Abstract
Background
Sexual dysfunction is a known complication of adjuvant therapy for breast cancer and an important determinant of quality of life, however few studies have explored how treatment and other factors affect sexual functioning in young breast cancer survivors.
Methods
461 pre-menopausal women with Stage 0–III breast cancer were surveyed an average of one year after diagnosis as part of a prospective cohort study of women aged 40 or younger at diagnosis. Sexual interest and dysfunction were assessed using the Cancer Rehabilitation Evaluation System (CARES). Mean CARES scores were compared and multiple regression models were fit to assess treatment and a range of menopausal and somatic symptoms in relation to sexual functioning.
Results
Mean CARES sexual interest and dysfunction scores were both highest (indicating poorer functioning) among women who received chemotherapy and were amenorrheic from treatment. After accounting for menopausal and somatic symptoms, treatment-associated amenorrhea remained associated with decreased interest but was no longer an independent predictor of dysfunction. In the multivariable analysis, independent predictors of dysfunction included vaginal pain symptoms, poorer body image and fatigue. Sexual interest was associated with vaginal pain symptoms, body image and weight problems.
Conclusion
Factors associated with decreased sexual functioning in young breast cancer survivors can often be ameliorated. Our findings have implications for pre-menopausal women with other types of cancer who might be experiencing amenorrhea due to chemotherapy or surgery. Increased awareness and early intervention is essential to help improve sexual functioning and associated quality of life for all young cancer survivors.
Keywords: Sexual dysfunction, breast cancer, amenorrhea, chemotherapy, pre-menopausal
Introduction
With over 200,000 incident cases of invasive carcinoma reported annually, breast cancer is the most frequently diagnosed cancer among women in the United States.1 Breast cancer is also the most common cancer among younger women, representing over one-quarter of all cancers diagnosed in women aged 20–39. 2 A breast cancer diagnosis at any age lends itself to numerous quality of life (QOL) concerns, however younger women are more likely to experience compromised QOL relative to older women. 3–5
Sexual functioning is an important QOL issue often overlooked during the early survivorship period, with studies reporting an association between adjuvant treatment and sexual dysfunction in breast cancer patients during the first year following diagnosis. 6, 7 Most findings relating to sexual dysfunction among breast cancer survivors are based on peri- and post-menopausal women; few studies have explored how treatment affects sexual functioning in women who are pre-menopausal at diagnosis. Using a large prospective cohort, we sought to: 1) describe treatment-associated differences in sexual functioning at one year post-diagnosis; 2) assess additional factors, including menopausal symptoms, body image and somatic symptoms, in relation to sexual functioning in younger women. Specifically, we hypothesized that women experiencing amenorrhea due to treatment would have worse functioning compared to women who were treated with chemotherapy but continued to menstruate, and women who did not receive chemotherapy. Evaluating whether certain factors are associated with worse sexual outcomes can help identify targets for intervention, with the goal of preventing and treating sexual dysfunction as early as possible, thus minimizing the risk of long term problems, such as vaginal atrophy.
Methods
Study population
Beginning in November 2006, we enrolled women from ten sites into the Helping Ourselves, Helping Others: The Young Women’s Breast Cancer Study, a prospective cohort study established to explore biological, medical, and QOL issues specific to young women with breast cancer. For the nine sites in Massachusetts, women were identified using the Rapid Case Identification Core of the Dana-Farber/Harvard Cancer Center. As of December 2012, 1511 women were invited to participate in the study; of these, 568 declined either actively or passively (participation rate of 62%). Eligibility requirements included diagnosis with breast cancer at or under 40 years of age and less than six months prior to enrollment. Following enrollment and informed consent, women are mailed surveys every six months for the first three years following diagnosis. This analysis includes survey data collected at approximately one year post-diagnosis. The study was approved by the Institutional Review Board at the Dana-Farber/Harvard Cancer Center as well as at other study sites.
