Abstract
Managing depression and anxiety during pregnancy and the postpartum period is challenging. Both pharmacological treatment and the lack thereof can pose threats to a fetus. SSRIs are the drugs of choice for use during pregnancy, but there is considerable evidence for the safety and efficacy of older antidepressants during pregnancy as well. This study highlights a single case of the use of the tricyclic nortriptyline during pregnancy and postpartum. The subject involved had an unexpectedly high ratio of serum level to drug dose during the postpartum period. We monitored the subject for a significantly greater portion of the postpartum period than has been done in previous studies, and explored medical and lifestyle changes that could account for the level-to-dose ratios we observed. Differences in smoking patterns, coupled with the patient’s status as a genetic poor metabolizer, were the most likely explanations.
Keywords: Pregnancy, Depression, Anxiety, Nortriptyline, Pharmacokinetics, Tricyclic antidepressants
Introduction
Depression is one of the most common illnesses among women, with a lifetime risk of 10–25 % (Wisner et al. 1999) and a peak onset during the childbearing years. As many as 75 % of those with depression also suffer from anxiety (Lamers et al. 2011). During pregnancy and the postpartum period, these disorders pose risks to both mother and fetus, and managing the disease optimally during these vulnerable periods is critical (Kessler et al. 1994; Spinelli 1998; Marcus et al. 2003). Many women and their babies benefit from the stable control of their disease that pharmacologic treatment can offer, but exposing the fetus to any drug is not a decision to be undertaken lightly (Cohen and Rosenbaum 1998; Weinberg and Tronick 1998; Wisner et al. 2000; Cohen et al. 2006).
Antidepressants are among the most studied classes of medications during pregnancy, and the bulk of the literature in recent years has focused on SSRIs. Most studies have concentrated on four major categories of risk: (1) congenital defects; (2) growth effects, spontaneous abortion, preterm birth, and stillbirth; (3) neonatal adaptation problems; and (4) long-term neurodevelopmental differences. The data are generally reassuring in all four of these categories. There is no consistent evidence of increased risk of congenital defects (Kulin et al. 1998; Nulman et al. 2002; Moses-Kolko et al. 2005; Cohen et al. 2006; Alwan et al. 2007; Louik et al. 2007), some evidence of increased rates of preterm birth that cannot be distinguished from the effect of depression (Hemels et al. 2005; Suri and Altshuler 2009; Wisner et al. 2009), no evidence of association with stillbirth and infant mortality (Stephansson et al. 2013), good evidence for self-limited neonatal adaptation problems (respiratory distress, feeding problems, neonatal abstinence syndrome, and jitteriness) (Nonacs and Cohen 2002; Levinson-Castiel et al. 2006), conflicting evidence about the risk of persistent pulmonary hypertension in the newborn (including most recently the FDA’s retraction of their warning about this risk) (Chambers et al. 2006; Källénm and Olausson 2008; Andrade et al. 2009; Wisner et al. 2009), and conflicting evidence of possible associations with modest long-lasting motor (but not mental) developmental differences, though it is unclear whether these differences may in fact be accounted for by maternal depression itself (Oberlander et al. 2009, Croen et al. 2011, Rai et al. 2013).
Although the risks of SSRIs overall are low, not all women will respond to these drugs, and some may experience side effects. It is therefore crucial for perinatal psychiatrists to consider the older tricyclic antidepressants (TCAs) for use during pregnancy. These drugs have a side effect burden that is similar to the SSRIs but a different profile, including a higher risk of orthostasis (amatter of particular concern during pregnancy). Moreover, they have a narrow therapeutic window and greater risks in overdosing—so what are the advantages? First, we have a reassuring body of evidence concerning their safety and efficacy in pregnancy and the postpartum (Nonacs and Cohen 2002; Cohen et al. 2006; Wisner et al. 2009). Numerous prospective and retrospective studies, taken together, fail to show any significant risk for major congenital anomalies (Wisner et al. 2006), preterm birth or low birth weight (Pearson et al. 2007), persistent pulmonary hypertension (Chambers et al. 2006), or long-term neurodevelopmental changes (Nulman et al. 2002). Several have reported a neonatal TCA withdrawal syndrome (jitteriness, irritability, seizure) (Wisner et al. 1999). There have also been reports of neonatal toxicity attributable to the anticholinergic side effects of these drugs, and for that reason, nortriptyline and desipramine (the least anticholinergic TCAs) are the preferred agents in pregnancy (Suri and Altshuler 2009). (It should be noted that fewer prospective safety data are available for a number of less commonly used agents, including mirtazapine, nefazodone, trazodone, and the monoamine oxidase inhibitors (Altshuler et al. 1996; Cohen and Rosenbaum 1998; Nonacs and Cohen 2002).
