Abstract
During apoptosis, proteolytic cleavage of Bax at the amino terminus generates a truncated Bax of ∼18 kDa (p18Bax) and an amino-terminal peptide of ∼3 kDa (p3Bax). Whereas extensive studies have shown that p18Bax behaves like a BH3 protein with enhanced pro-apoptotic function over that of the full-length Bax (p21Bax), little is known about the function of p3Bax in apoptosis. We have previously shown that Bax and Ca2+ play a synergistic role in amplifying apoptosis signaling and that store-operated Ca2+ entry (SOCE) contributes to Bax-mediated apoptosis in prostate cancer cells. Here we test whether p3Bax can contribute to regulation of Ca2+ signaling during apoptosis through use of a membrane-penetrating peptide to facilitate delivery of recombinant p3Bax into NRP-154 cells, a prostate epithelial cell line with tumorigenic capacity. We find that human immunodefficiency virus transactivator of transcription protein (TAT)-p3Bax fusion peptide can enhance thapsigargin-induced apoptosis in NRP-154 cells, elevate SOCE activity, and increase inositol 1,4,5-trisphosphate-sensitive intracellular Ca2+ stores. Our data indicates that p3Bax can modulate the entry of extracellular Ca2+ and thus regulate the amplification of apoptosis in prostate cancer cells.
Keywords: human immunodefficiency virus transactivator of transcription protein-p3Bax, transforming growth factor-β, calcium signaling, therapy, thapsigargin
targeting the interconnected cellular pathways controlling apoptosis and regulation of Ca2+ homeostasis are two avenues for treatment of human cancers. We previously examined NRP-154 cells, an androgen-independent prostate cancer cell line (4), as a model for investigating the role of apoptosis in prostate cancer. Using this model, we demonstrated that there is a functional interaction between store-operated Ca2+ entry (SOCE) and Bax in apoptosis of NRP-154 cells (8). Exploring the synergy between SOCE and Bax could potentially provide insights into the therapeutic treatment of androgen-independent prostate tumors.
One interesting finding in our earlier study is that extensive proteolysis of Bax is associated with apoptosis of NRP-154 cells (9). This is consistent with findings from other investigators that showed during apoptosis, the full-length Bax (p21Bax) is cleaved into a truncated Bax of ∼18 kDa (p18Bax) plus a short peptide containing the amino terminal 28 to 33 amino acids (p3Bax). This process is thought to be mediated by calpain, a Ca2+-dependent protease (5, 20–22). It was demonstrated that p18Bax behaved like a BH3 protein (2) with potent proapoptotic function greater than that of native p21Bax protein in a number of tumor cell lines (3, 16). Whereas the larger proteolytic fragment is know to promote apoptosis, the role of remaining small peptide p3Bax in apoptosis signaling remains to be examined.
In this study, we tested the hypothesis that generation of p3Bax during apoptosis can amplify apoptosis signaling by perturbing intracellular Ca2+ homeostasis in NRP-154 cells. We found that recombinant p3Bax coupled with a membrane-penetrating peptide, specifically a fragment of the human immunodefficiency virus transactivator of transcription protein (TAT) (1, 11), could enter the cells and enhance cell death induced by Ca2+-mediated apoptosis. The proapoptotic effect of p3Bax appears to correlate with its effect on Ca2+ signaling, leading to elevation of cytosolic Ca2+ concentration and increased endoplasmic reticulum (ER) Ca2+ storage. This discovery will have important implications for targeting the functional interaction between Bax and Ca2+ in therapy of prostate cancer.
MATERIALS AND METHODS
Cell culture.
The NRP-154 cell line was originally derived from the dorsal-lateral preneoplastic of a Lobund-Wistar rat (4). NRP-154 cells were maintained in a humidified environment at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM)-F12 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 15 mM HEPES, in the presence of 100 U/ml penicillin and 100 μg/ml streptomycin. The various clones of NRP-Bax and NRP-Con cells are described elsewhere (8). Hela cells were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 15 mM HEPES, in the presence of 100 U/ml penicillin and 100 μg/ml streptomycin. When appropriate, this medium was supplemented with 100 μM quinoline-Val-Asp-CH2-O-Ph (Oph-109, MP Biomedicals, Solon, OH), 25 μM N-acetyl-Leu-Leu-Nle-CHO (ALLN, Biomol International, Plymouth Meeting, PA), 20 μM (2S, 3S)-trans-epoxysuccinyl-leucylamido-3-methylbutanse ethyl ester (E-64d, Sigma-Aldrich, St. Louis, MO), 1 μM staurosporine (Sigma-Aldrich), or 0.1 to 2 μM thapsigargin (TG, Sigma-Aldrich).
