial components are critical activators of TLR2-mediated response (Lamkanfi et al., 2009a; Mariathasan et al, 2006; Sutterwala et al., 2006). Our previous studies showed that TLR2 mediates P. acnes induction of innate immune response in monocytes by inducing IL-8 and Il-12 production; however, whether TLR2 pathway is specifically involved in IL-1β regulation in the presence of P. acnes is not clear yet. Using TLR2 blocking Ab, we show that P. acnes induction of IL-1β is suppressed by approximately 50% at mRNA (Fig. 1d) and 40% at protein/secreted (Fig. 1e) levels respectively, indicating that TLR2 is at least partially but not soley involved in IL-1β induction.
Caspase-1 activation is required for P. acnes-induced IL-1β secretion
In order to determine other factors/pathways in addition to TLR2 that mediate P. acnes induction of IL-1β, we studied inflammasome complex involved in inducing innate immune response. Since mature IL-1β requires proteolytic cleavage by inflammatory caspases, we examined the expression of two caspases, caspase-1 and caspase-5 involved in inflammation (Martinon and Tschopp, 2007). We found that monocytes stimulated with P. acnes significantly induced the mRNA expression of both caspase-1 and caspase-5, by approximately 5-fold and 6-fold respectively, in comparison to cells cultured in media alone (p<0.01) (Fig. 2a).
Fig 2. P. acnes induction of IL-1β is dependent on caspase-1 in human monocytes.
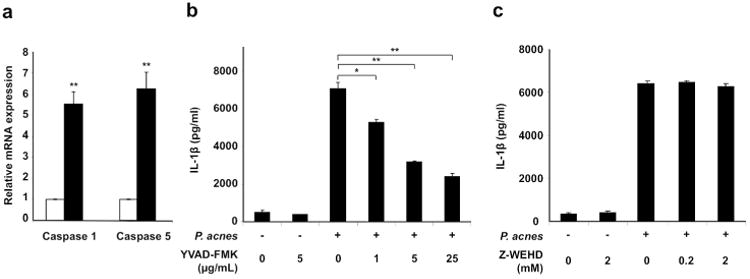
a) Cells were stimulated with P. acnes (MOI of 0.5) for 24 hours and the mRNA expression of caspase-1 and caspase-5 were determined using qRT-PCR and normalized against the expression of GAPDH. Cells were treated with b) Z-YVAD-FMK, caspase-1 inhibitor or c) Z-WEHD-FMK, caspase-5 inhibitor, for 30 minutes then stimulated with P. acnes (MOI of 0.5). Culture supernatants were collected after 24 hours and assayed with IL-1β ELISA. Data represent mean ± SD (n=3; * p ≤ 0.05, **p ≤ 0.01).
In order to determine whether caspase-1 and/or caspase-5 played a role in P. acnes-induced IL-1β secretion, we performed inhibitor studies. Monocytes were pre-treated with either Z-YVAD-FMK or Z-WED-FMK, specific inhibitors of caspase-1 and caspase-5, respectively, prior to stimulation with live P. acnes. IL-1β secretion following P. acnes stimulation was inhibited in a concentration-dependent manner by the caspase-1 inhibitor, Z-YVAD-FMK (p<0.01, p<0.05) (Fig. 2b). In contrast, there was no significant change in IL-1β secretion in the presence of the caspase-5 specific inhibitor (Fig. 2c). In comparison, P. acnes induced IL-6 in the presence of both caspase-1 and caspase-5 inhibitors (supplementary data, Fig 1) suggesting that specific induction of IL-1β by P. acnes is dependent on caspase-1.
P. acnes induction of IL-1β is dependent on NLRP3 inflammasome
In order to identify the specific inflammasome involved in P. acnes induction of IL-1β, we first analyzed the gene expression of major NLRs by RT-PCR in monocytes treated with or without P. acnes. Our results show that P. acnes induces gene expression of NLRP1 and NLRP3 in human monocytes (Fig. 3a). In contrast, AIM2 and IPAF were expressed in unstimulated monocytes and their expression was not affected by P. acnes treatment (Fig. 3a).