TCAs are clearly a viable choice for pharmacologic treatment during pregnancy and the postpartum—but how do we dose them during this challenging period? Treatment efficacy has been shown to be tied to serum level rather than dose, and the level-to-dose ratio varies greatly among individuals (Wisner et al. 1997; Sit et al. 2008). The TCAs undergo hepatic metabolism through the cytochrome p450 system, specifically through enzyme 2D6 (Rubin et al. 1985; Kvist et al. 2001). A moderator of change in serum levels and dose requirements during pregnancy will be CYP450 metabolizing status, ranked as follows, possibly due to different numbers of functional 2D6 genes: ultrarapid metabolizer (UM), extensive metabolizer (EM), intermediate metabolizer (IM), and poor metabolizer (PM). About 7 % of Caucasians are defined as PMs, and about 1–10 % are UMs (Kvist et al. 2001).
Genetic differences in rates of metabolism are not the only factor governing an individual’s response to drug doses. Physiologic changes may necessitate altered dosing of many drugs for the same individual at different times. In pregnancy, these changes include different levels of steroid hormones, which may affect the rate of metabolism through the cytochrome p450 system, expansion of plasma volume and total body water (and therefore of volume of distribution), reduction in albumin levels, leading to decreased protein binding of drugs, changes in hepatic blood flow, and increased glomerular filtration rate and renal excretion of drugs (Sit et al. 2008). Many drugs (including β-blockers, calcium channel blockers, anticonvulsants, and psychotropics) can be affected (Sit et al. 2008). For the p450 system, there is some evidence that the activity of CYP2D6 is increased by 25–50 % during pregnancy, and pregnancy doses are usually about 1.3–2 times higher than those used in the non-pregnant state (Wisner et al. 1993; Altshuler and Hendrick 1996;Wadelius et al. 1997; Anderson 2005; Tracy et al. 2005;Miller et al. 2008; Topletz et al. 2013). To avoid potential neonatal toxicity or withdrawal, some clinicians will lower pregnancy doses 10 to 14 days prior to the estimated delivery date (although evidence for this strategy is lacking and it may leave the pregnant woman vulnerable to increased symptoms) (Miller et al. 2008). The postpartum may also be a time of altered metabolism of the TCAs when compared to the non-pregnant, non-postpartum state. One study has demonstrated a relative refractory period for nortriptyline metabolism with an elevated level-to-dose ratios during the first postpartum weeks (Wisner et al. 1997). Little is known about the factors that affect altered metabolism in the postpartum period. It is therefore instructional to look to case studies such as the following that may not conform to our assumptions about these changes.
Case presentation
L.R., a 31-year-old married Caucasian professional with a history of panic disorder and depression and no other significant medical history, was referred for consultation about medication during pregnancy. She was the younger of two daughters of a day trader and a homemaker and had a family history significant for her father’s compulsive gambling (treated with fluoxetine), her mother’s depression (treated with amitriptyline and then sertraline), and her sister’s recent onset anxiety and depression (treated with bupropion).
L.R. experienced her first psychiatric symptoms 3 years before presentation, at the age of 27, in the context of her parents’ divorce and the patient’s moving in with her boyfriend. She experienced a panic attack with symptoms of a racing heart, diaphoresis, shortness of breath, and dizziness. After several similar episodes, she underwent an extensive medical workup, which included an electrocardiogram, an echocardiogram, and a 24-h Holter monitor. Both cardiac and endocrinological disease were ruled out, and the patient began to see a psychiatrist and a cognitive behavioral therapist about 3 months after the onset of symptoms. L.R. described the next several months as the “worst period of my life.” She had trouble sleeping, often could not leave the apartment, lost her appetite, and worried incessantly; she also began to feel depressed. Early in the course of her illness, she was treated with citalopram, but stopped after 2 days due to side effects (shortness of breath, vomiting, and chills). She next tried a low dose of nefazodone, which helped with the panic and sleep but left the patient feeling “deadened.” Several months later, she added bupropion 200 mg to the nefazodone 75 mg, along with occasional lorazepam as needed for breakthrough anxiety. Within a year of the first onset of symptoms, the patient felt “back to myself.” She married shortly thereafter and was virtually symptom free for the next year. At the time, she consumed about two to three alcoholic drinks per week, used no illicit drugs, and smoked about one pack of cigarettes per day.