Data and measures
Socio-demographic information, including age, race, ethnicity, and partner status, as well as treatment data, was self-reported by participants. Missing data was abstracted from the medical record when available. Pathology reports and medical records were reviewed to ascertain stage and estrogen receptor (ER) status.
Sexual functioning was assessed from responses to items from the Cancer Rehabilitation Evaluation System (CARES) Sexual Functioning Summary Scale, an instrument that has been validated and extensively used to evaluate QOL issues among cancer patients.8 The CARES demonstrates good validity and reliability; for the sexual functioning scale, internal consistency was reported to be high (α ≥ 0.80) and test-retest reliability was also very good (r =0.84).9 The CARES asks respondents to assess on a scale of 0–4 (0=not at all, 4=very much) how they have felt about different QOL scenarios over the past few weeks. The CARES Sexual Functioning Summary Scale is composed of two subscales: sexual interest and sexual dysfunction. Scores represent the mean of ratings for each individual item and range from 0–4, with a higher score indicative of more problems.8 The sexual dysfunction subscale items are only applicable to women who report sexual activity since diagnosis. Specifically, this subscale assesses frequency of intercourse, arousal, lubrication, and orgasm. The sexual interest subscale includes one item about feeling sexually attractive and another item about interest in having sex. An additional two items apply only to women who are in a “significant relationship” and asks whether the respondent perceives that her partner no longer finds her attractive and is not interested in having sex with her.
Assessment of treatment-associated amenorrhea
On each survey, women were asked to report the date of their last menstrual period (LMP). We then calculated the time between this date and the date the survey was returned to study investigators to approximate the duration of amenorrhea. Women who had chemotherapy and reported that six months or more had elapsed since their LMP or who reported current ovarian suppression (OS) were categorized as having treatment-induced amenorrhea. A second category included women who were treated with chemotherapy but were not amenorrheic (i.e., reported having a period within the last 6 months) and a third category included women who did not receive chemotherapy or OS.
Assessment of other covariates
Current tamoxifen treatment was dichotomized by reported use at the time of survey completion. Radiation treatment was also dichotomized as “any history” vs. “none.” Primary cancer surgery was divided into three groups: 1) mastectomy with reconstruction; 2) mastectomy only; 3) lumpectomy.
Menopausal and somatic symptoms were evaluated using items from the Breast Cancer Prevention Trial (BCPT) Symptom Checklist. Women were asked to report how much they were bothered by each symptom (scale of 0–4, “not at all” -“extremely”) over the past four weeks. Symptoms were grouped into the following composite scales (BCPT Symptom Scales)10: musculoskeletal pain (general aches, joint pains, muscle stiffness), vaginal problems (vaginal dryness, pain with intercourse), weight problems (unhappiness with appearance, weight gain) and hot flashes (night sweats, hot flashes). Fatigue/tiredness was assessed using the same scale (0=not at all; 4=extremely) and analyzed as “symptomatic” (rating of ≥ 1) vs. “asymptomatic” (rating of 0). General psychosocial wellbeing was measured using the 17 item psychosocial subscale from the CARES-SF; concern with body image was measured using three items from the CARES psychosocial scale. As with the CARES sexual functioning subscales, a score (range: 0–4) representing the mean item ratings was calculated, with higher scores signifying more problems.8
Statistical analysis
Frequencies and means were reported for categorical and continuous covariates, respectively. To compare CARES scores between groups while controlling for potential confounding by age, stage, race, partner status, time from diagnosis, and other treatment received, we fit multivariable models and estimated least squares means. Additional multiple linear regression models were fit to assess fatigue, BCPT symptoms, and body image, in relation to sexual functioning. Treatment-associated amenorrhea was also included as a covariate in the multivariable models to evaluate whether it was independently associated with sexual functioning after controlling for other factors. As a secondary analysis to evaluate the relationship between generalized psychosocial wellbeing and sexual functioning, we added the CARES psychosocial subscale to the multi-variable model.