Fig 3. P. acnes induction of IL-1β is NLRP3-dependent in human monocytes.
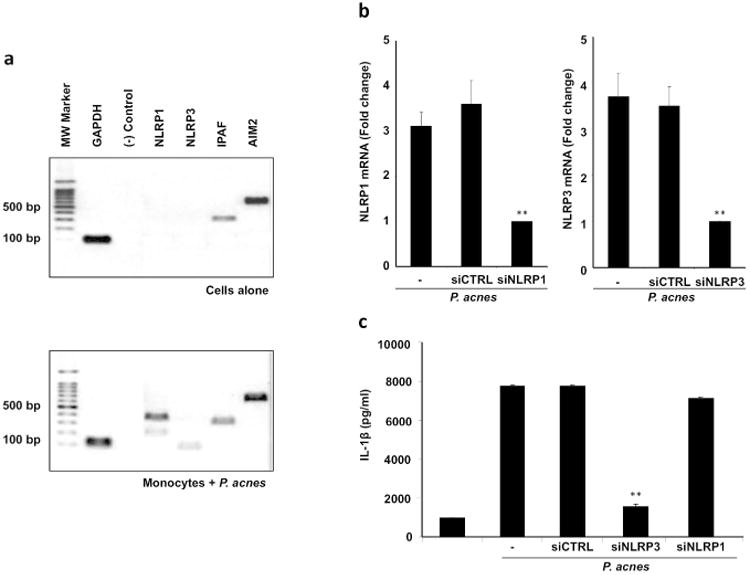
a) Monocytes were stimulated with P. acnes (MOI 0.5) and the gene expression of major NLRs was determined after 24 hours using RT-PCR. Data is representative of four independent experiments; b) Monocytes were transfected with siRNA oligos specific for NLRP3, NLRP1 or a non-specific siRNA oligo (siCTRL) prior to stimulations with P. acnes (MOI 0.5) and the levels of NLRP3 and NLRP1 were assessed by qRT-PCR and are represented as fold change versus media control; and c) siRNA transfected cells were then stimulated with P. acnes (MOI of 0.5) then culture supernatants were collected after 24 hours and assayed with IL-1β ELISA (n=3; ** p ≤ 0.01).
To determine whether NLRP1 and/or NLRP3 mediate P. acnes-induced IL-1β secretion we utilized siRNA to silence specific gene expression. Transfection of monocytes with specific siRNA reduced P. acnes-induced NLRP1 and NLRP3 expression by 68 and 73% (p<0.01) respectively, while a non-specific control siRNA (siCTRL) had no effect (Fig. 3b). Importantly, we found that the NLRP3-specific siRNA suppressed P. acnes-induced IL-1β secretion by approximately 90% (p<0.01) while the NLRP1-specific siRNA and siCTRL had no significant effect on IL-1β secretion (Fig. 3c). Taken together, these results suggest that the NLRP3 inflammasome is necessary for P. acnes-induced IL-1β generation and secretion.
Potassium efflux is required for P. acnes induced IL-1β secretion
In order to further study the mechanism of NLRP3 inflammsome activation by P. acnes, we used glybenclamide, a selective inhibitor of ATP-sensitive K+ channels that blocks maturation of caspase-1 (Lamkanfi et al., 2009b). Treatment of monocytes with glybenclamide prior to stimulation with P. acnes inhibited activation of caspase-1 as determined by western blot (Fig. 4a). While P. acnes stimulation induces conversion of procaspase-1 (45 kD) to the active forms, p10 and p20, in the presence of either caspase-1 inhibitor or glybenclamide, both p10 and p20 expression were downregulated (Fig. 4a).
Fig 4. P. acnes activation of caspase-1 and IL-1β secretion in human monocytes is regulated by K+ efflux.