Protein purification.
Oligonucleotides encoding the 32 amino acids of mouse p3Bax (1MDGSGEQLGSGGPTSSEQIMKTGAFLLQGFIQ32) were synthesized as following: sense oligonucleotide, 5′-AATTCGATGGACGGGTCCGGGGAGCAGCTTGGGAGCGGCGGGCCCACCAGCTCTGAACAGATCATGAAGACAGGGGCCTTTTTGCTACAGGGTTTCATCCAGTGAA-3′; antisense oligonucleotide, 5′-AGCTTTCACTGGATGAAACCCTGTAGCAAAAAGGCCCCTGTCTTCATGATCTGTTCAGAGCTGGTGGGCCCGCCGCTCCCAAGCTGCTCCCCGGACCCGTCCATCG-3′. All of the oligonucleotides contain EcoRI and HindIII restriction enzyme sites. After annealing was completed, these oligonucleotides were cloned inframe into the pET-28b-TAT vector (V2.1) between the EcoRI and HindIII sites (11, 18). The pET-28b-TAT vector contains the TAT sequence YGRKKRRQRRR.
pTAT-p3Bax and pTAT plasmids were transformed into the Escherichia coli strain BL21, bacterial cultures were grown overnight, and protein expressions were induced by isopropyl 1-thio-β-d-galactopyranoside treatment for 5–6 h followed by sonication in a buffer solution containing 300 mM NaCl, 10 mM Tris·HCl, 20 mM imidazole, and 8 M urea, pH 8.0 (binding buffer) in the presence of protease inhibitor cocktail (Sigma-Aldrich). The His-tagged fusion proteins were purified using Ni2+-nitrilotriacetic acid-agarose affinity column (Invitrogen, Carlsbad, CA) through a sequential wash with buffer containing 300 mM NaCl, 10 mM Tris·HCl, 50 mM imidazole, and 8 M urea, pH 8, followed by elution with a buffer containing 300 mM NaCl, 10 mM Tris·HCl, 200 mM imidazole, and 8 M urea, pH 8.0. The elution step was followed by dialysis against phosphate-buffered saline using the Slide-A-Lyser dialysis cassette. The TAT-fusion proteins were then desalted on a PD-10 column (GE Healthcare, Piscataway, NJ) into phosphate-buffered saline (PBS) or DMEM, flash frozen, and stored at −80°C.
Western blot analysis.
NRP-154 cells (5 × 105) were treated with various concentrations of TAT or TAT-p3Bax for 30 min. Hela cells were transfected with pGFP or pGFP-Bax (12) using Genejammer transfection reagent (Stratagene, Cedar Creek, TX) per manufacturer's directions. Cell lysates were prepared with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) buffer (150 mM NaCl, 1% CHAPS, and 10 mM HEPES, pH 7.2) in the presence of protease inhibitor cocktail (Sigma-Aldrich). Equal amounts of protein (100 μg) were resolved on 4–12% bis-Tris gel and blotted with antibodies of interest. For Western blot analysis, these studies used two different primary antibodies for Bax, a monoclonal anti-Bax 6A7 (Zymed Laboratories, San Francisco, CA) that recognizes an epitope at the amino-terminal end of Bax and a polyclonal anti-Bax Δ21 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) that recognizes cleavage products of Bax (8). Green fluorescent protein (GFP) was detected using a rabbit polyclonal anti-GFP (Invitrogen). Cleaved Caspase-3 was detected using a rabbit monocolonal antibody (Asp175) specific to the active cleaved fragment (Cell Signaling Technology, Beverly, MA). Goat anti-mouse or anti-rabbit secondary antibody conjugated with horseradish peroxidase (Thermo Fisher Scientific) were used to detect bands with the ECL chemiluminescence kit (GE Healthcare, Piscataway, NJ).
