Abstract
In the past Pb2+ has been used in many industries, including gasoline, piping, toys, paints, and more. The use of lead has led to a natural increase of lead concentration in the environment especially in air and water. According to the U.S. CDC “no level of lead in blood is considered safe.” Exposure to very low amounts of lead can cause several health complications including developmental and neurological disorders. Over the past several years an emphasis has been placed in developing systems that can detect lead at a very low concentration. A great deal of work has been accomplished in the development of Pb2+ sensors that can not only detect but also quantify the amount and in some cases in the presence of other metal ions. Herein, we describe current regulations, mode of exposure and recent development of sensing techniques.
Keywords: Lead Poisoning, Heavy Metal, Metal Ion Detection, Heavy Metal Contamination, Fluorescent Sensors
Introduction
Lead has no known natural biological role in humans. It is a persistent environmental contaminant and has been viewed as a common environmental health concern. Lead is a strong chalcophile, found naturally in the environment mainly in ores of zinc, silver, and copper. The major sources of lead are in galena (PbS), cerrussite (PbCO3), and anglesite (PbSO4) deposits.1 The primary cause of lead contaminated soils, air, and water systems are due to anthropogenic activities such as burning leaded gasoline, use of lead solder, lead piping, and lead based paints.1,2 The natural levels of lead in surface and ground waters is very low (< 5 ppb) but in some areas higher than 1000 ppb lead have been found in both water and soil.2
There has been reports of mass lead intoxication in Senegal and Nigeria, which caused significant lose in life and developmental issues with children.3,4 This lead-cycle initiated by anthropogenic activities is more extensive than the natural lead-cycle, causing lead pollution a worldwide issue. The median BLLs in children under age 6 fell from about 15–18 μg/dL in 1970 to 2–3 μg/dL in 1994. However, more than 250,000 children of the age 1–5 years have BLLs above the Center for Disease Control (CDC’s) recommend action limit of 5 μg/dL.5,6 Even at extremely low levels, lead has been linked with a range of health effects including behavioral problems and learning disabilities.7–13 Lead enters the body through a multitude of pathways and understanding these pathways, the process of storage and transport, and localization in cells can help better evaluate the biological effects of lead.
The environmental concerns and associated health effects at a very low levels of lead means tools to detect and quantify lead must be operable at low concentrations. Sensitive, selective, and simple methods of detection are crucial in not only preventing contamination but for better understanding of biological pathways of lead. Several reviews have focused on the sensing techniques of different groups of metals (e.g., heavy metal, alkali metal) or focus on a specific type of sensing (e.g. DNAzymes, nanoparticles, fluorescent molecules, etc).14–17
Lead Exposure
In general, exposure to toxic metals may occur through food, medications, the environment i.e., water and air, or during occupational and/or recreational activities. In terms of environmental contamination, levels of pollutants are usually small in waters but they can be higher in certain zones due to industrial and domestic waste discharge.18 Aquatic plants and animals, in particular some mollusks, can accumulate high levels of toxic elements such as arsenic and cadmium providing a vehicle to introduce toxic metals into the food chain.18 In Europe, it has been reported that 17 species of birds of prey have had lead poisoning.19 Some of these birds include the White-tailed Eagle and the endangered Spanish Imperial Eagle.19 Heavy metals, including lead, are absorbed through the roots and leaves of plants.20 This can then interfere with the levels of antioxidants in plants, and reduce the nutritive value of the produce.20 Individuals are exposed to lead and its harmful effects through two main routes: from air and water. Lead containing particles or aerosols generated from lead-based paints, gasoline and industrial manufacturing serve as the primary route to aerial lead exposure. The second route is from water, dispensed from lead based pipes.21–26 In adults, the deleterious effects of lead may be reversed through chelation therapy, but the effect on children is more permanent.
Young children are particularly sensitive to exposure to lead due to their developmental properties and hand-to-mouth behavior.27 Young children are more susceptible to the hazardous health effects of lead exposure because they absorb more of the ingested lead.28 Lead has been found in high concentrations in children’s products, especially toys and candy.29 Indeed, a widespread problem has been reported with high metal contamination in toys and certain jewelry as children ingest Pb-contaminated products. One of the main sources of Pb2+ in jewelry items is thought to be the use of recycled electronic wastes and lead battery.30,31 In the past few years millions of toys have been recalled because of chemical safety hazards, for violating lead paint standard.32,33 It has been reported that metal leaching from several plastics, including children’s toys, has toxic effects on fish.34 In addition to Pb, Ba Cd, and Cr have been found in baby toys.35 Weidenhamer and Clement examined 39 jewelry items and found they contain 90% or more lead by weight.36 Children living in urban areas typically exhibit elevated blood lead levels (BLLs) along with many major cities having recent spikes in the lead concentration of their water system due to disinfectant switches.26, 37–39
Another current issue with lead exposure accompanies changes in the water systems disinfectants utilized. Figure 1 shows two different common approaches for disinfecting waters from microbial species. Traditionally, chlorine has been used in the drinking water system as a disinfectant, but recent concerns of toxic disinfectant by-products has lead to the use of a substitute, chloramine.38 In addition to forming carcinogenic nitrosamines, the switch from chlorine to chloramine has also been connected to elevated levels of lead in drinking water.40 Higher levels of Pb2+ in drinking water has lead to an elevated level of lead in human blood samples in Washington DC, Portland OR, Providence RI, and other cities.41,42 Edwards and colleagues reported a significant increase in BLLs in Washington D.C.42 The use of chlorine typically inhibits the leaching of lead from pipes as it oxidizes Pb2+ to Pb4+ and creates a coating of slightly insoluble lead oxide (PbO2). Chloramine is a weaker oxidant than chlorine, which does not produce such protective coatings, and this in turn releases more Pb2+ into the drinking water.6
Figure 1.
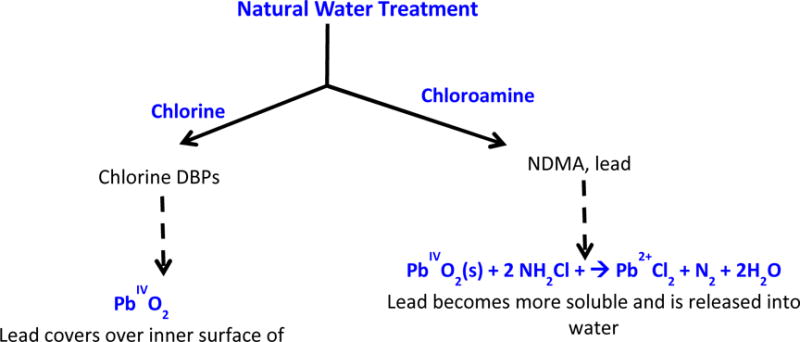
A schematic of purification processes for drinking water. Chlorination generates chlorine disinfection by-products (DBPs) but oxidizes lead to a slightly insoluble Pb4+ state. Chloramine causes increased release of lead from plumbing, it is a weaker oxidant that does not oxidize Pb2+ to Pb4+
Maximum Contamination Level and Lead Toxicity
Lead is a toxic metal that has been in use for many decades as discussed above, and different regulatory agencies have set a limit of maximum contamination level (MCL) for lead. However, the limits vary from agency to agency as well as the material they regulate (Table 1). According to the U.S. CDC a 100–190 ppb BLL poses a potential threat and that diagnostic testing is strongly encouraged.5 In 2010 the EPA set the maximum contaminant level goal (MCLG) for lead to zero ppb and the action level at 15 ppb, in drinking waters.43 The U.S. Food and Drug Administration (FDA) has set maximum level of 0.1 ppm for lead in candy.44 Additionally, the Consumer Product Safety Commission (CPSC) has recently lowered the limit of lead in children’s product to 100 ppm.45 This trend of lowering the maximum limit of lead in many consumer products is in response to the increasing recognition of the threat that lead poses. A low level exposure to lead can cause neurological, reproductive, cardiovascular, and developmental disorders.7–13
Table 1.