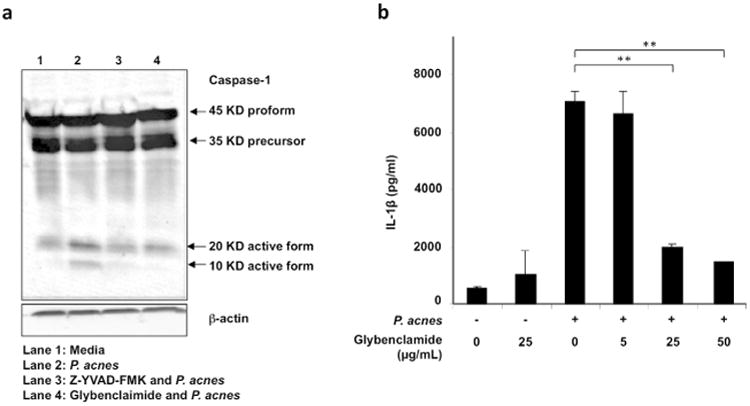
Primary human monocytes were treated with glybenclamide or with Z-YVAD-FMK for 30 minutes prior to stimulation with P. acnes (MOI of 0.5). a) Cells were lysed after 24 hours and caspase-1 activation was assessed by western blot; and b) Cells were treated with glybenclamide at various concentrations (5-50 μg) for 30 minutes prior to stimulation with P. acnes (MOI of 0.5). Culture supernatants were collected after 24 hours and assayed by IL-1β ELISA (n=3; **p ≤ 0.01).
Furthermore, P. acnes-induced IL-1β secretion was inhibited by glybenclamide in a concentration-dependent manner (p<0.01) (Fig. 4b). In contrast, secretion of IL-6 was unaffected by glybenclamide (supplementary data, Fig 2). Therefore, our data suggest that potassium efflux is required for NLRP3 activation of caspase-1 and subsequent IL-1β secretion in response to stimulation with P. acnes.
NLRP3 and active caspase-1 are expressed in acne lesions and co-localize in tissue macrophages
We examined acne lesions for in situ expression of NLRP3 and caspase-1 to correlate our in vitro findings and provide clinical relevance. Immunofluorescence labeling together with confocal laser microscopy using specific monoclonal antibodies revealed higher cellular expression of NLRP3 and active caspase-1 in the dermis surrounding the pilosebaceous follicles in acne lesions (Fig 5a, left and middle image) compared to normal skin control (Fig 5b, left and middle image). Additionally, a higher prevalence of CD68+ monocytes/macrophages in acne lesions (Fig 5a, right image) vs. normal skin control was noted (Fig 5b, right image). To confirm the cell lineage expressing NLRP3 and caspase-1 in acne lesions, we performed double immunofluorescence labeling utilizing monocyte/macrophage marker CD68. We first demonstrated that caspase-1 and NLRP3 co-localized (Fig. 5c, left image). In addition, both caspase-1 (Fig. 5c, middle) and NLRP3 (Fig. 5c, right) individually colocalized with CD68. These findings suggest that caspase-1 and NLRP3 are expressed in CD68+ monocytes/macrophages in acne lesions (Fig 5c). Triple immunofluorescence labeling showed that both NLRP3 and caspase-1 colocalized with the monocyte/macrophage marker CD68 (Fig. 5d, left and middle image). In contrast to acne lesions, staining of normal skin revealed few foci of NLRP3 and caspase-1 fluorescence within dermal macrophages (Fig 5d, right image).
Fig 5. NLRP3 and caspase-1 are expressed and colocalized with CD68+ macrophages in acne lesions.