Intracellular Ca2+ measurement.
NRP-154 cells were loaded with 5 μM fura-2 acetoxymethyl ester (AM, Invitrogen-Molecular Probes, Eugene, OR) for 45 min at 37°C in a balanced salt solution (BSS, in mM) containing 140 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 12 d-glucose, and 10 HEPES, pH 7.2. The cells were then collected from the culture dish by trypsin-EDTA digestion (Invitrogen), After wash out of fura-2-AM from the culture medium, cells were resuspended in BSS buffer and incubated with 5 μM TAT or TAT-p3Bax (dissolved in PBS) for 30 min at 37°C. For measurement of total intracellular Ca2+ content, the extracellular medium was replaced with BSS without Ca2+ 1 min before experimentation. A population of 1 × 106 cells was used for each assay, where the release of intracellular Ca2+ was measured after exposure of cells with ionomycin in a cuvette-based dual-wavelength spectrofluorometer (Photon Technology International, Monmouth Junction, NJ). Fura-2 fluorescence was recorded at excitation wavelengths of 340 nm (F340) and 380 nm (F380).
Mn2+ quenching as a measurement of SOCE.
For quantitative measurement of SOCE, the quenching of fura-2 fluorescence by Mn2+ ions was used (8). Briefly, a population of 1 × 106 cells suspended in the cuvette system was treated with ATP to produce Ca2+ depletion from the ER. During this time fura-2 fluorescence at excitation wavelengths of 360 nm (F360) and 380 nm (F380) were recorded to monitor both the resting Ca2+ (before ATP addition) and the total ER Ca2+ store (after ATP addition). After ATP treatment, 0.5 mM MnCl2 was added to the extracellular solution, and the rate of fura-2 fluorescence quenching by Mn2+ ions was monitored at F360. At the end of the experiment, 0.1% Triton-X100 was added to allow for complete quenching of fura-2 fluorescence by Mn2+ (Fmin). For quantification purpose, F360 was normalized by using the formula [F360-Fmin]/[Fmax-Fmin], where Fmax is the initial F360 fluorescence intensity before the addition of Mn2+. The slope of Mn2+ quenching was calculated for the first 20 s after the addition of Mn2+.
Cell survival assay.
To test the effect of TAT-p3Bax on apoptosis of NRP-154 cells, 5 μM of TAT or TAT-p3Bax (dissolved in DMEM) together with 0.1 μM TG were applied to NRP-Bax cells at 25–30% confluence grown on six-well culture plates. At various times after these treatments, the cells were washed with PBS twice to remove any unattached cells. Propidium iodine (PI, 1 μg/ml, Sigma-Aldrich) was then added to the culture medium to allow visualization of the number of cells in active apoptosis. Representative population of cells in the center of the culture dish was recorded under a digital microscope. Cell viability was defined by the percentage of attaching cells minus those that are positive for PI staining.
Statistical analysis.
Values are presented as means ± SE. Significance of difference was determined by Student's t-test using a P < 0.05 or 0.01 as criterion for statistical significance. In the figures, asterisks indicates P < 0.05, whereas double asterisks indicate P < 0.01.
RESULTS
TAT-mediated delivery of recombinant p3Bax protein into NRP-154 cells.
NRP-154 is a tumorigenic epithelial cell line derived from the preneoplastic dorsal-lateral prostate of rats. These cells lack androgen-receptor expression and are exquisitely sensitive to transforming growth factor (TGF)-β- and TG-induced apoptosis. Thus NRP-154 cells are an excellent model for investigating the mechanism of apoptosis in androgen-independent prostate cancer. We previously showed that stable overexpression of Bax can sensitize NRP-154 cells to Ca2+-induced apoptosis, suggesting a functional interplay between Bax and Ca2+ in apoptosis signaling (8).