About a year after her marriage, the patient began to taper her medications in anticipation of pregnancy. About 7 weeks after the medications were stopped, L.R. began once again to have panic attacks, palpitations, and muscle spasms, along with a great deal of anticipatory anxiety about the recurrence of her symptoms. She did not tolerate a 1-day trial of sertraline 25 mg (a “hellish reaction,” including rumination and nausea) and restarted bupropion and nefazodone.
Her reaction to the taper made it likely that the patient would remain on medication once she conceived, and she was opposed to SSRIs because of her history of adverse reactions to citalopram and sertraline. After an extensive risk-benefit discussion, the patient elected to begin nortriptyline. We started her on 10 mg with instructions for a slow cross-taper with nefazodone. The rate of increase was unusually slow due to the patient’s anxiety about the new drug and the nature of her response to it. She had an increased need for lorazepam prn (0.5–1 mg qd) during the first few weeks. Within 6 weeks, L.R. had discontinued nefazodone and had a serum level of 75 ug/L (therapeutic range 50–150) on 50 mg of nortriptyline. She was still experiencing some occasional negative thoughts, but no overt anxiety or panic. She required only occasional use of lorazepam. Side effects were mild dry mouth and dizziness. We then began a cross-taper of bupropion. Within 4 months of presentation, L.R. was completely off bupropion and was stable and asymptomatic on a nortriptyline dose of 70 mg (blood level 60 ug/L) (Table 1). The patient’s serum level on this higher dose was, surprisingly, lower than her level on the lower dose. All serum levels were measured at one of three labs owned by the same company and processed in the same facility. There were some discrepancies in time of collection. The patient was instructed to go within 12–15 h after taking her dose, but we are unable to verify that all blood draws were indeed collected within this time frame.
Table 1.
Nortriptyline doses and levels throughout study period
| Date | NTP dose | NTP level | Level/dose ratio |
Pregnancy status |
|---|---|---|---|---|
| 10/21/03 | 10 mg | n/a | n/a | |
| 11/11/03 | 20 mg | n/a | n/a | |
| 12/03/03 | 40 mg | n/a | n/a | |
| 12/17/03 | 50 mg | 75 ug/L | 1.5 | n/a |
| 12/22/03 | 50 mg | 75 ug/L | n/a | |
| 01/14/04 | 50 mg | 75 ug/L | n/a | |
| 02/18/04 | 70 mg | 60 ug/L | 0.86 | n/a |
| 02/19/04 | 70 mg | 60 ug/L | n/a | |
| 05/18/04 | 70 mg | Not drawn | n/a | |
| 08/31/04 | 100 mg | Not drawn | 8 weeks | |
| 09/21/04 | 100 mg | 110 ug/L | 1.1 | 11 weeks |
| 10/26/04 | 125 mg | Not drawn | 16 weeks | |
| 01/10/05 | 150 mg | Not drawn | 27 weeks | |
| 02/03/05 | 150 mg | 182 ug/L | 1.21 | 30 weeks |
| 03/01/05 | 150 mg | Not drawn | 34 weeks | |
| 03/17/05 | 150 mg | 151 ug/L | 1.01 | 36 weeks |
| 04/05/05 | 150 mg | 151 ug/L | 39 weeks | |
| 04/28/05 | 125 mg | Not drawn | 2 weeks pp | |
| 06/06/05 | 125 mg | Not drawn | 8 weeks pp | |
| 06/10/05 | Quant. not suff. | 8 weeks pp | ||
| 07/01/05 | 150 mg | 367 ug/L | 2.45 | 11 weeks pp |
| 07/18/05 | 125 mg | 375 ug/L | 3.0 | 14 weeks pp |
| 07/19/05 | 125 mg | 375 ug/L | 14 weeks pp | |
| 07/22/05 | 125 mg | 311 ug/L | 2.49 | 14.5 weeks pp |
| 07/26/05 | 125 mg | 258 ug/L | 2.06 | 15 weeks pp |
| 08/12/05 | 50 mg | 171 ug/L | 3.42 | 17 weeks pp |
| 09/06/05 | 50 mg | Not drawn | 21 weeks pp | |
| 09/12/05 | 50 mg | 113 ug/L | 2.26 | 22 weeks pp |
| 10/21/05 | 50 mg | 128 ug/L | 2.56 | 26.5 weeks pp |
| 10/25/05 | 50 mg | 128 ug/L | 27 weeks pp | |
| 11/08/05 | 75 mg | 98 ug/L | 1.31 | 29 weeks pp |
| 11/23/05 | 100 mg | 206 ug/L | 2.06 | 31 weeks pp |
| 11/28/05 | 125 mg | 322 ug/L | 2.58 | 32 weeks pp |
| 11/30/05 | 125 mg | 322 ug/L | 32 weeks pp | |
| 12/05/05 | 50–75 mg | 180 ug/L | 3.0 | 33 weeks pp |
NTP nortriptyline, n/a not available, pp postpartum
Upon completion of the cross-taper, the patient felt that her symptoms were back to baseline, and she began trying to conceive. She reported that she had cut her smoking in half (from about one pack per day to one half pack per day) in anticipation of pregnancy. When she did not become pregnant immediately, she began to experience some anxiety and muscle twitching, which she worried was a symptom of impending panic, and her dose was increased to 100 mg. Three months after beginning to try to conceive, L.R. began clomiphene citrate and intrauterine insemination. After 1 month, she moved on to injectable follistim and human chorionic gonadatropin, and became pregnant during the first month of treatment. The patient reported that her anxiety and mood were relatively stable during the hormone treatment, and she stopped smoking entirely once her pregnancy was confirmed.