Only women who identified as sexually active (n=386) since diagnosis were included in the sexual dysfunction analyses. All analyses were conducted in SAS version 9.2 (SAS Institute, Cary, N.C.).
Results
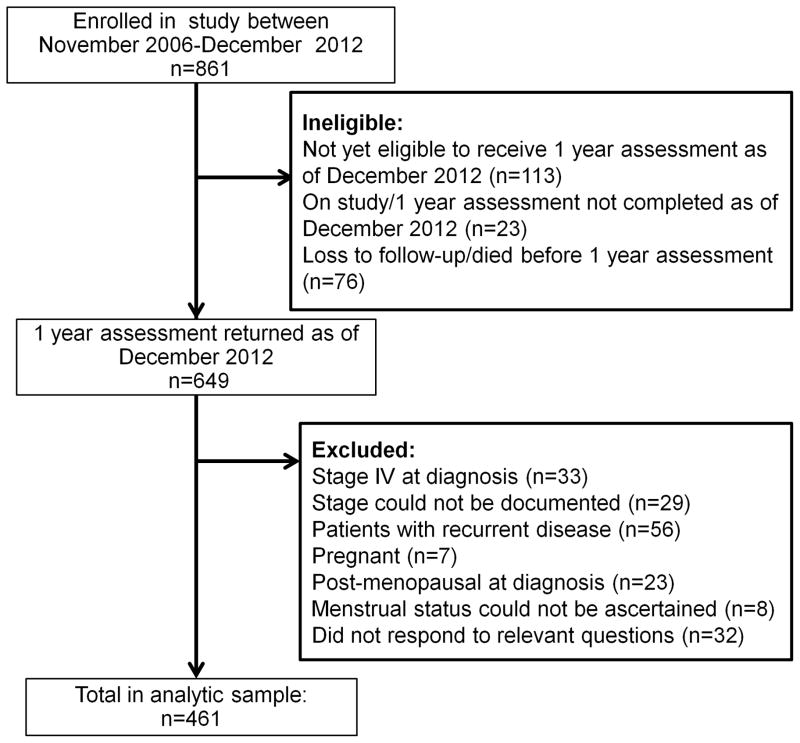
In total, 461 women were eligible for inclusion in our analysis (Figure 1). Table 1 includes socio-demographic, disease, and treatment characteristics for the whole study population and for the subsample of women who identified as sexually active since diagnosis. Mean age at diagnosis was 35.6 years. Mean time from diagnosis at assessment was 12.7 months.
Figure 1.
Study flow chart
Table 1.
Study population characteristics
| All women (n=461) | Sexually-active women (n=386) | |
|---|---|---|
| Mean age at diagnosis (SD) | 35.6 (4.0) | 35.5 (4.0) |
| Range | 17–40 | 22–40 |
| Mean time from diagnosis months (SD) | 12.7 (1.7) | 12.7 (1.7) |
| Range | 7.8–21.6 | 7.9–21.6 |
| Mean CARES sexual interest score (SD) | 1.11 (1.03) | 0.99 (0.97) |
| Range | 0–4 | 0–4 |
| Mean CARES sexual dysfunction score (SD) | ---- | 1.52 (1.22) |
| Range | --- | 0–4 |
| N (%) | N (%) | |
| Partner status | ||
| Partnered | 396 (86) | 359 (93) |
| Non-partnered | 65 (14) | 27 (7) |
| Race | ||
| WNH | 413 (90) | 349 (90) |
| Non-WNH | 48 (10) | 37 (10) |
| Incomea | ||
| <$25,000 | 23 (5) | 18 (5) |
| $25,000–$49,999 | 36 (8) | 25 (6) |
| $50,000–$99,999 | 135(29) | 111 (29) |
| ≥$100,000 | 225 (49) | 198 (51) |
| Don’t know | 4 (1) | 3 (1) |
| College educationb | ||
| Yes | 376 (85) | 315 (85) |
| No | 67 (15) | 56 (15) |
| Stage | ||
| 0 | 35 (8) | 32 (8) |
| I | 173 (38) | 150 (39) |
| II | 197 (43) | 157 (41) |
| III | 56 (12) | 47 (12) |
| Estrogen receptor statusc | ||
| Positive | 322 (70) | 276 (72) |
| Negative | 137 (30) | 108 (28) |
| Chemotherapy | ||
| Yes | 351 (76) | 288 (75) |
| No | 110 (24) | 98 (25) |
| Tamoxifen | ||
| Yes | 272 (59) | 231 (60) |
| No | 189 (41) | 155 (40) |
| Ovarian suppression | ||
| Yes | 104 (23) | 82 (21) |
| No | 357 (77) | 304 (79) |
| Treatment-associated amenorrhea | ||
| Amenorrhea (due to chemotherapy or ovarian suppression) | 210 (46) | 172 (45) |
| No amenorrhea/Chemotherapy | 164 (36) | 138 (36) |
| No amenorrhea/ No chemotherapy | 87 (19) | 76 (20) |
| Radiation | ||