List of Standardized Lead Levels.
| Agency | Focus | MCL | |
|---|---|---|---|
| United States | EPA | Drinking Water | 15 ppb |
| Air | 1.5 μg/m3 | ||
| CPSC | Children’s Toys (12 or younger) | 100 ppm | |
| Paint | 600 ppm reduced to 90 ppm | ||
| FDA | Food and Dishware | 0.5 – 7.0 ppm leaching solution | |
|
| |||
| Canada | Health Canada |
Drinking Water | 10 ppb |
| Air | N/A | ||
| Children’s Toys (12 or younger) | 90 ppm | ||
|
| |||
| EU | European Commission |
Drinking Water | 10 ppb |
| Air | 0.5 μg/m3 | ||
| Children’s Toys (12 or younger) | N/A | ||
|
| |||
| International | WHO | Drinking water | 10 ppb |
Lead is known to bioaccumulate in bones for up to 20 years and is not hazardous to human health unless it is released, this can occur when the bones experience turnover and remodeling by osteon activity.46,47 Lead is a likely target of calcium- and zinc-binding proteins (C2A domain of synaptotagmin I and cysteine-rich zinc finger proteins) that control cell signaling and gene expression, respectively, but the molecular and cellular mechanisms of lead toxicity remain an open question.48–52 Lead can also be taken up by the cell by copper and zinc transporters and have been observed to cause both oxidative stress and DNA damage.53,54 Lead is also known to interfere with the biosynthesis of heme.55 Bradman et. al. reported that iron deficiency increases BLLs perhaps due to an increase in absorption and retention of lead.56 In addition, Pb2+ may bind in vacant Fe2+ sites in the hematopoietic system, resulting in reduced lead excretion.56 Hopkins et. al. have demonstrated a close association between iron metabolism genes with lead exposure in children. As mentioned, iron deficiency has been implicated with an increased absorption and deposition of lead. However, iron supplement given to a control group did not change the BLL, and children with genetic variants may be more prone to the lead poisoning.57 Interest in explaining these pathways, as well as public concerns over toxic lead exposure, provides a need for devising new ways to track Pb2+ in natural samples.
Current Method of Detection
Metal ions enters into the cells through several ways and understanding these pathways of metal incorporation, the process of their storage and transport, and localization in cells can help for the better understand normal functioning as well as the diseased states. These methods with their detection limits are listed in table 2.
Table 2.
List of different Techniques used in lead detection.
| Class | Reagent (support matrix) | Method | Signal Properties |
Detection Limit |
Range | Selectivity | Year | Ref |
|---|---|---|---|---|---|---|---|---|
| Electrochemical | Bi-SPE | ASV | N/A | 0.15 ppb | 0.5 – 100 ppb | unknown | 2011 | 69 |
| G-quadruplex with crystal violet | DPV | N/A | 82.2 ppt | 0.2 – 205 ppb | good | 2011 | 70 | |
| 1,10-phenanthroline | ASV | N/A | 82.2 ppt | N/A | nonspecific | 2011 | 72 | |
| DNA/C-TiO2 NTs | ASV | TiO2 | 0.7 ppt | 2.1 ppt – 32.8 ppb | good | 2010 | 71 | |
| carbon composite PVC-based membrane | ASV | Pt wire | 65.8 ppb | 0.158 – 20,548 ppm | good | 2010 | 73 | |
| Nanoparticles | Au nanoparticles | Fluorescence Absorbance | Ex: 330nm Em: 600nm | 247 ppb | N/A | poor | 2012 | 74 |
| Au nanoparticles and DNAzymes | DLS | Adsorption at ~540 nm | 1.3 ppt, 7.2 ppt | 2.1 – 61.1 ppt | good | 2012, 2011 | 85,86 | |
| CdTe-QDs | Fluorescence | Ex: 525nm Em: 560nm (QY =0.07) | 30 ppb | 0.22–4.51 ppm | good | 2009 | 75 | |
| DNAzymes | cationic polythiophene (PT) DNAzyme | Fluorescence | N/A | 0.2 ppb | 0.002 – 20 ppm | good | 2012 | 82 |
| DNAzyme with graphene oxide | Fluorescence | Em: ~565 nm | 100 ppt | N/A | good | 2011 | 79 | |
| Computer-Readable DNAzyme on Disc | Error Distribution of CD | N/A | 2ppb | 0.002–205 ppm | good | 2011 | 81 | |
| GR-5DNAzyme | Fluorescence | N/A | 0.76 ppb | N/A | good | 2010 | 78 | |
| DNAzyme in amicrofluidic device | LIF | Ex: 488 nm Em: 518 nm | 2.3 ppb | 0.020.5 – 20.5 ppm | good | 2005 | 80 | |
| DNAzyme-Au-NPs | Absorbance | Abs: 522 & 700 nm | can be tuned | can be tuned | good | 2003 | 77 | |
| Small Molecules | Calix-DANS3-OH (PDMS microfluidic chip) | Fluorescence | Ex: 365 nm Em: 496 nm | 42ppb | N/A | good | 2012 | 102 |
| triazolo-thiadiazi (imbedded in PVC) | Fluorescence | Ex: 330 nm Em: 430 nm QY=0.039 | 4.5 ppb | 0.010.2 – 78.1 ppm | good | 2011 | 99 | |
| PDA-polymer | Fluorescence | Ex: 254nm Em: 540 nm | 0.8 ppm | N/A | good | 2011 | 104 | |
| LFS-1 | Fluorescence | Em: 469 nm | N/A | N/A | good | 2011 | 105 | |
| L2 (immobilized on PVC) | Fluorescence | N/A | 41 ppb | 0.062 – 5137 ppm | N/A | 2010 | 106 | |
| Arsenazo III (immobilized on XAD-16) | Reflectance | N/A | 10 ppb | 0.205–20.5 ppm | N/A | 2010 | 107 | |
| Lead-selective ionophore (immobilzed on PVC) | Absorbance | N/A | 5 ppb | 0.007 – 10.7 ppm | N/A | 2010 | 108 | |
| BODIPY-functionalized Fe3O4.SiO2 nanoparticles | Fluorescence | Ex: 326&596 Em: 456 nm (QY=0.042) | 1.5 ppb | 0 – 30 ppb | good | 2010 | 109 | |
| Calix-DANS4 | Fluorescence | Ex: 365 nm | 5 ppb | N/A | N/A | 2009 | 101 | |
| 1,8-diaminoanthraquinone | Absorbance | Abs: 524 nm | Eye: 2–3 ppm UVvis: 21 ppb | N/A | good | 2009 | 98 | |
| Imine-bridged TTF-pi-Py | Absorbance and Electrochemical | Abs: 508 nm | micromolar | N/A | Zn2+ interferes | 2009 | 110 | |
| Leadglow (LG) | Fluorescence | Ex: 389 nm Em: 423 nm | 10 ppb | 1 –50 ppb | good | 2009 | 100 | |
| LF1 | Fluorescence | LF1:Pb2+: Em:514 (QY=0.013) | <15 ppb | N/A | good | 2006 | 97 | |
| Ketoaminocoumarin (solution) | Fluorescence | Ex: 411nm Em:491 (QY=0.35) | N/A | N/A | good | 2002 | 96 |
Selectivity: good (Mn2+, Mg2+, K+, Fe2+, Fe3+, Co2+, Cd2+, Al3+, Ag+, Zn2+, Ca2+, Ba2+, Sr2+, and Hg2+), N/A (unknown - not known or only a few metal ions tested), nonspecific.