She returned to our office at 8 weeks gestation. She was maintained on 100mg of nortriptyline, with a level of 110 ug/L. At 13 weeks, we increased her dose to 125 mg to combat new muscle twitches, trouble sleeping, and anxiety; the patient did not comply with a request to have a level drawn at this time. At 22–23 weeks, the patient suffered increased anxiety following a frightening emergency room visit for a kidney stone, and we raised her dose to 150 mg. Although the level drawn on this new dose was outside the therapeutic range, at 182 ug/L, we decided to maintain the dose as the patient was no longer symptomatic and was experiencing no adverse effects of nortriptyline; her next level, at 36 weeks, was 151 ug/L. The patient also began to see a new cognitive behavioral therapist who specialized in transitions to motherhood. At 39 weeks, the patient remained symptom free, and we lowered her dose to 125 mg in anticipation of delivery.
With the exception of some facial edema and borderline blood pressures that did not meet the criteria for preeclampsia, L.R. had a medically uneventful pregnancy. Labor was induced with oxytocin and rupture of her amniotic membranes at 40 weeks, following dinoprostone for cervical ripening. She received epidural anesthesia, was fully dilated, and had been pushing for about 1 hour when the fetus developed an erratic heart rate. The baby was delivered by emergency C-section, with no obstetric complications. The baby was in excellent physical condition with no manifestation of neonatal adaptation syndrome or cardiotoxicity.
At an office visit 2 weeks postpartum, the patient described some minor symptoms following the dose decrease to 125 mg (muscle spasms), but was feeling essentially well. She had chosen not to breastfeed and reported no neonatal adaptation problems in the baby. We instructed the patient to have a nortriptyline level drawn then, but she was unable to get to the lab. At 8 weeks postpartum, the patient complained of increasing breakthrough anxiety symptoms. A blood draw at 125 mg was unfortunately of insufficient quantity, and the patient returned for a second level at 11 weeks postpartum (now on a dose of 150 mg). Her level then was 367 mg/mL. We hoped that the unusually high level was erroneous, but considered immediate (albeit temporary) cessation of the medication. The patient was extremely reluctant to discontinue, so we instead opted to lower the dose. At 14 weeks postpartum, now on 125 mg, her level was 375. The patient denied any side effects and was reluctant to consider a change of medication. Over the course of the next week, we rechecked her level twice, and it remained elevated (at 311 and 258). At 15 weeks postpartum, we dropped her dose to 50 mg. Her levels dropped to 171 at 17 weeks and 113 at 22 weeks (still considerably higher than her pre-pregnancy level of 75 at the same dose).
Her serum levels then remained stable, but at 25 weeks postpartum, the patient experienced her first menstrual period and her symptoms returned. She felt anxious and had heart palpitations and chest tightness; she saw her internist for a cardiac workup, including an EKG. The results were within normal limits, including a QT interval of 378 and a QTc of 393. As her level on 50 mg was still stable at 128, we cautiously began to increase the dose. Levels remained relatively stable but symptoms did not improve; at 31 weeks, we found that the patient’s levels had jumped again (to 206 on 100 mg and 322 on 125 mgs). The level improved modestly (to 180) when the dose was again dropped to 50 mg, but the patient continued to experience breakthrough anxiety and required lorazepam prn. Although the patient had not displayed signs of toxicity, we were nevertheless concerned about the persistently high levels and the potential for cardiac toxicity, and we agreed to taper nortriptyline in favor of another agent. Choice of another agent was limited by the patient’s unwillingness to take an SSRI. There are fewer safety data available for antidepressants during pregnancy outside of the SSRI and TCA families. After a detailed risk-benefit analysis with the patient, venlafaxine was chosen as the next agent. Animal studies and limited human data for this drug show no consistent risk for congenital anomalies or neurocognitive delays; there are reports of neonatal adaptation syndrome (as with all serotonergic agents) (http://reprotox.org/Members/AgentDetail.aspx?a=3791). The patient eventually was stabilized on 262.5 mg of venlafaxine XR and weekly cognitive behavioral therapy. She has remained stable for several years, and successfully completed another pregnancy and postpartum period while remaining on venlafaxine. Her dose was increased to 300 mg during the second pregnancy, and she has maintained that dose with no lorazepam requirements since her second birth.