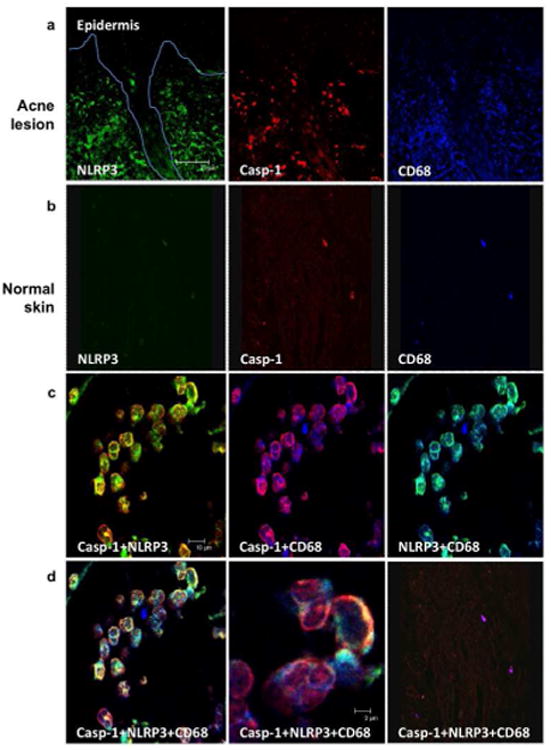
Three-color immunofluorescence confocal images were obtained for NLRP3 (green), caspase-1 (red), and CD68 (blue) in a) acne lesions and b) normal skin. Original magnification 20× (scale bar represents 75 μm). The image is a representative data of at least three skin lesions obtained from skin lesions; c) The images were then super-imposed and double positive cells are shown in yellow (caspase-1+NLRP3), pink (caspase-1+CD68) and turquoise (NLRP3+CD68), original magnification 40× (scale bar represents 10 μm); and d) The overlay of NLRP3, caspase-1, CD68, is seen in increasing magnification, 40× and 63× (scale bar represents 2 μm) respectively, (left and middle); d) right panel represents staining of normal tissue, magnification 20×. Data is representative of experiments with tissue obtained from three independent donors.
Discussion
Recognition of microbial pathogens by innate immune cells triggers inflammatory signals to combat infection and prevent disease. Yet the same mechanism can also lead to uncontrolled inflammation resulting in tissue injury and disease. In acne, P. acnes-induced inflammatory response, including the secretion of IL-1, represents a key pathogenic factor leading to disease manifestation (Ingham et al., 1992; Mastrofrancesco et al., 2010). Here we investigated the molecular mechanism by which P. acnes induces IL-1β generation in human monocytes. Our results demonstrate that P. acnes triggers IL-1β generation through an inflammasome pathway, specifically involving NLRP3 and caspase-1. The induction of IL-1β by P. acnes also involves a mechanism that is dependent on K+ efflux. Furthermore, we provide evidence in vivo that NLRP3 and active caspase-1 are expressed by CD68+ tissue macrophages in acne lesions, infiltrating around pilosebaceous follicles, further suggesting involvement of inflammasome complexes in acne pathogenesis. Our study suggests a mechanism by which P. acnes triggers innate immune response through activation of NLRP3 inflammasome and secretion of IL-1β.
In addition to inflammasome-mediated response, we also demonstrate that TLR2 signaling leads to a partial induction of IL-1β. We have previously demonstrated that P. acnes stimulates innate immune cells to induce inflammatory cytokines that contributes to disease pathology via TLR2 activity (Kim et al., 2002). The involvement of TLR2 in P. acnes-induction of IL-1β was not known. Here our results are consistent with other studies which have shown that TLR2 ligands such as zymosan and lipoteichoic acid induce inflammsome-mediated IL-1β release as well (Lamkanfi et al., 2009a; Mariathasan et al., 2006; Sutterwala et al., 2006). P. acnes TLR2 ligand has not been identified but previous studies provide clues and lipoteichoic acid on the cell wall of P. acnes could be a possible TLR2 ligand candidate.
While TLR2 involves recognition of P. acnes at the cell surface by a pattern recognition receptors (PRR), our study demonstrating NLRP3 requirement for P. acnes-induced IL-1β secretion suggest activation of a cytoplasmic PRR of innate immune cells by P. acnes. While P. acnes is an extracellular pathogen, studies suggest that the bacterium can be internalized by phagocytes and persist within the cells to induce chronic inflammation (Liu et al., 2008). Studies have shown that microbes genetically similar to P. acnes, such as Mycobacterium abscessus and M. tuberculosis can also activate NLRP3 (Dorhoi et al., 2012; Lee et al., 2012). The extracellular skin pathogen Staphylococcus aureus and intracellular pathogens such as Listenria monocytogenes also activate inflammasomes (Kim et al., 2010). Our results demonstrating that P. acnes activates NLR signaling, specifically NLRP3 in human monocytes is therefore consistent with what is known for other skin pathogens.