Techniques Approved by Regulatory Agencies
The United States EPA approved methods for lead detection are inductively coupled plasma mass spectrometry (ICP-MS)58 with a detection limit of 0.6 ppb, inductively coupled plasma atomic emission spectrometry (ICP-AES)59 with a detection limit of 42 ppb, and graphite furnace atomic absorption spectroscopy (GFAAS)60 with a detection limit of 0.7 ppb. The detection limits and linear working ranges are dependent on the sample matrix, instrumentation, and selected operating conditions. These methods provide means to determine dissolved lead in ground waters, surface waters, drinking water, wastewaters, sludges, and soil samples.58–60 There are four natural isotopes of lead and in ICP-MS three isotopes (206Pb, 207Pb, and 208Pb) are typically monitored, however the method suffers from interferences including isobaric elemental interferences, abundance sensitivity, isobaric polyatomic ion interferences, and physical interferences. Another EPA approved method, ICP-AES, experiences interferences from Co, Al, Ce, Cu, Ni, Ti, and Fe at 100 mg/L (100 ppm) at the recommended wavelength of 220.353 nm.59 Similar to ICP-MS, one can perform direct analysis of samples with GFAAS. Although GFAAS is a reliable and sensitive method, the necessity of additives and modifiers is considered to be a disadvantage. If the sample preparation involves digestion with HCl, for example, it can influence the sensitivity for lead detection. This effect can be corrected up to 10% with the addition of Pd/Mg/H2 as a modifier.60 Though the FDA and CPSC have significantly higher lead limits, they employ the analysis similar to the EPA.61,62
These methods usually require small sample size and sensitive, they are instrument intensive, non-portable, and require trained technicians. Also, the sample preparation of specific metal ions may require a long digestion process. First, the composite sample must be blended with water and/or nitric acid, and predigestion is often required for suspended samples. Generally, the microwave digestion is followed by analysis with GFAAS, ICP-AES, and ICP-MS. Overall, the most favorable EPA approved method of analysis for lead in an environmental water sample is ICP-MS.58 ICP-MS analysis often serves as the simplest sample preparation, with least interference, and lowest detection limit. For these reasons, ICP-MS has been viewed as a gold standard for other methods, including those described herein. These methods that have been approved by the regulatory agencies have a major limitation as they cannot be easily used to monitor the distribution of metal ions in real time. Thus, a simple, rapid, inexpensive, minimal sample handling, selective, and sensitive method that permits real time detection of metal ions is of interest.
Electrochemical Techniques
A traditional method of metal ion detection is the application of electrochemical properties including anodic stripping voltammetry (ASV), cathodic stripping voltammetry (CSV), and adsorptive stripping voltammetry (AdSV).63–65 These methods can be used determine metal ion concentrations in water to sub–parts-per-billion levels. They usually incorporate three electrodes, a working, auxiliary, and reference electrodes. The analytical species is reduced onto the working electrode and is then oxidized, “stripped”, back into the electrolyte solution.66 The stripping current due to oxidation of each analyte is proportional to the concentration of that analyte on or in the electrode and thus, in the solution in question.66 Mercury film electrodes (MFE) and the hanging mercury drop electrode (HMDE) have been traditionally used for ASV because of the negative potential range of mercury but due to the potential health hazards of mercury alternative electrodes have been sought.67 A bismuth-film electrode (BFE) has been successfully used to determine Zn, Cd, and Pb at low concentrations. It has mostly been used for the determination of Pb and Zn in tap water and in human hair.63 ASV has also been frequently used to determine BLLs.57,63,68
A recent development in electrochemical detection of Pb2+ utilizes the traditional bismuth electrode but also carbon composite, G-quadruplex, phenanthroline, and DNA combined with TiO2 (Table 2). Quintana et. al. reported a sensitive bismuth-modified screen-printed electrodes (Bi-SPE) for lead detection that avoids the use of toxic mercury containing films.69 Stripping voltammetry using SPEs allows the formation of an amalgam that enables the analyte to accumulate on the bismuth film, providing with a higher sensitivity and reproducibility. They carried out in situ or ex situ in the same frame as the European Union project. Ex situ involves putting SPE into the solution containing Bi3+ ions and then a potential to reduce bismuth to Bi0 prior to the addition of the analyte. In situ experiment uses the sample solution containing Bi3+ and thus both analyte and the bismuth are electrochemically deposited on the working electrode. In situ produced a linear working range for lead ion concentration from 0.5 to 100 ppb and a detection limit of 0.15 ppb, which proved to be more selective than ex situ measurements.69
Additionally, Li et. al. used differential pulse voltammetry (DPV) to monitor the G-rich DNA conformational switch, induced by Pb2+, from a random-coil to G-quadruplex (G4) with crystal violet as the G4-binding indicator.70 G-quadruplexes are four-stranded DNA structures stabilized by coordination cations and some K+-stabilized G-quadruplexes exhibit superior peroxidase-like activity. This effectively catalyzes a H2O2-mediated oxidation of 2,2′-azino-bis(3-ethlybenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), which causes a colorimetric and oxidative change. The technique has a detection limit of 82.2 ppt, well below the EPA limit. Another electrochemical DNA-based lead sensor has been developed, that involves vertically aligned conductive carbon hybridized TiO2 nanotube (NT) arrays (DNA/C-TiO2 NTs).71 In this case, TiO2 nanotubes were vertically aligned on a Ti substrate by anodic oxidation and then DNA was immobilized onto the substrate. This sensor exhibits a high sensitivity (2.1 ppt – 32.8 ppb), good selectivity (Mn2+, Mg2+, K+, Fe2+, Fe3+, Co2+, Cd2+, Al3+, Ag+, Zn2+, Ca2+, Ba2+, Sr2+, and Hg2+), and a wide pH range. The superior characteristics for lead sensing were attributed to the immobilization of abundant target biomolecules, DNA, and the enhanced bioelectrical activity. The controllable carbon hybridized of the TiO2 NTs increases the conductivity of the electrode, while retaining the tubular structure, biocompatibility and hydrophilicity.71 The electrode can then be regenerated for multiple uses including numerous environmental sites.
Bouw and colleagues reported a low cost electrochemical sensor based on clay modified by 1,10-phenanthroline within montmorillonite (MMT).72 Pb2+ is sensed by a carbon paste electrode (CPE) by adsorptive stripping voltammetry through the exchange of saturated sodium ions by Pb2+. The low cost materials, ease of preparation and use, and low detection limit (82.2 ppt) make this method an attractive one for testing environmental samples, however, the approach suffers from a 70% reduction in Pb2+ signal in the presence of Hg2+.72 Another low cost approach, developed by Abbaspour et. al., uses reusable and disposable carbon composite PVC-based membranes, where a Pt wire was coated with phenyl hydrazone derivative-carbon composite in PVC membrane.73 The membrane sensors have a fast response time and can be reused for up to 70 days, and is fairly sensitive with a detection limit of 65.8 ppb. The new developments in reusable, disposable, and affordable electrochemical sensors has been exciting, however, there is a room for improvement in their selectivity as well as sensitivity. Also, these electrochemical techniques do not provide any spatial information, which is of interest to better understand the cellular distribution of lead.