Discussion
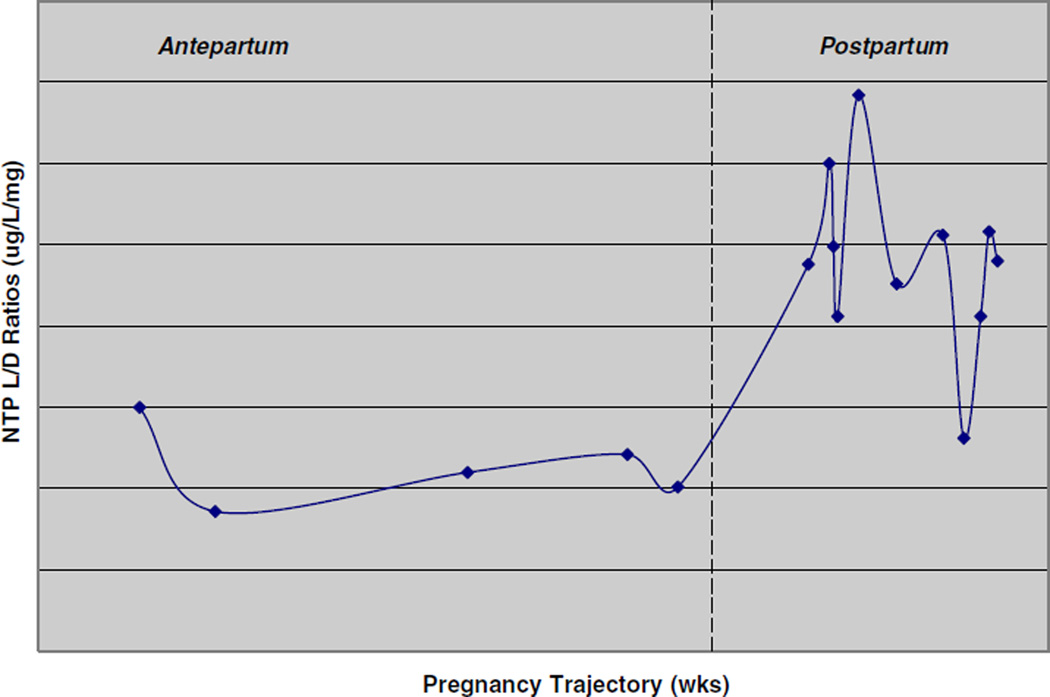
The case of L.R. is instructive in many ways. Her increased levels in the postpartum period are consistent in kind but not degree with previous reports. In the most comprehensive previous study of nortriptyline pharmacokinetics in the postpartum period, subjects (n=16) had a mean level-to-dose (L/D) ratio of 1.11 at week 2 postpartum, increasing to a peak of 1.21 at week 6. Mean ratios then declined steadily until reaching a stable level of 0.84 at week 17, but that mean does not reflect the considerable individual variability among study participants. Three participants, in fact, had levels that dropped after an initial peak at 4–8 weeks but then began to rise steadily again at 11–14 weeks and were continuing to rise upon termination of the study at 17 weeks (Wisner et al. 1997). No data are available on L.R.’s L/D ratio until the 11th week postpartum. From that point until we discontinued the drug at 33 weeks postpartum, her L/D ratio ranged from 2.06 to 3.42, with one exception (an L/D ratio of 1.31 at 29 weeks postpartum). These ratios are substantially higher than her ratios during pregnancy (1.01–1.21) and before pregnancy (0.86–1.5) (Fig. 1).
Fig. 1.
Antepartum and postpartum NTP level/dose (L/D) ratios of L.R.
The most obvious explanation for the variability in level-to-dose ratios is the patient’s decision to alter her dose. However, L.R. was a very responsible, adherent patient, whose great anxiety led her to be extremely precise in her doses. Altering the dose on her own appeared to be an unlikely explanation (though the patient’s occasional failure to get lab tests as requested may indicate that she was less reliable in her dosing than we had assumed). Another explanation would be her genetically determined rate of metabolism, but we initially dismissed this as unlikely given the difference between her pre-pregnancy and post-pregnancy levels.