| Yes | 266 (58) | 213 (55) |
| No | 195 (42) | 173 (45) |
| Surgery | ||
| Lumpectomy | 142 (31) | 113 (29) |
| Mastectomy | 60 (13) | 48 (12) |
| Mastectomy with reconstruction | 259 (56) | 225 (58) |
Abbreviations: CARES, Cancer Rehabilitation Evaluation System; SD, Standard Deviation; WNH, White non-Hispanic
n=38/461 missing;
n=18/461 missing;
n=2/461 missing
Treatment and sexual functioning
Adjusted mean CARES sexual interest and dysfunction scores are presented in Table 2. Treatment-induced amenorrhea was associated with both decreased sexual interest and dysfunction (p=0.002). Women with treatment-induced amenorrhea had significantly higher mean sexual interest scores compared to both women who were treated with chemotherapy but were not amenorrheic (1.41 vs. 1.18, p=0.03) and women who did not have chemotherapy or OS (1.41 vs. 0.88, p=0.0008). Similarly, amenorrheic women had significantly higher mean sexual dysfunction scores compared to women who had chemotherapy but continued to menstruate (1.58 vs. 1.17, p=0.004), and compared to women who did not have chemotherapy or OS (1.58 vs. 0.99, p=0.004).
Table 2.
Mean CARES sexual interest and sexual dysfunction scores by treatment groups
| N | Mean CARES sexual interest a,b (n=461) | N | Mean CARES sexual dysfunction a,b (n=386) c | |||
|---|---|---|---|---|---|---|
| p-value | p-value | |||||
| Treatment-associated amenorrhea | 0.002 | 0.002 | ||||
| Amenorrhea | 210 | 1.41 | 172 | 1.58 | ||
| No amenorrhea/Chemotherapy | 164 | 1.18 | 138 | 1.17 | ||
| No amenorrhea/ No chemotherapy | 87 | 0.88 | 76 | 0.99 | ||
| Tamoxifen | 0.07 | 0.52 | ||||
| Yes | 272 | 1.07 | 231 | 1.21 | ||
| No | 189 | 1.25 | 155 | 1.29 | ||
| Radiation | 0.75 | 0.34 | ||||
| Yes | 266 | 1.14 | 213 | 1.16 | ||
| No | 195 | 1.18 | 173 | 1.33 | ||
| Breast surgery | 0.34 | 0.08 | ||||
| Lumpectomy | 142 | 1.10 | 113 | 1.31 | ||
| Mastectomy | 60 | 1.10 | 48 | 0.99 | ||
| Mastectomy + Reconstruction | 259 | 1.28 | 225 | 1.44 | ||
Abbreviations: CARES, Cancer Rehabilitation Evaluation System
Higher mean CARES scores are indicative of greater sexual interest problems (i.e., decreased interest) and sexual dysfunction
Adjusted for stage, age, race/ethnicity, partner status, time from diagnosis, and treatment variables included in table.