Nanoparticles
Gold nanoparticles have been used in sensors because of their extremely high extinction coefficient in the visible wavelength range. Typically, even at nanomolar concentrations, the color change of the particles can be observed by the naked eye. Recently, Yang et. al. reported a bromide capped gold nanoparticles for sensing lead.74 In this case, Pb2+ reacts with bromide ions to form PbBr2 on the surface of the gold nanoparticles that exhibits luminescence by absorbing in the UV region (300 nm) with visible emission (600 nm). With an increase in Pb2+ concentration the color of the particles changes from red to light blue and even become colorless which can be visualized with naked eyes. Alkali metals cause no spectral changes. However, alkaline earth and heavy metals show small changes, and even with 4 to 5 equivalents of these ions, Pb2+ has been detected.74 In this case, no rigorous synthetic or biological procedures to be adopted; the sensor is water soluble, and has the very desirable visible region optical properties but the detection limit is above EPA limit. Wang and Guo reported a new nanoparticle based lead sensor that utilizes fluorescence resonance energy transfer (FRET) for Pb2+ detection.75 It comprised of a positively charged CdTe-QDs capped with cystamine (CA-CdTe-QD) and negatively charged AuNPs capped with 11-mercaptoundecanoic acid (MUA-AuNPs).75 The lead assay utilizes FRET efficiency of the positively charged quantum dots (QDs) and the negatively charged gold nanoparticles (AuNPs) which in the presence of Pb2+ the electrostatic interaction of inhibited. They are water soluble and biocompatible, easy to operate, and little interference from physiological metal ions but the working range of this sensor is parts per million although the detection can be as low as 30 ppb. Fe3+ and Ag+ ions interfere with Pb2+ detection, which can be improved in this exciting sensor.
DNAzymes
DNAzymes were first reported in 1994 showing approximately an 100-fold increase in a lead ion dependent cleavage process of a double stranded DNA that is linked with a flurophore or a chromophore and a quencher, and when the double strand is cleaved a fluorescence or a difference in absorption is observed.76 In general, DNAzyme-based Pb2+ sensors are composed of an enzyme and substrate strand and the addition of Pb2+ enables the DNAzyme to cleave its substrate. Several lead-specific DNAzyme were built on the 8–17 base pair that are capable of catalyzing a phosphodiester bond cleavage in the presence of Pb2+. In 2003 Liu introduced a DNAzyme adhered to gold nanoparticles that allows tuning of the detection level over several orders of magnitude.77 In this case, the 3′ strand of a DNA is binds to gold nanoparticles (diameter, 13 nm), which causes aggregation of the nanoparticle exhibiting a blue color. In the presence of Pb2+, the complementary 5′ strand that can recognize the Pb2+ catalyzes a hydrolytic cleavage preventing the formation of the NP aggregates. This results in a red color, sensing the presence of Pb2+. This system allows detection with different dynamic range that are present in various environments in question; for example the EPA has a limit of 15 ppb while the CPSC has limits in the part per million (ppm). Lan et. al. developed a catalytic beacon sensor for Pb2+ based on the first 8–17 DNAzyme that exhibited a higher metal ion selectivity (~ 40,000 times), than the previously reported Pb2+ sensors.78 Wen developed a “mix-and-detect” fluorescent sensor for Pb2+ ions by using graphine oxide nanoprobes.79 Upon addition of Pb2+, the substrate strand was cleaved causing significant quenching of the fluorescence. As the Pb2+ concentration was increased, the fluorescence signal decreased in response to increased cleaved DNAzymes. The dynamic range was tuned to high concentration range by appropriate mixing of enzyme strands.79
There has been considerable interest in developing portable and small devices for onsite testing. Chang and colleagues developed a miniaturized DNAzyme in a nanocapillary interconnected microfluidic device.80 They combined a lead-specific DNAzyme with a microfabricated device containing a network of microfluidic channels that are fluidically coupled via nanocapillary array interconnect.80 This sensor is a unique combination of DNAzyme and microfluid-nanofluid and it has been applied to the determination of lead onsite of an electroplating sludge and reference material. Another small and easy to use device was developed by Wang, a computer-readable DNAzyme Pb2+ assay on disc.81 A conventional compact disc (CD) was used as the platform for preparing DNAzyme assays and an unmodified optical drive of ordinary computers as the readout device. Pb2+ specific DNAzyme was immobilized on the “transparent side” of a conventional CD-R via mild surface reactions. The concentration of Pb2+ was determined with a diagnostic program that checks the error distribution on the CD. The errors increase linearly over a wide range of concentrations, 2 ppb to 200 ppm.81
A recent report in lead specific DNAzymes took advantage of the optical properties of a water-soluble cationic polythiophene (PT) and designed a fluorometric sensing assay for the detection of Pb2+.82 A simple “mix-and-detect” approach enables the detection of Pb2+ within 20 minutes due to the distinguishable optical properties of PT–dsDNA and PT–ssDNA. The detection was well below the standard EPA at 0.2 ppb but this methods has a wide detection range, 2 ppb to 20 ppm, enabling diverse functional.82 This method avoids modification and separation thus making this simple, sensitive, specific, and cost-effective approach showed great potential in environmental monitoring, waste management and cellular understanding.
DNAzymes and Nanoparticles
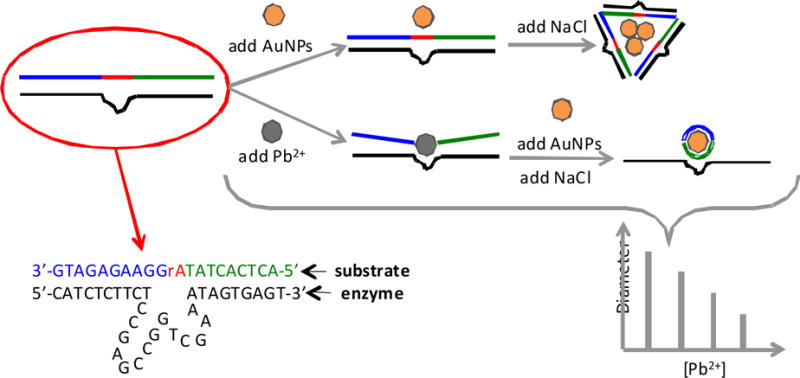
Recent developments have been made using a combination of nanoparticles and DNAzymes. Aside from the optical properties of the gold nanoparticles and DNAzymes, nanoparticles’ diameters are related to their disperse state. Thus a change of the disperse state from discrete nanoparticles to aggregates will lead to the increase of their average diameter, which can be monitored by dynamic light scattering (DLS).83,84 Miao and colleagues reported a dynamic light scattering sensor for Pb2+, which was constructed with oligonucleotide-modified gold nanoparticles based upon its cleavage property for DNAzyme.85,86 The basis for this method was to determine the signal of the average diameter of AuNPs (Figure 3). There are two strands, the enzyme strand and the substrate strand, which can hybridize with each other to form duplex structure DNAzyme. This structure can then be adsorbed on the surface of the AuNPs, preventing them from aggregating in the presence of NaCl. In the presence of Pb2+ the substrate strand of DNAzyme can be cleaved into single strand DNA (ssDNA) fragments and these ssDNA fragments are adsorbed on the surface of the AuNPs to inhibit aggregation in the presence NaCl. Thus, as the concentration of Pb2+ increases the diameter of the aggregates decreases linearly from 2.1 to 61 ppt. This method is relatively simple and inexpensive because the DNA is unmodified and not separated.