We therefore made a close examination of L.R.’s lifestyle before, during, and after pregnancy, in order to identify any factors unique to the postpartum period. There were few differences. At no time was L.R. on long-term regular medications in addition to nortriptyline. She did use both clomiphene citrate and injectable follistim and gonadotropins prior to pregnancy, but for only 1 month each. Her nortriptyline levels were not measured while she was on those drugs. L.R. did not use oral contraceptives at all, either before or after pregnancy. Her alcohol consumption was similar and low in both periods (about two drinks per week, occasionally three), with no alcohol consumption during pregnancy. There was no use of illicit substances and no use of herbal supplements. Exercise levels were similar, with about three workouts per week before, during, and after pregnancy, with the exception of a 6-week hiatus in the early postpartum period.
L.R. used several medications postpartum that she had not used during or before pregnancy; these included acetaminophen with codeine for about 3–4 days, ibuprofen for about 2 weeks, and docusate sodium for several weeks. None of these was used for a long enough period to be responsible for the observed differences. L.R.’s thyroid and liver functions were both checked at 14.5 weeks postpartum, and all values were within normal limits: thyroid stimulating hormone 1.82 µU/mL, free T4 1.1 ng/dL; total bilirubin 0.35 mg/dL, alkaline phosphatase 108 U/L; aspartate aminotransferase 28 U/L; alanine aminotransferase 36 U/L. She did not have a urine drug screen at any point during her treatment but she denied substance use.
The one identifiable difference in L.R.’s lifestyle before and after pregnancy was smoking. Before pregnancy, L.R. estimated that she had been smoking one pack per day for about 10 years and had cut down to about one half pack on some (but not most) days shortly before pregnancy. More recently, she has acknowledged that her smoking was heavier than one pack per day, particularly when she was experiencing periods of anxiety. She did not smoke at all during pregnancy, but resumed smoking within 3 weeks of delivery. She states that post-delivery, she smoked a maximum of one half pack per day (but acknowledged that she is not sure of this amount). Smoking is a known inducer of some CYP450 enzymes, most notably 1A2, but also 2D6 (Bondolfi et al. 2005; Mann et al. 2008). The polyaromatic hydrocarbons in cigarette smoke bind to a soluble intracellular receptor, which then enters the nuclear compartment and binds to the promoter for the relevant p450 gene (Okey et al. 1986). Induction of 2D6 due to heavy smoking could certainly have resulted in low serum levels of nortriptyline before pregnancy. The physiological changes of pregnancy would have then maintained those low levels. If the patient smoked considerably less after pregnancy than before, she might have had less induction of 2D6 and therefore higher serum levels.
Because we do not know exactly how much her smoking changed, we were uncertain whether the change could account for the marked differences seen. The recent advent of affordable genetic testing allowed us to test this hypothesis by genotyping L.R. with regard to p450 enzymes (see Table 2). Testing conducted on July 15, 2013, indicated that L.R. is a poor metabolizer of 2D6. This status can explain her very high serum level postpartum, with the assumption that her heavy smoking prepartum may have induced metabolism then while lower smoking in the postpartum period revealed her true poor metabolizer state. In addition, the patient was found to be heterozygous for the T allele of the methylenetetrahy drofolate reductase (MTHFR) gene. Individuals with this allele may have an impaired ability tometabolize folate. This impairment can lead to elevated homocysteine levels, which have been found to be a risk factor for mood and anxiety disorders (Bjelland et al. 2003). We do not have any information on L.R.’s homocysteine levels.
Table 2.