The precise mechanism involved in activation of the NLRP3 inflammasome remains unclear. However, we demonstrate that K+ efflux is necessary for NLRP3 activation of caspase-1 in human monocytes and subsequent IL-1β release in response to stimulation with P. acnes. Activation of the NLRP3 inflammasome by extracellular bacteria including Staphylococcus aureus and Escherichia coli has been shown to require potassium efflux through ATP-sensitive K+ channels in response to ATP (Lamkanfi et al., 2009b). In contrast, activation of NLRP3 inflammasome by intracellular bacteria such as Salmonella and Listeria in murine macrophages does not require K+ efflux, suggesting different mechanisms mediating NLRP3 activation in response to intracellular and extracellular bacteria (Franchi et al., 2012).
Although a P. acnes ligand for NLRP3 is not known, there are some clues from recent studies, which have identified specific bacterial ligands directly activating NLRP3. For example, M. tuberculosis protein ESAT 6 was recently shown to directly activate the NLRP3 inflammasome and trigger IL-1β release (Mishra et al., 2010). Martinon et al. identified bacterial muramyl dipeptide as a direct activator of the NLRP3 inflammasome (Martinon et al., 2004). Given that the P. acnes cell wall also contains muramyl dipeptide (Girvan et al., 2011), it is possible that this might be considered a potential ligand that activates NLRP3.
While the cellular structure of P. acnes may contain NLRP3 ligand(s) and directly activate NLRP3, it is also possible that P. acnes instead stimulate cellular stress through activation and release of reactive-oxygen species (ROS) that can then activate NLR signaling. NLRP3 has been hypothesized to sense cellular stress through activation by ROS in response to stimulation with several pathogens including Respiratory Syncytial Virus (RSV), Aspergillus fulmigatus, and Plasmodium falciparum (Dostert et al., 2009; Said-Sadier et al., 2010; Tschopp and Schroder, 2010). A recent study demonstrated that the production of ROS by keratinocytes initiates P. acnes-induced inflammation in skin (Grange et al., 2009). Whether the mechanism of this inflammation requires NLR signaling remains elusive.
TLR2 signaling has been shown to contribute to rapid inflammasome activation during Francisella novicida infection (Jones and Weiss 2011). In addition TLR activation regulates NLRP3 gene expression in monocytes infected with respiratory syncytial virus (Segovia et al., 2012). In this study, we demonstrate that TLR2 partially mediates induction of IL-1β in response to P. acnes. However, further studies are required in order to assess whether TLR2 activation by P. acnes regulates NLRP3 expression and inflammasome activation.
In this study we focus on monocytes and tissue macrophages since we have previously demonstrated that tissue macrophages surrounding the pilosebaceous unit in acne lesions expressed high levels of TLR2 and correlated with the degree of inflammatory nature of the clinical lesions (Kim et al., 2002). Similarly, here we observed that the expression of a cytosolic PRR, NLRP3 inflammasome, is expressed in tissue macrophages and the macrophages are abundant in inflammatory acne. It is likely that other innate cells, which make up the pilosebaceous unit such as keratinocytes and sebocytes and other dermal inflammatory cells such as neutrophils also express inflammasome proteins and mediate inflammation in acne. In fact, keratinocytes express inflammasome complexes and induce inflammation in other disease models (Johansen et al., 2007; Watanabe et al., 2007). While some of our experiments are limited as we use peripheral monocytes, there is more evidence to suggest that acne maybe a systemic condition as diet, stress and metabolic conditions have been associated with acne (Lolis et al., 2009). The use and study of peripheral monocytes therefore maybe more relevant than initially thought.