In NRP-154 cells stably overexpressing exogenous Bax (NRP-Bax), prominent proteolysis of Bax can be clearly observed, in particular when triggered to undergo apoptosis. With the use of a polyclonal antibody, more Bax cleavage products can be observed in NRP-Bax cells compared with NRP-154 cells transfected with mock plasmid (NRP-Con) (Fig. 1A). Similar Bax cleavage products have been previously identified in other cell lines (5, 20–22). We also observe cleavage of GFP-Bax to produce a small NH2-terminal fragment of Bax (GFP-p3Bax) in transfected Hela cells (Fig. 1B). The quantity of GFP-p3Bax greatly produced increases in cells induced to undergo apoptosis due to exposure to TG. These cleavage events have been linked to the action of the Ca2+-dependent protease calpain, which cleaves full-length Bax to produce various degradation products (2, 5, 16, 21, 22). Cleavage at one calpain site located at amino acids 30–33 Phe-Ile-Gln-Asp (FIQD) of Bax (22) leads to generation of a 3-kDa peptide containing the first 32 amino acids of Bax (p3Bax, Fig. 1C). A specific role for calpain in the generation of p3Bax was confirmed as generation of the GFP-p3Bax fragment in Hela cells can be prevented by the addition of the calpain inhibitors (ALLN and E64D, Fig. 1B).
Fig. 1.
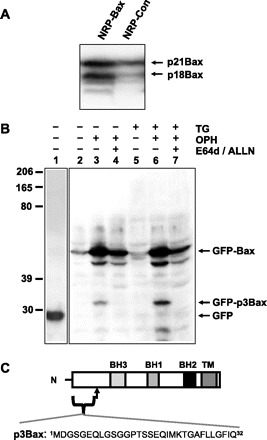
NRP-154 cells display cleavage of Bax during apoptosis. A: Western blot with anti-Bax (Δ21 antibody) reveals proteolysis of Bax in NRP-154 cells undergoing transforming growth factor (TGF)-β-induced apoptosis. Cells were treated with 10 ng/ml TGF-β for 48 h. B: Hela cells were transfected with either green fluorescent protein (GFP, lane 1) or GFP-Bax (lanes 2–7). Three hours after transfection, media were changed to either growth media without thapsigargin (TG, lanes 2–4) or with 2 μM TG (lanes 5–7). Transfected cells were treated with additional caspase inhibitor Oph-109 (lanes 3, 4, 6, and 7) to allow for cell survival until the end of the assay. Calpain inhibitors (ALLN and E-64d) were applied to lanes 4 and 7. Total cell lysates were collected 18 h after treatment and probed with anti-GFP (lane 1) or anti-Bax 6A7 (lanes 2–7). C: schematic diagram illustrates that a 32 amino-acid peptide (p3Bax) is generated from Bax (p21) via cleavage by calpain.
To test the function of p3Bax in apoptosis of prostate cancer cells, we first attempted to express the p3Bax peptide encoding amino acids 1–32 of Bax by transfection of p3Bax cDNA into cultured NRP-154 cells. Multiple trials with plasmid-DNA-based gene transfection did not produce detectable protein expression, possibly reflecting degradation of such small peptides by cellular quality control mechanisms. As an alternative approach to test the in vivo function of p3Bax, we produced recombinant TAT-p3Bax fusion proteins (1, 11). Published studies showed that a TAT peptide can facilitate penetration of various proteins across the cell membrane (11, 18). We generated the TAT-p3Bax constructs and used TAT for control purpose (Fig. 2A). Ample recombinant fusion protein of appropriate sizes can be produced in E. coil (Fig. 2B).
Fig. 2.
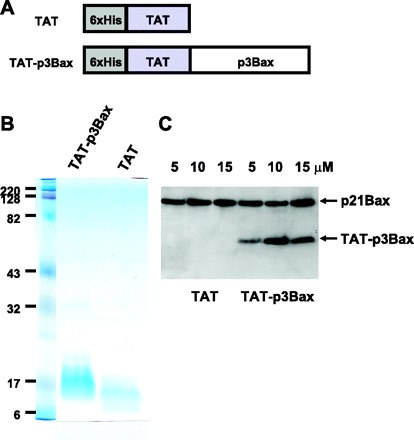
Purification and delivery of TAT-p3Bax into NRP-154 cells. A: TAT was fused to the amino-terminal end of p3Bax to facilitate the delivery of fusion proteins into cells. As a control, TAT vector was used. B: after isopropyl 1-thio-β-d-galactopyranoside (IPTG) induction of Escherichia coli, large amounts of TAT-p3Bax and TAT proteins could be expressed and purified using the Ni-affinity column. Purified proteins were separated on a 4–15% SDS-PAGE and stained with Coomassie blue. C: penetration of TAT-p3Bax into NRP-154 cells was analyzed by immunoblot using anti-Bax 6A7 monoclonal antibody. A 30-min incubation of cells with 5, 10, or 15 μM TAT-p3Bax led to detectable amount of TAT-p3Bax in the cell lysate. Endogenous p21Bax was used as loading control (top band).