Sexual dysfunction analysis is restricted to women who reported being sexually active since diagnosis
Mean CARES sexual dysfunction and interest scores were lower (indicating fewer problems) among women taking tamoxifen relative to women who were not, however the difference was not statistically significant. There were no significant differences between scores on either subscale between radiation groups. Women who had reconstruction had the highest mean scores on both subscales however this difference was significant only in comparison to women who had mastectomies without reconstruction on the dysfunction subscale (1.44 vs. 0.99, p=0.02).
To account for a potential interaction between tamoxifen and chemotherapy we further divided women with treatment-induced amenorrhea into two groups: those who were on tamoxifen and those who were not. In this analysis (Table 3), compared to women with amenorrhea who were on tamoxifen, amenorrheic women who were not taking tamoxifen had higher mean CARES sexual interest (1.58 vs. 1.31, p=0.06) and sexual dysfunction (1.82 vs. 1.45, p=0.05) scores.
Table 3.
Mean CARES sexual interest and sexual dysfunction scores accounting for interaction between tamoxifen and amenorrhea
| N | Mean CARES sexual interest a,b (n=461) | N | Mean CARES sexual dysfunction a,b (n=386) c | |||
|---|---|---|---|---|---|---|
| p-value | p-value | |||||
| 0.0009 | 0.0009 | |||||
| Amenorrhea/Tamoxifen | 134 | 1.31 | 114 | 1.45 | ||
| Amenorrhea/No Tamoxifen | 76 | 1.58 | 58 | 1.82 | ||
| No amenorrhea/Chemotherapy | 164 | 1.20 | 138 | 1.18 | ||
| No amenorrhea/No chemotherapy | 87 | 0.86 | 76 | 1.00 | ||
Abbreviations: CARES, Cancer Rehabilitation Evaluation System
Higher mean CARES scores are indicative of greater sexual interest problems (i.e., decreased interest) and sexual dysfunction
Adjusted for stage, age, race/ethnicity, partner status, time from diagnosis, and other treatment (surgery and radiation)
Sexual dysfunction analysis is restricted to women who reported being sexually active since diagnosis
Additional factors associated with sexual functioning
To evaluate additional factors in relation to sexual interest and dysfunction, we added the BCPT symptoms, fatigue, and the CARES body image score to the models that had originally included treatment, socio-demographic characteristics, stage, time from diagnosis, and partner status (Table 4). Body image, weight problems, and vaginal pain symptoms were independently associated with decreased interest in the fully adjusted model. Treatment-associated amenorrhea remained significantly associated with sexual interest after accounting for symptoms.
Table 4.
Multivariable analysis of factors associated with sexual interest and sexual dysfunction
| CARES Sexual interesta | CARES Sexual dysfunctiona | |||
|---|---|---|---|---|
| β (se)b | p-value | β (se)b | p-value | |
| Treatment-associated amenorrhea | ||||
| No amenorrhea/ No chemotherapy | Reference | Reference | ||
| Amenorrhea | 0.37 (0.12) | 0.003 | 0.20 (0.16) | 0.21 |
| No amenorrhea/Chemotherapy | 0.30 (0.12) | 0.02 | 0.20 (0.16) | 0.23 |
| Symptoms | ||||
| Vaginal painc | 0.11 (0.04) | 0.002 | 0.62 (0.05) | <0.0001 |
| Hot flashes | −0.02 (0.04) | 0.52 | −0.05 (0.05) | 0.28 |
| Weight problems | 0.32 (0.05) | <0.0001 | 0.05 (0.06) | 0.47 |
| CARES body image | 0.36 (0.04) | <0.0001 | 0.17 (0.05) | 0.002 |
| Fatigue | 0.14 (0.09) | 0.12 | 0.35 (0.11) | 0.003 |
| Musculoskeletal pain | 0.07 (0.04) | 0.10 | 0.04 (0.05) | 0.43 |
Abbreviations: CARES, Cancer Rehabilitation Evaluation System; se, standard error
Adjusted for stage, age, race/ethnicity, treatment, partner status, time from diagnosis, and all variables included in table.