In conclusion, we have shown that stimulation of monocytes with P. acnes in vitro results in IL-1β secretion in a partially NLRP3 inflammasome-mediated mechanism. We have further demonstrated that caspase-1 and NLRP-3 are expressed in vivo in dermal macrophages surrounding the pilosebaceous follicles in acne lesions. In light of the potential role of IL-1β in acne pathogenesis, anti-IL-1β and/or molecules that could regulate NLRP3, caspase-1 or K+ efflux should be considered in treatments of acne. Further understanding of P. acnes-induced IL-1β regulation through inflammasome pathways should allow for methods of immunologic intervention in acne and in other IL-1β-mediated skin diseases.
Materials and Methods
Antigens and Reagents
P. acnes strain ATCC 6919 was obtained from American Type Culture Collections and was grown in the miniMACS anaerobic workstation (Don Whitley Scientific. West Yorkshire, UK). Briefly, Brucella agar plates supplemented with blood, hemin and vitamin K (Remel-Fisher scientific) were streaked with P. acnes overnight. Single colonies of bacteria were isolated and used to inoculate reinforced Clostridium medium. The cultures were grown in an anaerobic chamber for 4-5 days. A spectrophotometer OD600 was used to determine the bacterial log phase. Bacterial cultures were diluted at a final concentration of 0.1, 0.5 and 1 Multiplicity of Infection (MOI) and used to stimulate cells. We have selected live P. acnes as opposed to sonicated preparations of P. acnes due to the pathophysiological relevance of our model and consistency on the observed responses.
Caspase-1 and caspase-5 inhibitors (Z-YVAD-fmk and Z-WED-fmk, respectively) were purchased from Biovision (Milpitas, CA). Conditions and effectiveness of caspase inhibitors were established based on previous studies and according to manufacturer's recommendations (Garcia-Calvo et al., 1998; Thornberry et al., 1997). Glybenclamide was purchased from InvivoGen (San Diego, CA). Anti-Human TLR-2 monoclonal antibody and appropriate IgG1 isotype antibody were purchased from eBioscience (San Diego, CA).
For western blot analysis, we used the following specific antibodies; pro-IL-1β (R&D systems), caspase-1 (Santa Cruz Biotechnology), and β-actin (Cell Signaling Technology). Secondary antibodies were horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgGs (R&D systems).
Isolation of monocytes and cytokine ELISA
PBMCs were isolated from whole blood of normal healthy donors as approved by the Institutional Review Board at UCLA using Ficoll-Paque gradients (GE Healthcare. Piscataway, NJ) and plated onto 6 well tissue culture plates (20 × 106 to 30 × 106/well) for 2 hours in RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 1% FCS (Omega Scientific, Tarzana, CA). Nonadherent cells were removed by rigorously washing three times with RPMI. Adherent monocytes were cultured in 10% FCS with P. acnes at MOI 0.1, 0.5 and 1.0, for 24 hours at 37°C. Supernatants were collected and assayed by IL-1β specific ELISA with quantification by reference to a recombinant IL-1β standard (eBioscience). Samples were assayed in triplicates. Results are expressed as mean ± SD of at least 3 independent experiments, with monocytes obtained from 3 independent donors.
RNA isolation, cDNA synthesis and real-time PCR
Monocytes were stimulated with media or with P. acnes at MOI 0.1, 0.5 or 1.0, for 24 hours at 37°C. Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) following manufacturer's recommendations and treated with RNase-free DNase. RNA concentration and quality were determined by spectrophotometry. RNA samples were reverse-transcribed to cDNA using iScript cDNA synthesis kit (BIO-RAD). Reactions were done at 25°C for 5 min, 42°C for 30 min and 85°C for 5 min.
cDNA was used as templates for subsequent PCR amplification using gene-specific primers (Table S1). Conventional PCR was performed in a cycling condition as follows: 5 min at 95°C followed by 30 cycles of 20 sec at 95°C, 20 sec at 60°C and 30 sec at 72°C with a final step at 72°C for 8 min to allow complete extension of all amplified fragments. The amplified products were visualized by running on 2% agarose gel electrophoresis.