Efficient entry of TAT-p3Bax can be observed in NRP-154 cells following brief incubation with the protein in the culture media (Fig. 2C). Western blot analysis using monoclonal antibody that recognizes the amino-terminus of Bax detected the endogenous full-length p21Bax as well as the presence of recombinant TAT-p3Bax. Note that this antibody does not detect any endogenous p3Bax cleavage product in cells treated with either TAT or TAT-p3Bax recombinant proteins. This suggests that either endogenous p3Bax levels are too low to detect in these unstimulated cells or that small Bax cleavage products may be degraded by the cellular control mechanisms when present in low concentration before the induction of apoptosis increases the concentration of p3Bax.
TAT-p3Bax enhances TG-induced apoptosis in NRP-154 cells.
Incubation of NRP-Bax cells with TAT-p3Bax alone does not appear to affect cell viability as the cells continue to proliferate (Fig. 3A). Interestingly, NRP-Bax cells pretreated with TG (0.1 μM), which produces Ca2+-dependent apoptosis (8), displayed increased apoptosis in the presence of TAT-p3Bax (Fig. 3B). Cells positive for PI staining were considered to be apoptotic; thus the percentage of living cells represent the number of cells that remain attached to the culture dish and are negative for PI staining. The percentage of living cells was normalized to the reading before treatment was begun (at 0 h). Preincubation of NRP-Bax cells with TAT-p3Bax alone did not cause cleavage of caspase-3, an important step in the induction of apoptosis. Significant cleavage of caspase-3 was observed in the presence of TG and TAT-p3Bax (Fig. 3C). Since TAT-p3Bax only increases cell death when applied with TG, a compound known to disrupt intracellular Ca2+ homeostasis, we hypothesize that p3Bax and Ca2+ must act synergistically to induce apoptosis. To further test this possibility, we must address how p3Bax may affect Ca2+ homeostasis in cancer cells.
Fig. 3.
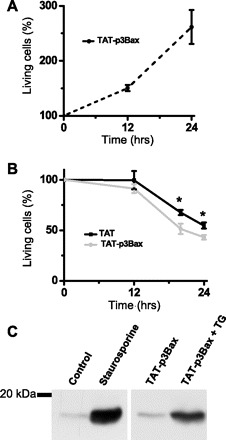
TAP-p3Bax enhances TG-induced apoptosis in NRP-Bax cells. A: NRP-Bax cells were incubated with 5 μM TAT-p3Bax in the culture medium. Time-dependent changes in viability of NRP-Bax cells (n = 5) were plotted and represented by a dotted line. B: NRP-Bax cells treated with 5 μM TAT or TAT-p3Bax together with 0.1 μM TG ware plotted. Data from 4 independent experiments of both cell lines were averaged and plotted as means ± SE; *P < 0.05. C: NRP-Bax cells were incubated with 1 μM staurosporine, 5 μM TAT-p3Bax, and/or 0.1 μM TG and then used for Western blot analysis for the active cleavage product of Caspase-3. Molecular weight markers are provided on the left.
TG acts on the sarco-endoplasmic reticulum Ca2+-ATPase pump to deplete the intracellular Ca2+ store, leading to activation of SOCE. Previous studies link apoptosis of prostate cancer cells to SOCE (8, 17), and our published data showed that SOCE activity in NRP-154 cells was partially activated by 0.1 μM TG (8). The enhancement of TG-induced apoptosis in NRP-Bax cells by TAT-p3Bax could be the result of perturbation of intracellular Ca2+ homeostasis by several possible mechanisms. First, increased cell death could result from TAT-p3Bax facilitating the ER Ca2+ release process and increasing the synergy between Bax and Ca2+ in the progression of apoptosis (12). Alternatively, TAT-p3Bax could alter Ca2+ entry across the plasma membrane and increase Ca2+-dependent apoptosis. For the later possibility, one would predict to observe changes in resting Ca2+ concentration as well as total Ca2+ storage in the ER. We tested these possibilities by measuring intracellular Ca2+ levels and SOCE following application of TAT-p3Bax.