Figure 3.
Organic Molecules
In the past 20 years fluorescent sensors have been investigated intensely for their potential in real-time monitoring that helps in understanding cellular metal accumulation, trafficking, protein trafficking, small-molecule signaling, organelle distribution, and cell viability, along with the simple, quick, and determining environmental contamination.87–93 Fluorescence-based sensors can offer unparalleled sensitivity and thus have garnered significant interest. Traditional methodologies require collection, transportation, occasional pretreatment of the sample, and in many cases, expensive instrumentation operated by trained personnel.28,56–58,94 Small molecule sensors are valuable not only as they are cost effective and user-friendly, but also for real time monitoring and garnering special information.94 Among the different chemical sensors, fluorescence-based ones present many advantages: fluorescence measurements are very sensitive, it is possible to detect a single molecule, typically low cost, easily performed, versatile, and allow subnanometer spatial resolution.94,95 This approach has contributed greatly to explaining the roles of calcium and related s-block metals in biology,95 but similar chemical tools for d- and p-block transition and heavy metals remain somewhat open for study.96
Chen et. al. reported a ratiomentric fluorescent sensor based on polypeptide scaffolds equipped with a microenvironment-sensitive fluorophore.96 This sensor emits at visible wavelength and has a quantum yield of 0.35.96 He et. al. reported another fluorescent based sensor called, Leadfluor-1 (LF1), that shows promise in probing lead in biological samples.97 It has a selective turn-on response, visible excitation and emission profile useful for real time monitoring of living cells.97 Ranyuk et. al. reported a anthraquinone-based receptor that functions as a colorimetric chemosensor for the selective recognition of lead in water.98 This sensor was developed using a systematic approach based on single modification of a one-step available chromogenic macrocyclic backbone, thus this is convenient for the development of selective sensors for different metal ions.98 By changing the nature and number of side arms this backbone can be adapted to the nature of the metal of interest. In the presence of 2–3 ppm lead, a distinct change in color can be observed by the naked eye; a detection limit of 21 ppb can be achieved with a spectrophotometer.98 Visible detection with the naked eye holds a great prospect for quick detection.
Alongside the development of devices, small organic molecules have been imbedded and immobilized in various substances. A most recent example of this immobilization was reported by Aksuner et. al.,99 in which a novel triazolo-thiadiazin derivative was immobilized in PVC. The sensor displays a response for Pb2+ over a concentration range of 10 ppb to 78 ppm with the detection limit of 4.5 ppb.99 In addition to high reproducibility and reversibility of the fluorescence signal, the sensor also exhibits good selectivity over common metal ions (Na+, K+, Ca2+, Mg2+, Cu2+, Co2+, Ni2+, Zn2+, Cd2+, Mn2+, Fe3+). The membrane is easily prepared, stable, rapid, and is simple. The accuracy of the proposed sensor was confirmed by analyzing standard reference materials of natural water and surface water.
Another small organic sensor was developed in 2009 by Marbella et. al.100 It is a unique fluorescent sensor both in structure and binding. Many fluorescent sensors have a flexible framework allowing the coordination to the metal ion, while this molecule has a rigid structure allowing alterations to be imposed without affecting the binding site. Intestingly, only a few metal ion sensors have solely a rigid framework. This sensor, called ‘leadglow’ (LG) was found to have a ‘turn-on,’ ratiometric response to Pb2+ in aqueous solution.100 The free ligand has an excitation at 415 nm and an emission at 465 nm. Conversely, the Pb2+ bound ligand has an excitation at 389 nm and an emission at 423 nm.100 Additionally, LG is able to detect Pb2+ at a wide pH range (4–10). This allows for the determination of Pb2+ in a variety of samples: slightly acidic, neutral, and basic pH levels. LG was compared with the EPA approved method. Determination of trace elements in waters and wastes by ICP-MS. By comparing the quantification of Pb2+ in SRM® 3128 Lead Standard Solution by both LG and ICP-MS, the two methods were found to be statistically equivalent. LG offers a wide variety of choices from tuning the excitation with the addition of functional groups to the incorporation into a matrix for ease of use. LG shows promise in becoming a simple method to detect Pb2+ in environmental samples.
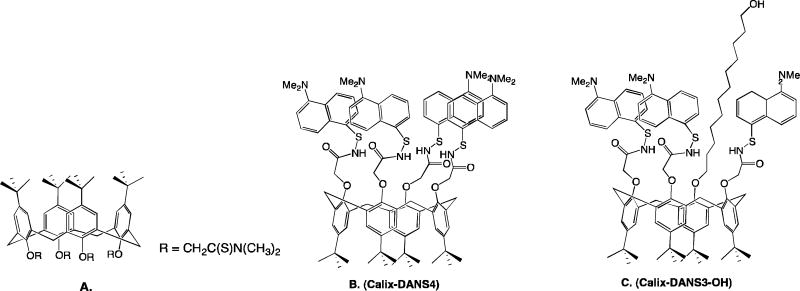
As environmental and on site testing is of great interest to prevent and monitor lead contamination, advance in small and simple devices has grown. Zhao et. al. developed a micro-fabricated device for detection in water.101 It was based on a selective and sensitive fluorescent molecular sensor for Pb2+, Calix-DANS4.101 The micro-chip based lead sensor detected by using a configuration in which the sensing molecules are excited by two optical fibers each one connected to a 365 nm UV LED source, and the light collection is made by another optical fiber with a photomulitplier tube. The detection limit is below the EPA limit, permitting onsite testing of rivers and lakes. A recently developed devices used a calixarene bearing three dansyl groups and one long alkyl chain terminated by an alcohol function.102 Calix-DANS3-OH was grafted on the wall of a PDMS microfluidic device. It has emission properties in the visible region and a detection limit ~42 ppb.
Polymeric materials with chromophore or flurophores have also garnered interest because of their potential applications. In 2005 Kim and co-workers reported a structurally simple polymer that was sensitive for lead in aqueous environments.103 In the presence of lead, the polymer fluorescence is quenched. More recently, a polydiacetylenes (PDAs) were reported for lead sensing.104 In the presence of lead the polymers displayed a selective colorimetric change and a significant fluorescence enhancement. PDAs have an intense blue color and when in the presence of lead a transition to a bright pink occurs, a change in color that can be easily seen with the naked eye.104
Concluding remarks
Lead, has been a major threat to human and environmental health and it continues to be a persistent problem, though its industrial usage has decreased drastically. Several countries are dealing with outbreaks of lead poisoning while others are dealing with high levels of lead in their water systems. New findings highlight the detrimental effect of lead even at a very low concentration. In order to better manage these problems new techniques for detection have been developed, and or modifications to more established techniques have been made, which also helps in understanding its mechanism of action.
Selection of the best probe is based on the testing environment and purpose. Detection limit of probe and necessary sensitivity, state of sample (solution, solid, biological, etc.), testing environment (access to laboratory or on-site testing), and information desired (real-time imaging, concentration, or simple detection) all must be considered when selecting a probe. Electrochemical techniques have been extensively used but with the development of new probes (DNA, organic, and Bismuth based) this method has become safer, easier, and significantly more accurate. Electrochemical probes are easily used in the analysis of aqueous samples and with the development of portable electronics electrochemical probes can now be applied to on-site testing. Among various detection techniques optical detection appear to be the most convenient methods due simplicity and detection limits. Among these techniques fluorimetry is highly impressive for its high sensitivity, fast kinetics, and high spatial resolution among in situ application, real-time monitoring in both environmental and in vivo imaging. DNAzymes and nanoparticles have become a widely growing field for their unprecedented detection abilities, the combination of the two also can produce personalized sensors. DNAzymes are usually resistant to hydrolysis and allow for easy modification for the detection of a range of metal ions.