Positive coefficients with a p-value ≤0.05 are associated with higher CARES scores, which indicates significantly more sexual functioning concerns.
In non-sexually active women, “vaginal pain” includes the single item measuring vaginal dryness severity. In sexually-active women, “vaginal pain” includes items measuring both vaginal dryness and dyspareunia severity.
In the multivariable analysis, vaginal pain symptoms, body image, and fatigue were all independently associated with sexual dysfunction. Treatment-associated amenorrhea was no longer an independent predictor of sexual dysfunction after accounting for symptoms.
The CARES psychosocial subscale (data not shown) was significantly related to both sexual interest and dysfunction, however including it in the model did not substantially change other results, with the exception of body image, which was no longer a significant predictor of dysfunction.
Discussion
To the best of our knowledge, this study represents the largest analysis of sexual functioning during the post-treatment period in young women with breast cancer. Our findings indicate that treatment-associated amenorrhea is an important determinant of both sexual functioning and interest, a finding consistent with results of other studies that have evaluated the relationship between adjuvant chemotherapy and sexual functioning in both pre- and post-menopausal women. 6, 7, 11–16
In the multi-variable analysis, several symptoms were associated with sexual interest, however treatment-associated amenorrhea remained an independent predictor of decreased interest even after accounting for these factors, indicating that additional causes, unaccounted for in this analysis, are likely playing a role in mediating this effect. These might include, but are not limited to, stress, anxiety, and side effects from anti-depressants. Additionally, the strong association between sexual dysfunction and both vaginal dryness and dyspareunia is consistent with other studies that have assessed the role of treatment while accounting for symptoms 6, 16, 17 and represent the most important explanatory factors related to differences in sexual functioning by amenorrheic status.
Our findings indicate that tamoxifen does not adversely affect sexual functioning at least in the short term. Though the difference between groups was non-significant, current tamoxifen users had lower CARES scores on both subscales, relative to non-users, indicating a trend towards fewer problems among users. When we assessed the interaction between amenorrhea and tamoxifen, those who were on tamoxifen had lower mean CARES scores than those who were not, also suggesting a trend towards better sexual functioning among tamoxifen users. This finding is supported by some evidence that tamoxifen can help improve vaginal lubrication, thereby potentially having a beneficial effect on sexual functioning. 18
Results from prior studies have been mixed with some finding that tamoxifen negatively impacts sexual function while others report no effect. 16, 19, 20 We cannot exclude the possibility that tamoxifen will have a long-term detrimental impact on sexual functioning. Because this was a cross-sectional analysis of data collected at an average of one year after diagnosis, additional follow-up of the cohort when many women will have been receiving adjuvant endocrine treatment for an extended period of time will allow us to more accurately evaluate tamoxifen and its side effects.
We had hypothesized that additional symptoms might explain some of the effect of treatment on sexual functioning. In our analysis, poor body image was associated with decreased sexual interest and dysfunction, which is consistent which findings from several other studies. 16, 17, 21, 22 When we added musculoskeletal symptoms, body image, and fatigue to the treatment models, however, the mean CARES scores did not change measurably, indicating that while some of these side effects might be independently associated with sexual problems in the cohort, they are not explicit mediators between treatment and sexual interest or dysfunction.