TAT-p3Bax increases intracellular Ca2+ content in NRP-154 cells.
To examine whether p3Bax may be a modulator of intracellular Ca2+ homeostasis, we first measured the total intracellular Ca2+ store by adding 2 μM ionomycin, a Ca2+ ionophore, to an extracellular solution containing 0 Ca2+ (10). A population of 1 × 106 NRP154 cells was loaded with 5 μM fura-2 AM in the presence of 2 mM Ca2+ BSS, then incubated with 5 μM TAT (for control) or TAT-p3Bax in 2 mM Ca2+ BSS for 30 min. Upon changing the extracellular solution to 0 Ca2+, the addition of 2 μM ionomycin led to a transient elevation of intracellular Ca2+, as reflected by an increase in the ratio of F340/F380 (Fig. 4A). Our data showed that cells treated with TAT-p3Bax displayed significantly elevated levels of cytosolic Ca2+ at resting state when compared with those treated with the control TAT peptide (Fig. 4B), whereas the peak of ionomycin-induced Ca2+ release from intracellular stores was only marginally increased in TAT-p3Bax-treated cells compared with those treated with TAT (Fig. 4C).
Fig. 4.

TAT-p3Bax increases resting cytosolic [Ca2+] and inositol 1,4,5-trisphosphate (IP3)-releasable Ca2+ from ER in NRP-Bax cells. A: population of fura-2 AM-loaded NRP-154 cells were pretreated with 5 μM TAT or TAT-p3Bax for 30 min, and the releases of intracellular Ca2+ in NRP-154 cells were triggered by 2 μM ionomycin in an extracellular solution containing 0 Ca2+. Changes in fura-2 fluorescence represented by F340/F380 were used as an indicator of changes in cytosolic Ca2+. B: basal value of F340/F380, measured before the addition of ionomycin, displayed significant differences between TAT- (n = 6) and TAT-p3Bax-treated cells (n = 5); **P < 0.01. This indicates an elevated resting cytosolic Ca2+ concentration ([Ca2+]cyt) in TAT-p3Bax-treated NRP-Bax cells. C: data from multiple experiments with ionomycin-induced changes in total intracellular Ca2+ storage, ΔF340/F380, were averaged (n = 6 for TAT, n = 4 for TAT-p3Bax). The difference was not significantly different (P = 0.23). D: 0.2 mM ATP was applied to an extracellular solution containing 0 Ca2+, which lead to activation of IP3-receptor-mediated Ca2+ release from the ER store. Pretreatment of NRP-154 cells with 5 μM TAT-p3Bax (30 min) significantly increased the amount of ATP-induced Ca2+ release when compared with cells treated with the TAT control peptide. These data were produced during experiments to measure SOCE detailed in Fig. 5. The ratio of F360/F380 was used for measurement of cytosolic Ca2+ as described in Li et al. (8). E: statistical result for ATP-induced release of Ca2+ from ER in NRP-154 cells with TAT (n = 8) and TAT-p3Bax (n = 5) treatment; *P < 0.05.
We next tested the effect of TAT-p3Bax on inositol 1,4,5-trisphosphate (IP3)-receptor-mediated Ca2+ release by using 0.2 mM ATP to activate the purinergic pathway. We found that pretreatment of NRP-154 cells with 5 μM TAT-p3Bax (30 min) significantly increased the amount of Ca2+ released from the ER by ATP compared with NRP-154 cells that were treated with TAT (Fig. 4, D and E). In addition, the resting cytosolic Ca2+ was elevated in TAT-p3Bax-treated cells, consistent with the data shown in Fig. 4B. These results show that TAT-p3Bax pretreatment leads to enhanced IP3-mediated Ca2+ release from the ER. The increase of resting intracellular Ca2+ concentration ([Ca2+]i) in NRP-154 cells with TAT-p3Bax treatment may reflect the perturbation of Ca2+ movement across the plasma membrane.
TAT-p3Bax enhances SOCE in NRP-154 cells.