Genetic variants of L.R. (as outlined in Genomind Assay Report)
| Clinically significant variations | |||
|---|---|---|---|
| Gene | Result | Clinical Factors | Relevant therapies |
| SLC6A4 | L(A)/S | Associated with poor response, slow response, and adverse events with SSRI medications test | Caution with: SSRIs |
| 5HT2C | C/C | Associated with increased incidence of weight gain and metabolic syndrome with atypical antipsychotics | Caution with: atypical antipsychotics Therapeutic options: myo-inositol |
| CACNA1C | G/A | Common variations shows very modest association to schizophrenia and bipolar disorder, but is not diagnostic for either disorder and is also observed in individuals without those disorders | Therapeutic options: mood stabilizers, atypical antipsychotics, omega-3 fatty acids |
| MTHFR | C/T | Patients with the T allele have reduced enzyme activity resulting in reduced conversion of folic acid/folate to methylfolate. Methylfolate is a precursor to serotonin, norepinephrine, and dopamine synthesis | Therapeutic options: l-methylfolate |
| CYP2D6 | Poor metabolizer (PM) [low activity] *4/*4 | Increased risk of elevated serum levels, drug interactions, and reduced production of active moieties | Use increased caution when prescribing substrates in patients who are poor or intermediate metabolizers and, if clinical response and/or blood levels warrant, a dose adjustment may be considered |
Implications for practice L.R.’s case offers many lessons for clinicians who use antidepressants during pregnancy and the postpartum. First, as we have seen, the medication was well tolerated in pregnancy with a minimum of both side effects and breakthrough symptoms. Since only 60–70 % of people with depression respond to the first medication tried, it is vital that we do not lose sight of the efficacy and safety of older medications for use during pregnancy (particularly as these older drugs can also serve a two-in-one function of helping the sleep difficulties that are so common in pregnancy). Second, the issues experienced with the level/dose relationship across childbearing teach us that we must be especially vigilant about doses during this period of altered metabolism. Third, the possible relationship of smoking to the patient’s elevated serum levels cautions us to be mindful of lifestyle issues that may affect the p450 system during a period in which 2D6 activity plummets (compared to pregnancy). We should also note that a number of antidepressants, including some TCAs, are metabolized by 1A2 rather than 2D6—the enzyme more powerfully affected by smoking. Similarly, the postpartum period warrants especially careful monitoring of any other drugs that are inducers, inhibitors, or substrates of the p450 system, even if doses have already been adjusted for interactions in the pregnant or pre-pregnant state. Examples of such drugs among psychotropic agents include fluvoxamine, fluoxetine, diphenhydramine, and paroxetine (potent inhibitors), carbamazepine and St. John’s Wort (potent inducers), and amitriptyline, clozapine, haloperidol, risperidone, alprazolam, diazepam, and zolpidem (substrates). Finally, we may also take from L.R.’s story a lesson about the therapeutic index of TCAs. Though clinicians have long been reassured by our ability to relate dosage to serum level in these drugs, in this case, serum levels that were far above the accepted range resulted in no observed toxicity. Whether such an observation is unique to L.R., or unique to postpartum women, is unclear. Given how well L.R. had done on the drug, however, our results do prompt us to ask how concerned we need to be about levels in the toxic range if the patient exhibits no symptoms of toxicity or medical complications. In this case, we discontinued the drug due to concern about these high numbers, even though the medication was efficacious in terms of symptom remission. In retrospect, however, we must wonder whether individual signs of toxicity might not be more meaningful indicators of the necessity of stopping a drug than serum levels alone.
Footnotes
Previously presented as a poster, “Case report on nortriptyline levels in a postpartum woman,” at the 4th World Congress of Women’s Mental Health, March 2011
Disclosures None
Contributor Information
Lauren M. Osborne, Division of Behavioral Medicine, Department of Psychiatry, Columbia University Medical Center, 630 W. 168th Street, PH 1540G, New York, NY 10032, USA, lmo17@columbia.edu
Catherine A. Birndorf, Payne Whitney Women’s Program, Departments of Psychiatry and Obstetrics and Gynecology, Weill Medical College of Cornell University, New York, NY, USA
Lauren E. Szkodny, Department of Psychology, The Pennsylvania State University, University Park, PA, USA
Katherine L. Wisner, Departments of Psychiatry and Behavioral Sciences and Obstetrics and Gynecology, Northwestern University, Chicago, IL, USA
References
- Altshuler LL, Hendrick VC. Pregnancy and psychotropic medication: changes in blood levels. J Clin Psychopharmacol. 1996;16(1):78–80. doi: 10.1097/00004714-199602000-00015. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606. doi: 10.1176/ajp.153.5.592. [DOI] [PubMed] [Google Scholar]
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedmen JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- Andrade SE, McPhillips H, Loren D, Raebel MA, Lane K, Livingston J, Boudreau DM, Smith DH, Davis RL, Willy MEm, Platt R. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009 doi: 10.1002/pds.1710. Published online in Wiley InterScience ( www.interscience.wiley.com) [DOI] [PubMed] [Google Scholar]
