Abstract
Forty years ago, Judah Folkman (Folkman. N Engl J Med 285: 1182–1186, 1971) proposed that tumor growth might be controlled by limiting formation of new blood vessels (angiogenesis) needed to supply a growing tumor with oxygen and nutrients. To this end, numerous “antiangiogenic” agents have been developed and tested for therapeutic efficacy in cancer patients, including prostate cancer (CaP) patients, with limited success. Despite the lack of clinical efficacy of lead anti-angiogenic therapeutics in CaP patients, recent published evidence continues to support the idea that prostate tumor vasculature provides a reasonable target for development of new therapeutics. Particularly relevant to antiangiogenic therapies targeted to the prostate is the observation that specific hormones can affect the survival and vascular function of prostate endothelial cells within normal and malignant prostate tissues. Here, we review the evidence demonstrating that both androgen(s) and vitamin D significantly impact the growth and survival of endothelial cells residing within prostate cancer and that systemic changes in circulating androgen or vitamin D drastically affect blood flow and vascularity of prostate tissue. Furthermore, recent evidence will be discussed about the expression of the receptors for both androgen and vitamin D in prostate endothelial cells that argues for direct effects of these hormone-activated receptors on the biology of endothelial cells. Based on this literature, we propose that prostate tumor vasculature represents an unexplored target for modulation of tumor growth. A better understanding of androgen and vitamin D effects on prostate endothelial cells will support development of more effective angiogenesis-targeting therapeutics for CaP patients.
Keywords: androgens, androgen receptor, vitamin D, endothelium, prostate cancer
Lack of Success in Targeting Angiogenesis in Prostate Cancer
prostate cancer is a common malignancy in humans, representing the second leading cause of cancer-related deaths in men (87). The pervasiveness of prostate cancer in the male population has stimulated extensive efforts to develop better therapeutics to treat this disease, especially when the disease has progressed to an advanced stage and can no longer be controlled by surgery or radiation. Increasing evidence has demonstrated recently that the tumor microenvironment has a role equally important to cancer cells in the progression of the tumor (35, 49). One of the key components in the tumor microenvironment thought to have a critical role in tumor progression is the vasculature. In 1971, Judah Folkman proposed a new approach for elimination of tumors by targeting the blood vessels that supply oxygen and nutrients to the tumor (24). He hypothesized that tumor growth is facilitated by constant expansion of the vascular network (a process referred to as angiogenesis) to support the expanding tumor mass and that “antiangiogenic” therapeutics might be used singularly, or in conjunction with other therapeutics, to control tumor growth. Antiangiogenic therapies promised a rational approach, and multiple druggable targets were identified in experimental model systems. Based upon preclinical studies in vivo, pharmaceutical companies developed several novel antiangiogenic agents that extended the survival of patients, but only marginally. For example, bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF), was approved by the FDA as a first-line therapy for colorectal cancer, non-small cell lung cancer, and metastatic renal cell carcinoma and as a second-line therapy for colorectal cancer and glioblastoma multiforme (19, 41). Moreover, small-molecule tyrosine kinase inhibitors such as sunitinib, sorafenib, and pazopanib, which target VEGF receptor, platelet-derived growth factor (PDGF) receptor, and other kinases (KIT, Ret, BRAF, and Flt-3), were approved as monotherapies for the treatment of metastatic renal cell carcinoma (19). However, despite some clinical successes, antiangiogenic agents do not appear to be the “magic bullet” for treatment of solid tumors that was anticipated. In a phase III trial, treatment with bevacizumab in combination with either docetaxel plus prednisone or prednisone alone in patients with advanced prostate cancer did not improve the overall survival significantly (19). Despite such disappointing results, research continues to indicate that prostate vasculature has an important role in regulating the size and function of prostate malignancies (3, 11, 36, 37, 45, 89, 94). This review investigates the potential biological role of two hormone receptors (androgen and vitamin D receptors) in modulating angiogenesis in prostate cancer. A better understanding of the direct role of these receptors in human prostate endothelial cells may further justify their potential as new targets for antiangiogenic therapies.