Because one important mechanism for the extracellular Ca2+ entry is through SOCE, we directly evaluated the effect of TAT-p3Bax on SOCE in NRP-154 cells by applying 0.2 mM ATP in an extracellular solution without Ca2+ to deplete the ER Ca2+ store through activation of IP3 receptor. After ATP-induced ER Ca2+ depletion (200 s treatment), 0.5 mM MnCl2 was added into the cuvette containing 1 × 106 NRP-154 cells loaded with fura-2 (Fig. 5A). The rate of fura-2 quenching by Mn2+ is significantly enhanced in NRP-154 cells pretreated with TAT-p3Bax compared with cells treated with the TAT peptide (Fig. 5B). This result indicates that TAT-p3Bax can increase SOCE activity in NRP-154 cells. Enhanced SOCE function provides a potential mechanism that would result in elevation of resting cytosolic Ca2+ concentration ([Ca2+]cyt) and enhanced Ca2+-dependent apoptosis following treatment with TAT-p3Bax.
Fig. 5.
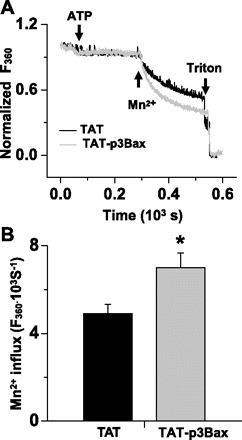
Increased store-operated Ca2+ entry (SOCE) activity in NRP-154 cells with TAT-p3Bax treatment. A: NRP-154 cells were loaded with 5 μM fura-2 AM, and their ER Ca2+ storages were depleted with the addition of 0.2 mM ATP (10 min). Addition of 0.5 mM Mn2+ lead to quenching of fura-2 fluorescence measured at excitation wavelength of 360 nm. Significantly faster rate of Mn2+ quenching of fura-2 was observed in TAT-p3Bax-treated cells. B: plot shows the statistical result for the effect of TAT (n = 7) and TAT-p3Bax (n = 5) on the rate of Mn2+ quenching of fura-2 fluorescence. *P < 0.05.
DISCUSSION
Posttranslation modification of Bax is an important cellular signaling process in the regulation of apoptosis, in which proteolysis of Bax has been shown to contribute to amplification of apoptosis (2, 16, 20–22). Ca2+-dependent cleavage of Bax has been reported to occur at different sites by various groups. For example, Wood et al. (22) showed that calpain recognizes a motif at amino acids 30–33 Phe-Ile-Gln-Asp (FIQD) of Bax. Hiroko et al. (16) reported that calpain cleaved Bax at glutamine-28 after Leu-Leu-Leu-Gln. It is possible that both of these sites could be active in vivo, as we observe multiple Bax cleavage products in NRP-154 cells undergoing apoptosis (9). However, our studies here indicate that one cleavage product, p3Bax, is preferentially produced in cells treated with TG in a calpain-dependent manner. Studies of amino-terminal truncation products of Bax, including Bax (Δ1–19), Bax (Δ1–38) (2, 21), Bax (Δ1–28) (16), and Bax (Δ1–32) (5), all indicate such truncations produce a more toxic effect than full-length (p21) Bax. However, the function of the amino-terminal peptide produced by these cleavage events has not been explored to this point.
In this study, we investigated the effect of p3Bax peptide on apoptosis and Ca2+ homeostasis in NRP-154 cells. Technical challenges in using DNA transfection to introduce small peptides into living cells (13, 14) required the use of TAT-mediated protein transduction to introduce the recombinant TAT-p3Bax proteins into NRP-154 cells. Our data showed that TAT-p3Bax could not induce significant apoptosis when applied to NRP-154 cells at resting conditions. Instead, we found that TAT-p3Bax could amplify TG-induced apoptosis in NRP-Bax cells by perturbing intracellular Ca2+ homeostasis. These findings suggest that p3Bax and intracellular Ca2+ can act synergistically to modulate cells death in prostate cancer cells.
Our studies also indicate the mechanistic basis for altered Ca2+ homeostasis in cells treated with p3Bax. We found that TAT-p3Bax elevated resting cytosolic Ca2+ concentration and increased the IP3-mediated Ca2+ from the ER in NRP-154 cells. In addition, SOCE activity was significantly enhanced by the presence of TAT-p3Bax, which could contribute to the observed elevation of intracellular cytosolic Ca2+ levels in NRP-Bax cells. Increased IP3-mediated Ca2+ release from the ER observed following TAT-p3Bax treatment is consistent with previous work by Foskett and colleagues (7, 19) who demonstrate that Bax can interact with the IP3 receptor to enhance its Ca2+ release channel function.