- Bjelland I, Tell GS, Vollset SE, REfsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C→T Polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618–626. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Morel F, Crettol S, Rachid F, Baumann P, Eap CB. Increased clozapine plasma concentrations and side effects induced by smoking cessation in 2 CYP4501A2 genotyped patients. Ther Drug Monit. 2005;27(4):539–543. doi: 10.1097/01.ftd.0000164609.14808.93. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Lyons Jones K, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Rosenbaum JF. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry. 1998;59(suppl 2):18–28. [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Henrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psych. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- Hemels ME, Einarson A, Koren G, Lanctot KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta-analysis. Ann Pharmacother. 2005;39(5):803–809. doi: 10.1345/aph.1E547. [DOI] [PubMed] [Google Scholar]
- Källénm B, Olausson PO. Maternal use of selective serotonin reuptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2008;17(8):801–806. doi: 10.1002/pds.1570. [DOI] [PubMed] [Google Scholar]
- Kessler R, McGonagle K, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry. 1994;51:8. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kulin N, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279(8):609–610. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- Kvist EE, Al-Shurbaji A, Dahl M-L, Nordin C, Alvan G, Stahle L. Quantitative pharmacogenetics of nortriptyline: a novel approach. Clin Pharmacokinet. 2001;40(11):869–877. doi: 10.2165/00003088-200140110-00005. [DOI] [PubMed] [Google Scholar]
- Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety. NESDA J Clin Psychiatry. 2011;72(3):341–348. doi: 10.4088/JCP.10m06176blu. [DOI] [PubMed] [Google Scholar]
- Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160:173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- Mann A, Miksys S, Lee A, Mash DC. Tyndale RF. Induction of the drug metabolizing enzyme CYP2D in monkey brain by chronic nicotine treatment. Neuropharmacology. 2008 Jul 19; doi: 10.1016/j.neuropharm.2008.07.017. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Flyne HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetric settings. J Women's Health. 2003;12(4):373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- Miller LJ, Bishop JR, Fischer JH, Geller SE, Macmillan C. Balancing risks: dosing strategies for antidepressants near the end of pregnancy. J Clin Psychiatry. 2008;69(2):323–324. doi: 10.4088/jcp.v69n0220. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293(19):2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- Nonacs R, Cohen LS. Depression during pregnancy: diagnosis and treatment options. J Clin Psychiatry. 2002;63(suppl 7):24–30. [PubMed] [Google Scholar]
- Nulman I, Rovet K, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective controlled study. Am J Psychiatry. 2002;159(11):1889–1895. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86(6):672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB, Roberts EA, Harper PA, Denison MS. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132–141. doi: 10.1016/s0009-9120(86)80060-1. [DOI] [PubMed] [Google Scholar]
- Pearson KH, Nonacs RM, Viguera AC, Heller VL, Petrillo LF, Brandes M, Hennen J, Cohen LS. Birth outcomes following prenatal exposure to antidepressants. J Clin Psychiatry. 2007;68(8):1284–1289. doi: 10.4088/jcp.v68n0817. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Biggs JT, Preskorn SH. Nortriptyline pharmacokinetics and plasma levels: implications for clinical practice. J Clin Psychiatry. 1985;46(10):418–424. [PubMed] [Google Scholar]
- Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69(4):652–658. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli MGS. Antepartum and postpartum depression. J Gender- Specific Med. 1998;1:33. [PubMed] [Google Scholar]
- Stephansson O, Kieler H, Haglund B, Artama M, Engeland A, Furu K, Gissler M, Norgaard M, Nielsen RB, Zoega H, Valdimarsdottir U. Selective serotonin reuptake inhibitors during pregnancy and risk of stillbirth and infant mortality. JAMA. 2013;309(10):48–54. doi: 10.1001/jama.2012.153812. [DOI] [PubMed] [Google Scholar]
- Suri R, Altshuler LL. No decision is without risk. J Clin Psychiatry. 2009;70(9):1319. doi: 10.4088/JCP.09com05529. [DOI] [PubMed] [Google Scholar]
- Topletz AR, Le HN, Lee N, Chapman JD, Kelly EJ, Wang J, Isoherranen N. Hepatic Cyp2d and Cyp26a1 mRNAs and activities are increased during mouse pregnancy. Drug Metab Dispos. 2013;41:312–319. doi: 10.1124/dmd.112.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy TS, Venkataraman R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6, and CYP3A activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62:400–407. doi: 10.1016/S0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Wheeler SB. Tricyclic dose requirements across pregnancy. Am J Psychiatry. 1993;150(10):1541–1542. doi: 10.1176/ajp.150.10.1541. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Findling RL, Hanusa BH. Effects of the postpartum period on nortriptyline pharmacokinetics. Psychopharmacol Bull. 1997;33(2):243–248. [PubMed] [Google Scholar]
- Wisner KL, Gelenberg AJ, Leonard H, Zarin D, Frank E. Pharmacologic treatment of depression during pregnancy. JAMA. 1999;282:1264–1269. doi: 10.1001/jama.282.13.1264. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Zarin DA, Holmbow ES, Appelbaum PS, Gelenberg AJ, Leonard HL, Frank E. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157(12):1933–1940. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DKY, Findling RL, Moses-Kolko EL. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Perel JM, Jones-Ivy S, Bodnar LM, Singer LT. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]