Real-time PCR was performed using iQ SYBR Green supermix (BIO-RAD). 40 cycles were carried out at 95°C for 5 min, then 95°C for 10 sec, 55°C for 20 sec, 72°C for 20 sec. GAPDH amplification was used as internal standard for normalization. Primers were designed using Primer Express (Applied Biosystems, Foster City, CA) and listed in Table 1 in Supplementary Materials and Methods. Gene expression level was quantified by the comparative method 2-ΔΔCT.
siRNA transfection
siRNA transfection into monocytes was accomplished by using the Amaxa Nucleofector system and the Human Monocyte Nucleofector Kit according to the manufacturer's recommendations with program U-001 for high viability. siRNA constructs were used at 1 μg per transfection. Transfection efficiency of expression constructs was assessed using the pMAX-GFP construct, and yielded an average of 70% transfection rate (Lonza Inc. Allendale, NJ).
Western blot analysis
Detection of proIL-1β and caspase-1 cleavage was performed by western blot analysis. Briefly, 24 hours after treatment with P. acnes, cells were harvested and lysed in 100 μL of lysis buffer (M-PER Mammalian Protein Extraction Reagents, Thermo Scientific) with added protease inhibitors. Protein profiles were separated by electrophoresis in 8 to 12% SDS-polyacrylamide gels and transferred onto Millipore PVDF membranes, and specific proteins were detected by the appropriate primary and secondary antibodies before visualization using VersaDOC MP5000 (BIO-RAD).
Immunofluorescence labeling and confocal microscopy
Three typical inflammatory acne lesions and three normal skin tissues from independent individuals were obtained from the Dankook University Hospital in accordance and approval of the Institutional Review Board. After deparaffinization and antigen retrieval with citrate buffer, acne sections were blocked with 10% goat serum for 30 min. Triple immunofluorescence was performed with a cocktail solution of mouse anti-human caspase-1 IgG2a (0.5 μg/ml, R&D) and mouse anti-human CD68 IgG1 (1:300, abcam). After overnight incubation, the sections were incubated for 1 hour in a cocktail of Alexa fluor 568 conjugated goat anti-mouse IgG2a and Alexa fluor 647 conjugated goat anti-mouse IgG1. The sections were incubated with mouse anti-human NLRP3 IgG2b (1: 300, Adipogen) for 1 hour and followed with Alexa fluor 488 conjugated goat anti-mouse IgG2b. Sections were mounted in mounting medium (Vector Laboratories) and images were obtained and analyzed using a Leica SP1 confocal microsystem (Leica Microsystems, Inc. Buffalo Grove, IL).
Statistical analysis
Results are expressed as the means ± standard deviation (SD) for the number of separate experiments indicated in each case (n ≥ 3). One-way analysis of variance was used to compare variances within groups and among them. Post hoc two-tailed Student's t test was used for comparison between two groups. Significant differences were considered for those probabilities ≤5% (p ≤ 0.05)
Supplementary Material
Fig S1. P. acnes induction of IL-6 is not affected by Z-YVAD-FMK in human monocytes. Cells were treated with Z-YVAD-FMK or Z-WEHD-FMK for 30 minutes prior to stimulation with P. acnes (MOI of 0.5). Culture supernatants were collected after 24 hours and assayed with IL-6 ELISA. Data are representative of 3 independent experiments.
Fig S2. P. acnes induction of IL-6 is not affected by glybenclamide in human monocytes. Primary human monocytes were treated with glybenclamide (5-50 μg) for 30 minutes, then stimulated with P. acnes (MOI of 0.5). Culture supernatants were collected after 24 hours and assayed by IL-1β ELISA. Data are representative of 3 independent experiments.
Table S1
Acknowledgments
This work has been partially supported by NIAMS R01 AR053542 [J.K.] and The American Acne and Rosacea Society, Research Grant [A.P.].