Organic molecules, especially fluorescent molecules, offer the necessary real-time and sensitivity desired for living specimen. These molecules can be modified for use in multiple solvents. Small molecules also have been synthesized with high selectively, great for mechanistic understanding of in vivo studies. Through the compilation of the some of the most promising techniques for the detection of Pb2+, it is concluded that customized sensors can be developed using many aspects (DNAzyme, nanoparticle, fluorescent, organic, electrochemical) depending are their intended usage. More generally the development of sophisticated detection methods can lead to a better understanding of Pb2+ toxicity, through the real-time monitoring of lead in organisms.
Figure 2.
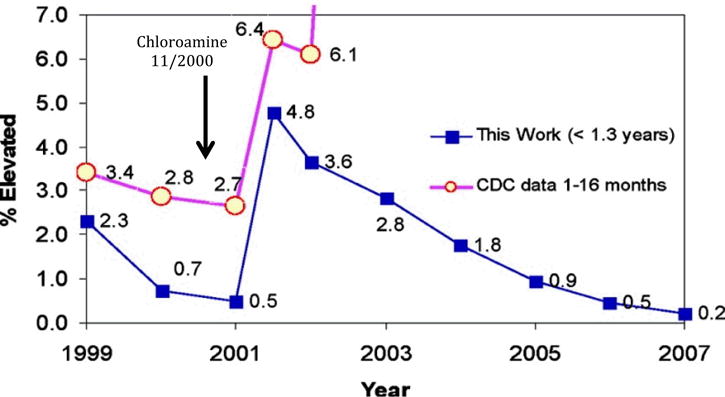
Trends in elevated blood lead levels (EBL) incidence for children aged ≤1.3 years taken from reference40 with permission.
Figure 4.
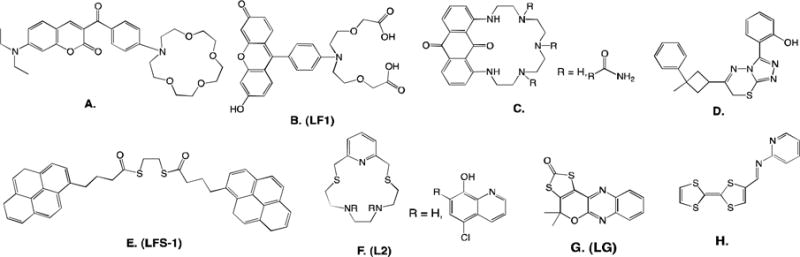
Small organic lead sensors. A.) Ketoaminocoumarin96 B.) Leadfluor-1, LF197 C.) 1,8-diaminoanthraquinone98 D.) Triazolo-thiadiazi, imbedded in PVC99 E.) Lead fluorescent sensor, LFS-1104 F.) 5,8-bis((5′-chloro-8′-hydroxy-7′-quinolinyl)methyl)-2,11-dithia-5,8-diaza-2,6-pyridinophane, L2, imboilized in PVC105 G.) Leadglow, LG100 H.) Imine-bridged TTF-pi-pyridine derivative109
Figure 5.
Macro-organic lead sensors. A.) Ionophore imbedded in PVC107 B.) Calix-DANS4101 C.) Calix-DANS3-OH incorporated into a PDMS microfluidic chip102
Acknowledgments
We appreciate helpful discussions with Ms. Lauren Marbella and Dr. Yi Lu. We thank the National Institutes of Health and Innovation Works for supporting our research.
Acronyms
- CDC
Center of Disease Control
- BLLs
Blood lead levels
- DBPs
Disinfectant by-products
- EBLs
Elevated blood levels
- EPA
Environmental Protection Agency
- MCGL
Maximum containment level goal
- FDA
Food and Drug Administration
- CPSC
Consumer product safety commission
- ICP-MS
Inductively coupled plasma mass spectrometry
- ICP-AES
Inductively coupled plasma atomic emission spectrometry
- GFAAS
Graphite furnance atomic adsorption spectroscopy
- ASV
Anodic stripping voltammetry
- CSV
Cathodic stripping voltammetry
- AdSV
Adsorptive stripping voltammetry
- MFE
Mercury film electrode
- HMDE
Hanging mercury drop electrode
- Bismuth film electrode
- SPEs
Screen-printed electrodes
- DPV
Differential pulse voltammetry
- MMT
Montmorillonite
- CPE
Carbon paste electrode
- NP
Nanoparticles
- DLS
Dynamic light scattering
- AuNPs
Gold nanoparticles
- ssDNA
Single strand DNA
- PVC
Polyvinyl chloride
- PVC
Polydiacetylenes
References
- 1.Galena. Missouri Department of Natural Resources, Division of Geology and Land Survey; 2006. (Publication No. 000658). [Google Scholar]
- 2.Cox PA. Inorganic Chemistry in the Environment The Elements on Earth. Oxford: 1997. [Google Scholar]
- 3.Clune AL, Falk H, Riederer AM. Blacksmith Inst J Health Poll. 2011;1 [Google Scholar]
- 4.Mamtani R, Stern P, Dawood I, Cheema S. J Toxic. 2011;2011 doi: 10.1155/2011/319136. ID 319136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. MMWR. 1997;46:141–146. [Google Scholar]
- 6.Meyer P, Pivert T, Dignam T, Homa D, Schoonover J, Brody D. MMWR. 2003;52 [PubMed] [Google Scholar]
- 7.Lanphear BP, Dietrich K, Auinger P, Cox C. Public Health Rep. 2000;115:521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich KN, Douglas RM, Succop PA, Berger OG, Bornschein RL. Neurotoxicol Teratol. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 9.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. New Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Lu Y. J Am Chem Soc. 2000;122:10466–10467. [Google Scholar]
- 12.Jain AK, Gupta VK, Singh LP, Raisoni JR. Electrochim Acta. 2006;51:2547–2553. [Google Scholar]
- 13.Xiao Y, Rowe AA, Plaxco KW. J Am Chem Soc. 2006;129:262–263. doi: 10.1021/ja067278x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XB, Kong RM, Lu Y. Ann Rev Anal Chem. 2011;4:105–128. doi: 10.1146/annurev.anchem.111808.073617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oehme I, Wolfbeis OS. Microchimica Acta. 1997;126:177–192. [Google Scholar]
- 16.Kim HN, Ren WX, Kim JS, Yoon J. Chem Soc Rev. 2011;41:3013–3442. [Google Scholar]
- 17.Domaille DW, Que EL, Chang CJ. Nat Chem Bio. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 18.Caroli S. Chemical Analysis: Element Speciation in Bioinorganic Chemistry. Vol. 135. Wiley-Interscience Publication: John Wiley and Sons, Inc.; 1996. [Google Scholar]
- 19.Mateo R. In: Watson RT, Fuller M, Pokras M, Hunt WG, editors. Idaho, USA: The Peregrine Fund; 2009. [Google Scholar]
- 20.Sharma RK, Agrawal M. J Environ Biol. 2005;26:301–313. [PubMed] [Google Scholar]
- 21.Stanbury M, Rosenman K. Work-Related Health Disparities in Michigan. 2011 [Google Scholar]
- 22.David JJ. J Urban Eco. 2012;71:151–164. [Google Scholar]
- 23.Lin GZ, Peng PF, Chen Q, Wu ZG, Du L. Environ Res. 2009;109:1–5. doi: 10.1016/j.envres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Mielke HW, Gonzales CR, Mielke PW., Jr Environ Res. 2011 [Google Scholar]
- 25.Xie Y, Giammar DE. Water Res. 2011;45:6525–6534. doi: 10.1016/j.watres.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 26.Smith P, Nriagu JO. Environ Res. 2011;111:1, 81–86. doi: 10.1016/j.envres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Trejo-Acevedo A, Díaz-Barriga F, Carrizales L, Domínguez G, Costilla R. Chemosphere. 2009;74:974–980. doi: 10.1016/j.chemosphere.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 28.The Center for Disease Contol (U.S.) Preventing lead poisoning in young children: a statement [5th revision] The Centers; Atlanta, GA: 2005. [Google Scholar]
- 29.Guney M, Zagury GJ. Envir Sci Tech. 2012;48:4265–4274. doi: 10.1021/es203470x. [DOI] [PubMed] [Google Scholar]
- 30.Weidenhamer JD, Clement ML. Chemosphere. 2007b;69:1670–1672. doi: 10.1016/j.chemosphere.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Weidenhamer JD, Clement ML. Chemosphere. 2007c;69:1111–1115. doi: 10.1016/j.chemosphere.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Morrison WM. Health and Safety Concerns over U.S. Imports of Chinese Products: An Overview. RS22173. Congressional Research Service; 2009. [Google Scholar]
- 33.http://service.mattel.com/us/recall.asp.