Our findings underscore the need for early recognition of the sexual side effects associated with treatment-induced amenorrhea. Early intervention is essential because hypoestrogenism leads to chronic vaginal atrophy and discomfort. Fortunately, many of these symptoms are amenable to interventions that can help restore lubrication, a natural pH, and increased elasticity to the genital tissue. Estrogen-based treatments, including vaginal rings and tablets, as well as topical creams, can reduce vaginal dryness and dyspareunia, though decisions to use localized estrogen therapy in women with a history of an estrogen-responsive cancer should be made in consultation with a patient’s oncologist. 18, 23, 24 Testosterone-based therapies have been investigated but have not shown any demonstrable benefit in the absence of estrogen replacement therapy, which is generally contra-indicated in women with a history of breast cancer. 23
Non-hormonal vaginal moisturizers can help symptoms by hydrating the vaginal mucosa, improving the balance of fluids in the vaginal epithelium and restoring a premenopausal vaginal pH. 23–25 Women should also receive information about optimal use of vaginal lubricants, which are meant to minimize dryness and promote comfort during sexual activity. Psycho-educational interventions have also demonstrated some benefit. Trials that reported a beneficial impact on sexual functioning include both individual and couple–based therapies, which focus on problem-solving, facilitating coping, and helping couples deal with potential intimacy issues.26, 27 Evidence also exists regarding the beneficial effects of interventions to improve associated fatigue, weight gain and body image after treatment for early stage breast cancer. 28 Focusing on these areas may be particularly important for some women. However most participants in intervention trials have been peri- or post-menopausal, and it is unclear how generalizable findings are to younger women, underscoring the need to test interventions that target both physiological and psychological domains in pre-menopausal women.
We acknowledge that this study has limitations. Women are enrolled in the cohort following diagnosis and we have no measure of sexual functioning prior to this time. It is possible that some women experienced some degree of dysfunction before their diagnosis. Additionally, since only women who reported sexual activity since diagnosis respond to the CARES sexual dysfunction items, selection bias cannot be excluded, as those who are experiencing the worst functional outcomes might not be answering these questions. This might result in the underestimation of the overall prevalence of dysfunction in the cohort, but we believe any bias is minimal, given that the proportion of sexually active women (84%) is high; among partnered women, only 9% reported no sexually activity since diagnosis.
Our study highlights the importance of considering the negative QOL consequences of treatment during the early survivorship period. These findings not only have implications for breast cancer survivors but also apply to pre-menopausal women with other types of cancer who might be experiencing amenorrhea either due to chemotherapy or surgery. Concerns about how treatment might impact sexuality and intimacy should be addressed early on, potentially during the informed consent process, before chemotherapy and the onset of associated amenorrhea, and women reassured that symptoms associated with sexual dysfunction can often be improved. This is crucial given that the onset of amenorrhea, whether temporary or permanent, can be especially distressing among young women. 3 Furthermore, there is a need to develop, test, and evaluate interventions to improve short and long term sexual outcomes and other dimensions of QOL in young women.
Acknowledgments
Funding: This work was supported by Susan G. Komen for the Cure and the National Cancer Institute (NIH 5 R25 CA057711; R25 CA92203).
The authors would like to thank Meghan Meyer and Bryce Larsen for their help with data management.
Funding support: The Young Women’s Breast Cancer Study is supported by Susan G. Komen for the Cure. Dr. Rosenberg was supported by the National Cancer Institute (NIH 5 R25 CA 057711; R25 CA92203).
Footnotes
Financial disclosures/conflicts of interest: None reported
References
- 1.American Cancer Society Breast Cancer Key Statistics. Available from URL: www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-key-statistics.