Androgens are a class of steroid hormones that primarily determine prostate development and prostate tumor growth. Therapies designed to lower circulating and tissue levels of androgen in prostate cancer patients remain the most effective therapy for advanced disease (65). Cellular responses to androgens are mediated mainly by the androgen receptor (AR) protein, which is expressed highly in both normal prostate luminal epithelial cells and in prostate cancer cells. In fact, expression of endogenous AR is important in regulation of the proliferative and differentiation state in prostate cancer cells (38, 76). Even in the absence of circulating testicular androgens, the maintenance of AR expression/function is critical for proliferation of castration-resistant prostate cancer cells (104). In addition, AR activity is involved directly in regulation of the propensity of prostate cancer cells to undergo apoptosis in vitro in response to noxious stimuli; growth of prostate cancer cells in androgen-free medium renders the cells more sensitive to apoptotic death in response to radiation or chemotherapy (60). Consequently, the prostate cancer research community has focused on developing therapeutic modalities to control aberrant AR activity in prostate cancer cells, particularly castration-recurrent prostate cancer cells, to both reduce proliferation and block the inhibition of apoptosis in response to therapeutic modalities. However, the epithelial cell-specific focus neglects compelling evidence that endothelial cells within the prostate tumor microenvironment also express AR (30). AR expression in prostate endothelial cells may directly regulate their differentiation state and viability and, therefore, indirectly regulate the viability and differentiation of the epithelial compartment of benign and malignant prostate tissue. Indeed, Cunha et al. (16) showed many years ago that AR expression in the mesenchymal compartment, not the epithelial compartment, during prostate organogenesis was indispensable for the development and growth of normal prostate epithelium.
Likewise, vitamin D receptor (VDR) is expressed in both prostate epithelial and prostate cancer cells, and there is extensive evidence that vitamin D-induced signaling mediated through binding to VDR can significantly slow the growth of prostate cancer cells in vitro and in vivo (in animal models) (21, 91, 105). Not surprisingly, VDR is also expressed in nonepithelial cells of prostate cancers as well as in the benign stroma around prostate tumors (53). However, the relevance of VDR within the nonepithelial cells of prostate cancer or benign stromal cells to tumor cell growth or differentiation, respectively, is not clear.
Androgen Action on Endothelium of the Prostate and Prostate Tumor
More than 10 years ago, Ralph Buttyan and Anders Bergh independently demonstrated that the first physiological effect of androgen deprivation in the rat prostate gland was a drastic reduction in blood flow (55, 82, 83). Perturbation of the prostatic blood flow was evident as early as 18 h after castration and coincided with the appearance of apoptotic endothelial cells in the rat prostate (83, 85). The reduction of blood flow was associated with the induction of an hypoxic environment in the prostate, with a 20-fold increase in hypoxia-inducible factor-1α (HIF-1α) protein observed at 48 h postcastration (84). Also, the reduction in blood flow was associated with major reductions in epithelial “weight,” stromal weight, blood vessel luminal weight, and number of endothelial cells 1 wk after castration (26). Both groups hypothesized that a large proportion of prostate epithelial cell loss was in fact an indirect effect caused by hypoxic/ischemic conditions that resulted from castration-induced endothelial cell death. Rat prostate endothelial cells were reported to lack expression of AR (77); therefore, both groups concluded that an androgen-regulated intermediary paracrine molecule synthesized by AR-expressing prostate epithelial or stromal cells regulated endothelial cell survival (23).
In humans, AR expression in endothelial cells has been observed in several benign tissues, including skin (8, 57), salivary gland (54), bone (1), bone marrow (61), corpora cavernosa (81), and skeletal muscle (90). Our group and others (20, 30) reported expression of functional AR in endothelial cells from benign prostate and prostate cancer (CaP), suggesting a potential role for AR in regulation of human prostate vascular endothelial cell homoeostasis. Supporting this hypothesis, withdrawal of androgenic signaling by AR antagonists (e.g., flutamide and bicalutamide) and inhibitors of steroid metabolism (e.g., finasteride and dutasteride) reduced hematuria during prostate surgery and in patients with benign prostatic hyperplasia (BPH) (18, 31, 59, 73, 74, 95). More recently, two clinical studies (4, 52) showed that combination therapy with bicalutamide-goserelin (a gonadotropin-releasing hormone agonist) and dutasteride (inhibitor of 5α-reductase isoenzyme types 1 and 2) induced profound vascular collapse in human CaP patients and reduced prostatic tissue vascularity. Our group demonstrated that acute prostate vascular involution was induced by androgen withdrawal in primary xenografts of benign or malignant human prostate tissue transplanted into severe combined immunodeficiency mice in which the vasculature was of human origin (29). In this preclinical model, vascular involution was correlated temporally with the induction of apoptosis in prostate endothelial cells, indicating that testicular androgen signaling (testosterone → dihydrotestosterone → AR activation) had an important role in the maintenance of prostate endothelial cell homeostasis in intact men. This observation also suggested that androgen ablation can negatively affect endothelial cell viability in human prostate tissue independent of epithelial cell death (29).