- 34.Lithner D, Damberg J, Dave G, Larsson A. Chemosphere. 2009;74:1195–1200. doi: 10.1016/j.chemosphere.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura Y, Kawasaki C, Mine S, Mutsuga M, Tanamoto K. J Food Hyg Soc Jpn. 2006;47:51–57. doi: 10.3358/shokueishi.47.51. [DOI] [PubMed] [Google Scholar]
- 36.Weidenhamer JD, Clement ML. Chemosphere. 2007a;67:961–965. doi: 10.1016/j.chemosphere.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 37.Pirkle JL, Kaufman RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Environ Health Perspect. 1998;106:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedlak DL, von Gunten U. Science. 2011;331:6013, 42. doi: 10.1126/science.1196397. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Korshin GV, Ferguson JF. Environ Sci Tech. 2009;43:3278–3284. doi: 10.1021/es803179b. [DOI] [PubMed] [Google Scholar]
- 40.Miranda M, Kim D, Hull A, Paul C, Galeano M. Envir Health Persp. 2007;115 [Google Scholar]
- 41.Edwards M, Dudi A. J Am Water Works Assoc. 2004;96:69–81. [Google Scholar]
- 42.Edwards M, Triantafyllidou S, Best D. Environ Sci Technol. 2009;43:1618–123. doi: 10.1021/es802789w. [DOI] [PubMed] [Google Scholar]
- 43.http://water.epa.gov/drink/contaminants/index.cfm
- 44.http://www.fda.gov/food/foodsafety/foodcontaminantsadulteration/metals/lead/ucm172050.htm (05/14/2012)
- 45.http://www.cpsc.gov/cpscpub/prerel/prhtml11/11278.html (05/14/2012)
- 46.Bannon DI, Murashchik C, Zapf CR, Farfel MR, Chisolm JJJ. Clin Chem. 1994;40:1730–1734. [PubMed] [Google Scholar]
- 47.Rabinowitz MB. Environ Health Perspect. 1991;91:33–37. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouton C, Frelin LP, Forde CE, Godwin HA, Pevsner J. J Neurochem. 2001;76:1724–1735. doi: 10.1046/j.1471-4159.2001.00168.x. [DOI] [PubMed] [Google Scholar]
- 49.Bressler J, Kim KA, Chakraborti T, Goldstein G. Neurochem Res. 1999;24:595–600. doi: 10.1023/a:1022596115897. [DOI] [PubMed] [Google Scholar]
- 50.Claudio ES, Godwin HA, Magyar JS. Prog Inorg Chem. 2003;51:1–144. [Google Scholar]
- 51.Hanas JS, Rodgers JS, Bantle JA, Cheng YG. Mol Pharmacol. 1999;56:982–988. doi: 10.1124/mol.56.5.982. [DOI] [PubMed] [Google Scholar]
- 52.Magyar JS, Weng TC, Stern TC, Dye CM, Rous DF, Payne BW, Bridgewater JC, Mijovilovich BM, Parkin A, Zaleski GJM, Penner-Hahn J, Godwin H. J Am Chem Soc. 2005;127:9495–9505. doi: 10.1021/ja0424530. [DOI] [PubMed] [Google Scholar]
- 53.Jin T, Lu J, Nordberg M. Neurotoxicology. 1998;19:529–535. [PubMed] [Google Scholar]
- 54.Sharma RK, Agrawal M. J Environ Bio. 2005;26:301–313. [PubMed] [Google Scholar]
- 55.Labbe RF, Vreman HJ, Stevenson DK. Clin Chem. 1999;45:2060–2072. [PubMed] [Google Scholar]
- 56.Bradman A, Eskenazi B, Sutton P, Athanasoulis M, Goldman LR. Environ Health Perspect. 2001;109:1079–84. doi: 10.1289/ehp.011091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopkins MR, Ettinger AS, Hernandez-Avila M, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Bellinger D, Hu H, Wright RO. Environ Health Perspect. 2008;116:1261–1266. doi: 10.1289/ehp.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creed JT, Brockhoff CA, Martin TD. Determination of trace elements in waters and wastes by inductively coupled plasma-mass spectrometry. EPA (U.S.); Cincinnati, OH: 1994. Revision 5.4. [Google Scholar]
- 59.Martin TD, Brockhoff CA, Creed JT, Group EMW. Determination of metals and trace elements in water and wastes by inductively coupled plasma-atmoic emission spectrometry. EPA (U.S.); Cincinnati, OH: 1994. Revision 4.4. [Google Scholar]
- 60.Creed JT, Martin TD, O’Dell JW. Determination of trace elements by stabilized temperature graphite furnace atomic absorption. EPA (U.S.); Cincinnati, OH: 1994. Revision 2.2. [Google Scholar]
- 61.Hepp NM, Mindak WR, Cheng J. J Cosmetic Sci. 2009;60:405–414. [PubMed] [Google Scholar]
- 62.CPSC. Testing Method: CPSC-CH-E1002-08. http://www.cpsc.gov/about/cpsia/CPSC-CH-E1002-08.pdf (05/17/2012)
- 63.Bannon DI, Chisolm JJ. Clin Chem. 2001;47:1703–1704. [PubMed] [Google Scholar]
- 64.Yang WR, Chow E, Willett GD, Hibbert DB, Gooding JJ. Analyst. 2003;128:712–718. doi: 10.1039/b212881k. [DOI] [PubMed] [Google Scholar]
- 65.Baldo MA, Daniele S, Ciani I, Bragto C, Wang J. Electroanalysis. 2004;16:360–366. [Google Scholar]
- 66.Copeland TR, Skogerboe RK. Anal Chem. 1974;46:1257–1268. [Google Scholar]
- 67.Kefala G, Economou A, Voulgaropoulos A, Sofoniou M. Talanta. 2003;61:603–610. doi: 10.1016/S0039-9140(03)00350-3. [DOI] [PubMed] [Google Scholar]
- 68.Bannon DI, Murashchik C, Zapf CR, Farfel MR, Chisolm JJ. Clin Chem. 1994;40:1730–1734. [PubMed] [Google Scholar]
- 69.Quintana JC, Arduini F, Amine A, Punzo F, Destri GL, Bianchini C, Zane D, Curulli A, Palleschi G, Moscone D. Analytica Chimica Acta. 2011;707:171–177. doi: 10.1016/j.aca.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 70.Li F, Feng Y, Zhao C, Tang B. Chem Comm. 2011;47:11909–11911. doi: 10.1039/c1cc15023e. [DOI] [PubMed] [Google Scholar]