- 2.Bleyer A, Barr R. Cancer in young adults 20 to 39 years of age: overview. Semin Oncol. 2009;36(3):194–206. doi: 10.1053/j.seminoncol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of Life, Fertility Concerns, and Behavioral Health Outcomes in Younger Breast Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel LB, Fairclough DL, Brady MJ, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86(9):1768–74. [PubMed] [Google Scholar]
- 5.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22(10):1849–56. doi: 10.1200/JCO.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 6.Burwell SR, Case LD, Kaelin C, Avis NE. Sexual problems in younger women after breast cancer surgery. J Clin Oncol. 2006;24(18):2815–21. doi: 10.1200/JCO.2005.04.2499. [DOI] [PubMed] [Google Scholar]
- 7.Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96(5):376–87. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 8.Schag CA, Heinrich RL. Cancer Rehabilitation Evaluation System (CARES) Manual. CARES Consultants; Santa Monica, CA: 1988. [Google Scholar]
- 9.Schag CA, Heinrich RL, Aadland RL, Ganz PA. Assessing problems of cancer patients: psychometric properties of the cancer inventory of problem situations. Health Psychol. 1990;9(1):83–102. doi: 10.1037//0278-6133.9.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–56. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 11.Arora NK, Gustafson DH, Hawkins RP, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma: a prospective study. Cancer. 2001;92(5):1288–98. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psychooncology. 2004;13(5):295–308. doi: 10.1002/pon.744. [DOI] [PubMed] [Google Scholar]
- 13.Berglund G, Nystedt M, Bolund C, Sjoden PO, Rutquist LE. Effect of endocrine treatment on sexuality in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol. 2001;19(11):2788–96. doi: 10.1200/JCO.2001.19.11.2788. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert E, Ussher JM, Perz J. Sexuality after breast cancer: a review. Maturitas. 2010;66(4):397–407. doi: 10.1016/j.maturitas.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Schover LR, Yetman RJ, Tuason LJ, et al. Partial mastectomy and breast reconstruction. A comparison of their effects on psychosocial adjustment, body image, and sexuality. Cancer. 1995;75(1):54–64. doi: 10.1002/1097-0142(19950101)75:1<54::aid-cncr2820750111>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Ganz PA, Desmond KA, Belin TR, Meyerowitz BE, Rowland JH. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol. 1999;17(8):2371–80. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- 17.Fobair P, Stewart SL, Chang S, D’Onofrio C, Banks PJ, Bloom JR. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15(7):579–94. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 18.Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26(5):753–8. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
- 19.Mortimer JE, Boucher L, Baty J, Knapp DL, Ryan E, Rowland JH. Effect of tamoxifen on sexual functioning in patients with breast cancer. J Clin Oncol. 1999;17(5):1488–92. doi: 10.1200/JCO.1999.17.5.1488. [DOI] [PubMed] [Google Scholar]
- 20.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17(9):2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 21.Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8(1):294–302. doi: 10.1111/j.1743-6109.2010.02034.x. [DOI] [PubMed] [Google Scholar]
- 22.Bredart A, Dolbeault S, Savignoni A, et al. Prevalence and associated factors of sexual problems after early-stage breast cancer treatment: results of a French exploratory survey. Psychooncology. 2011;20(8):841–50. doi: 10.1002/pon.1789. [DOI] [PubMed] [Google Scholar]
- 23.Melisko ME, Goldman M, Rugo HS. Amelioration of sexual adverse effects in the early breast cancer patient. J Cancer Surviv. 2010;4(3):247–55. doi: 10.1007/s11764-010-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickey M, Saunders CM, Stuckey BG. Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet Oncol. 2005;6(9):687–95. doi: 10.1016/S1470-2045(05)70316-8. [DOI] [PubMed] [Google Scholar]
- 25.Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med. 2011;8(2):549–59. doi: 10.1111/j.1743-6109.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- 26.Taylor S, Harley C, Ziegler L, Brown J, Velikova G. Interventions for sexual problems following treatment for breast cancer: a systematic review. Breast Cancer Res Treat. 2011;130(3):711–24. doi: 10.1007/s10549-011-1722-9. [DOI] [PubMed] [Google Scholar]
- 27.Allen SM, Shah AC, Nezu AM, et al. A problem-solving approach to stress reduction among younger women with breast carcinoma: a randomized controlled trial. Cancer. 2002;94(12):3089–100. doi: 10.1002/cncr.10586. [DOI] [PubMed] [Google Scholar]
- 28.Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30(30):3697–704. doi: 10.1200/JCO.2012.42.0638. [DOI] [PubMed] [Google Scholar]