Recent advances have identified several components of the molecular machinery that facilitate SOCE. STIM1 acts as a Ca2+ sensor in the ER (6, 15), whereas Orai1 channels provide for extracellular Ca2+ entry in response to ER Ca2+ depletion (23). Our data suggests that p3Bax may directly influence this SOCE machinery, perhaps by altering the coupling between STIM1 and Orai1. Future studies are required to identify if direct interactions take place between p3Bax and SOCE machinery and the molecular basis of such putative interactions.
Another unique observation of this study is that TAT-p3Bax is not toxic to NRP-Bax cells under resting conditions; it only enhances the process of apoptosis initiated by exposure to TG. This is particularly important when considering the exogenous p3Bax peptide as a therapeutic agent for prostate cancer. In such a case, p3Bax would not produce cytotoxic effects in cells with normal Ca2+ homeostasis; it could be used in combination with other cytotoxic agents to amplify apoptosis in targeted cancer cells.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods 24: 247–256, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Cartron PF, Oliver L, Juin P, Meflah K, Vallette FM. The p18 truncated form of Bax behaves like a Bcl-2 homology domain 3-only protein. J Biol Chem 279: 11503–11512, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Choi WS, Lee EH, Chung CW, Jung YK, Jin BK, Kim SU, Oh TH, Saido TC, Oh YJ. Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J Neurochem 77: 1531–1541, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Danielpour D, Kadomatsu K, Anzano MA, Smith JM, Sporn MB. Development and characterization of nontumorigenic and tumorigenic epithelial cell lines from rat dorsal-lateral prostate. Cancer Res 54: 3413–3421, 1994 [PubMed] [Google Scholar]
- 5.Gao G, Dou QP. N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem 80: 53–72, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42: 173–182, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Jones RG, Bui T, White C, Madesh M, Krawczyk CM, Lindsten T, Hawkins BJ, Kubek S, Frauwirth KA, Wang YL, Conway SJ, Roderick HL, Bootman MD, Shen H, Foskett JK, Thompson CB. The proapoptotic factors Bax and Bak regulate T Cell proliferation through control of endoplasmic reticulum Ca(2+) homeostasis. Immunity 27: 268–280, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Zheng L, Lin P, Danielpour D, Pan Z, Ma J. Overexpression of Bax induces down-regulation of store-operated calcium entry in prostate cancer cells. J Cell Physiol 216: 172–179, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Lin PH, Pan Z, Zheng L, Li N, Danielpour D, Ma JJ. Overexpression of Bax sensitizes prostate cancer cells to TGF-beta induced apoptosis. Cell Res 15: 160–166, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem 253: 5892–5894, 1978 [PubMed] [Google Scholar]
- 11.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 4: 1449–1452, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Pan Z, Bhat MB, Nieminen AL, Ma J. Synergistic movements of Ca(2+) and Bax in cells undergoing apoptosis. J Biol Chem 276: 32257–32263, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285: 1569–1572, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol 10: 290–295, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW Jr. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett 189: 221–230, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Vanden Abeele F, Shuba Y, Roudbaraki M, Lemonnier L, Vanoverberghe K, Mariot P, Skryma R, Prevarskaya N. Store-operated Ca2+ channels in prostate cancer epithelial cells: function, regulation, and role in carcinogenesis. Cell Calcium 33: 357–373, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Vocero-Akbani AM, Heyden NV, Lissy NA, Ratner L, Dowdy SF. Killing HIV-infected cells by transduction with an HIV protease-activated caspase-3 protein. Nat Med 5: 29–33, 1999 [DOI] [PubMed] [Google Scholar]
- 19.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol 7: 1021–1028, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood DE, Newcomb EW. Caspase-dependent activation of calpain during drug-induced apoptosis. J Biol Chem 274: 8309–8315, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Exp Cell Res 256: 375–382, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17: 1069–1078, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA 103: 9357–9362, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]