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- Bojar RA, Holland KT. Acne and Propionibacterium acnes. Clin Dermatol. 2004;22:375–9. doi: 10.1016/j.clindermatol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3:82–a38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhoi A, Nouailles G, Jorg S, Hagens K, Heinemann E, Pradl L, et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol. 2012;42:374–84. doi: 10.1002/eji.201141548. [DOI] [PubMed] [Google Scholar]
- Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, et al. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13:449–56. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–13. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- Girvan RC, Knight DA, O'Loughlin CJ, Hayman CM, Hermans IF, Webster GA. MIS416, a non-toxic microparticle adjuvant derived from Propionibacterium acnes comprising immunostimulatory muramyl dipeptide and bacterial DNA promotes cross-priming and Th1 immunity. Vaccine. 2011;29:545–57. doi: 10.1016/j.vaccine.2010.10.040. [DOI] [PubMed] [Google Scholar]
- Grange PA, Chereau C, Raingeaud J, Nicco C, Weill B, Dupin N, et al. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009;5:e1000527. doi: 10.1371/journal.ppat.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98:895–901. doi: 10.1111/1523-1747.ep12460324. [DOI] [PubMed] [Google Scholar]
- Jalian HR, Liu PT, Kanchanapoomi M, Phan JN, Legaspi AJ, Kim J. All-trans retinoic acid shifts Propionibacterium acnes-induced matrix degradation expression profile toward matrix preservation in human monocytes. J Invest Dermatol. 2008;128:2777–82. doi: 10.1038/jid.2008.155. [DOI] [PubMed] [Google Scholar]
- Johansen C, Moeller K, Kragballe K, Iversen L. The activity of caspase-1 is increased in lesional psoriatic epidermis. J Invest Dermatol. 2007;127:2857–64. doi: 10.1038/sj.jid.5700922. [DOI] [PubMed] [Google Scholar]
- Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–41. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–51. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009a;284:20574–81. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009b;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Yuk JM, Kim KH, Jang J, Kang G, Park JB, et al. Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1-Syk and p62/SQSTM1. Immunol Cell Biol. 2012;90:601–10. doi: 10.1038/icb.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Phan J, Tang D, Kanchanapoomi M, Hall B, Krutzik SR, et al. CD209(+) macrophages mediate host defense against Propionibacterium acnes. J Immunol. 2008;180:4919–23. doi: 10.4049/jimmunol.180.7.4919. [DOI] [PubMed] [Google Scholar]
- Lolis MS, Bowe WP, Shalita AR. Acne and systemic disease. Med Clin North Am. 2009;93:1161–81. doi: 10.1016/j.mcna.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–34. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Mastrofrancesco A, Kokot A, Eberle A, Gibbons NC, Schallreuter KU, Strozyk E, et al. KdPT, a tripeptide derivative of alpha-melanocyte-stimulating hormone, suppresses IL-1 beta-mediated cytokine expression and signaling in human sebocytes. J Immunol. 2010;185:1903–11. doi: 10.4049/jimmunol.0902298. [DOI] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–42. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–63. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Robert C, Kupper TS. Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol. 2000;114:602–8. doi: 10.1046/j.1523-1747.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, et al. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–11. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–63. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. P. acnes induction of IL-6 is not affected by Z-YVAD-FMK in human monocytes. Cells were treated with Z-YVAD-FMK or Z-WEHD-FMK for 30 minutes prior to stimulation with P. acnes (MOI of 0.5). Culture supernatants were collected after 24 hours and assayed with IL-6 ELISA. Data are representative of 3 independent experiments.
Fig S2. P. acnes induction of IL-6 is not affected by glybenclamide in human monocytes. Primary human monocytes were treated with glybenclamide (5-50 μg) for 30 minutes, then stimulated with P. acnes (MOI of 0.5). Culture supernatants were collected after 24 hours and assayed by IL-1β ELISA. Data are representative of 3 independent experiments.
Table S1