- 71.Liu M, Zhao G, Tang Y, Yu Z, Lei Y, Li M, Zhang Y, Li D. Environ Sci Tech. 2010;44:4241–4246. doi: 10.1021/es1003507. [DOI] [PubMed] [Google Scholar]
- 72.Bouw RGB, Tonl IK, Letaief S, Ngameni E, Detellier C. Applied Clay Sci. 2011;52:258–265. [Google Scholar]
- 73.Abbaspour A, Mirahmadi E, Khalafi-Nejad A, Babamohammadi S. J Haz Mat. 2010;174:656–661. doi: 10.1016/j.jhazmat.2009.09.101. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Zhou C, Liu C, Li Y, Liu H, Zhu D. Analyst. 2012;137:1446–1450. doi: 10.1039/c2an16148f. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Guo X. Analyst. 2009;134:1348–1354. doi: 10.1039/b822744f. [DOI] [PubMed] [Google Scholar]
- 76.Breaker RR, Joyce GF. Chem Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 77.Liu J, Lu Y. J Am Chem Soc. 2003;125:642–6643. [Google Scholar]
- 78.Lan T, Furuya K, Lu Y. Chem Comm. 2010;46:3896–3898. doi: 10.1039/b926910j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wen Y, Peng C, Li D, Zhuo L, He S, Wang L, Huang Q, Xu QH, Fan C. Chem Comm. 2011;47:6278–6280. doi: 10.1039/c1cc11486g. [DOI] [PubMed] [Google Scholar]
- 80.Chang IH, Tulock JJ, Liu J, Kim WS, Cannon DM, Jr, Lu Y, Bohn PW, Sweedler JV, Cropek DM. Envir Sci Tech. 2005;39:3756–3761. doi: 10.1021/es040505f. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Ou LML, Suo Y, Yu HZ. Anal Chem. 2011;83:1557–1563. doi: 10.1021/ac103177w. [DOI] [PubMed] [Google Scholar]
- 82.Chen X, Guan H, He Z, Zhou X, Hu J. Anal Meth. 2012;4:1619–1622. [Google Scholar]
- 83.Berne BJ, Pecora R. Bio Phy Dover. 2000 [Google Scholar]
- 84.Pecora R. J Nanopart Res. 2000;2:123–131. [Google Scholar]
- 85.Miao X, Ling L, Shuai X. Anal Biochem. 2012;421:582–586. doi: 10.1016/j.ab.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 86.Miao X, Ling L, Shuai X. Chem Comm. 2011;47:4192–4194. doi: 10.1039/c0cc05344a. [DOI] [PubMed] [Google Scholar]
- 87.Chang MY, Pralle A, Isacoff EY, Chang CJ. J Am Chem Soc. 2008;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gabe Y, Urano Y, Kikuchi K, Kojima H, Nagano T. J Am Chem Soc. 2008;126:3357–3367. doi: 10.1021/ja037944j. [DOI] [PubMed] [Google Scholar]
- 89.Griffin BA, Adams SR, Tsien RY. Science. 1998;281:269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 90.Haugland RP. Molecular Probes, Inc.; Eugene, Oregon: 2002. pp. 55–767. [Google Scholar]
- 91.Lim MH, Xu D, Lippard SJ. Nat Chem Bio. 2006;2:375–380. doi: 10.1038/nchembio794. [DOI] [PubMed] [Google Scholar]
- 92.Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Nat Chem Bio. 2007;3:263–267. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- 93.Rosania GR, Lee JW, Ding L, Yoon HS, Chang YT. J Am Chem Soc. 2003;125:1130–1131. doi: 10.1021/ja027587x. [DOI] [PubMed] [Google Scholar]
- 94.Geddes CD, Lakowicz JR. Springer Verlag; 2005. [Google Scholar]
- 95.Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. Nat Chem Bio. 2007;3:423–431. doi: 10.1038/nchembio.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen CT, Huang WP. J Am Chem Soc. 2002;124:6246–6247. doi: 10.1021/ja025710e. [DOI] [PubMed] [Google Scholar]
- 97.He Q, Miller EW, Wong AP, Chang CJ. J Am Chem Soc. 2006;128:9316–9317. doi: 10.1021/ja063029x. [DOI] [PubMed] [Google Scholar]
- 98.Ranyuk E, Douaihy CM, Bessmertnykh A, Denat F, Averin A, Beletskaya I, Guilard R. Org Let. 2009;11:987–990. doi: 10.1021/ol802926m. [DOI] [PubMed] [Google Scholar]
- 99.Askunar N. Sensors Actuators B: Chem. 2011;157:162–168. [Google Scholar]
- 100.Marbella L, Serli-Mitasev B, Basu P. Angew Chem Int Ed. 2009;48:3996–3998. doi: 10.1002/anie.200806297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao L, Wu T, Lefevre JP, Leray I, Delaire JA. Lab on a Chip. 2009;9:2818. doi: 10.1039/b904641k. [DOI] [PubMed] [Google Scholar]
- 102.Faye D, Lefevre JP, Delaire JA, Leray I. J Photochem Photobio A: Chem. 2012;234:115–122. doi: 10.1039/c2pp25103e. [DOI] [PubMed] [Google Scholar]
- 103.Kim IB, Dunkjorst A, Gilbert J, Bunz UHF. Macromolecules. 2005;38:4560–4562. [Google Scholar]
- 104.Lee KM, Chen X, Fang W, Kim JM, Yoon J. Macromol Rapid Comm. 2011;32:497–500. doi: 10.1002/marc.201000671. [DOI] [PubMed] [Google Scholar]
- 105.Hou C, Xiong Y, Fu N, Jacquot CC, Squier TC, Cao H. Tetra Let. 2011;52:2692–2696. [Google Scholar]
- 106.Shamsipur M, Sadeghi M, Alizadeh K, Bencini A, Valtancoli B, Garau A, Lippolis V. Talanta. 2010;80:2023–2033. doi: 10.1016/j.talanta.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 107.Yanaz Z, Filik H, Apak R. Sensors Actuators B: Chem. 2010;147:15–22. [Google Scholar]
- 108.Bualom C, Ngeontae W, Nitiyanontakit S, Ngamukot P, Imyim A, Tuntulani T, Aeungmaitrepirom W. Talanta. 2010;82:660–667. doi: 10.1016/j.talanta.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 109.Son H, Lee HY, Lim JM, Kang D, Han WS, Lee SS, Jung JH. Chem-A Euro J. 2010;16:11549–11553. doi: 10.1002/chem.201001772. [DOI] [PubMed] [Google Scholar]
- 110.Wang JJ, Wang XJ, Geng Y, Tung CH, Wi LZ. Sci China Series B: Chem. 2009;52:765–770. [Google Scholar]