Our studies strongly suggested that direct regulation of endothelial cell survival and proliferation may be a new biological role of AR in humans (30). Although possibly not a phenomenon general in all human organs, this concept has been validated in other human endothelial cell models (92, 93). A recent study in primary cultures of human aortic endothelial cells showed that AR activation led to moderate (a less than 2-fold increase) upregulation of VEGF-A, cyclin A, and cyclin D1 mRNA expression measured by real-time PCR; the latter two factors are involved in cell cycle progression (9). Nevertheless, the molecular targets of AR signaling in human endothelial cells are largely unknown (Fig. 2), and further elucidation of the mechanism of androgen action in human prostate endothelial cells will provide a better understanding of the effects of androgen ablation therapy on the tumor microenvironment.
Fig. 2.
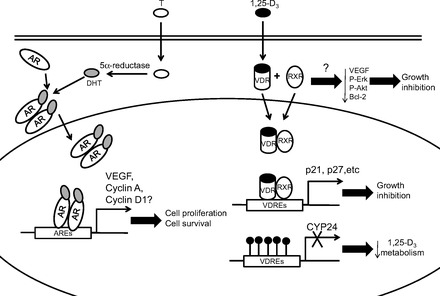
Effects of androgen receptor (AR) and vitamin D receptor (VDR) signaling activation in prostatic tumor endothelium. When T enters the cell, it is converted to DHT before binding to the AR. Homodimers of ligand-bound AR translocate into the nucleus and bind to androgen-responsive elements (AREs), inducing transcription of AR target genes that modulate endothelial cell proliferation and survival. Similarly, when 1,25-dihydroxycholecalciferol (1,25-D3) enters a cell, it binds to the VDR in the cytoplasm. Activated VDR heterodimerizes with retinoid X receptor (RXR) before the complex translocates into the nucleus. In tumor endothelium, the ligand-bound VDR-RXR-heterodimer binds to DNA harboring vitamin D response elements (VDREs) and induces expression of genes that inhibit cell proliferation. Due to DNA methylation, this heterodimer cannot bind to the promoter of CYP24, leading to reduced CYP24 expression and reduced calcitriol metabolism in the cells.
Potential Role of Adrenal Androgens in Modulation of Prostate Endothelial Cell Homeostasis
Circulating androgens are formed primarily in the testis (testosterone) and in the adrenal gland [dehydroepiandrosterone (DHEA) and androstenedione] (44) (Fig. 1). The biological role of adrenal androgens (DHEA and DHEA derivatives) has not been studied in prostate endothelial cells. However, an increasing body of evidence in other endothelial cell models suggests that DHEA (and metabolites) may directly activate intracellular signaling pathways to mediate biological actions in human endothelial cells. (25, 58, 88). Binding of DHEA to cell surface receptors stimulated phosphorylation of Akt and sustained activity of endothelial nitric oxide synthase, which resulted in increased production of the vasodilator nitric oxide (25). Akt is known to phosphorylate the forkhead transcription factor FoxO1, leading to its nuclear exclusion (51, 99). Phosphorylated FoxO1 regulates cellular proliferation, differentiation, apoptosis, and glucose homeostasis (2, 32, 70) and has an important role in modulation of vascular tone and proliferation (10). Moreover, mice that lack FoxO1 expression die in utero due to improper vascular development (27).
Fig. 1.
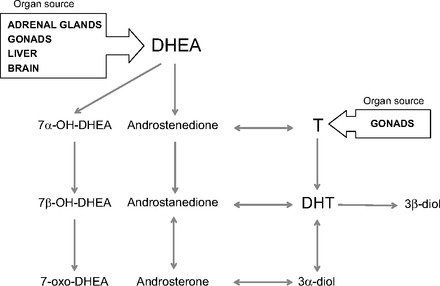
Metabolism of circulating androgens in peripheral human cells. Conversion of testosterone to dihydrotestosterone (DHT) is the preferred androgen metabolism pathway before androgen deprivation therapry (ADT) and in androgen-stimulated prostate cancer. After ADT, metabolism of the adrenal androgen dehydroepiandrosterone (DHEA) is the major source of DHT and 5α-reduced androgen metabolites. Androgens [testosterone (T), DHEA, and their metabolites] might exert nuclear receptor-mediated genomic effects (DHT, androsterone) and plasma membrane receptor-mediated nongenomic effects [DHEA, T, and 3β-androstenediol (3β-diol)] on the biology of endothelial cells.
Whereas DHEA has profound direct effects on endothelial cell function mediated through direct binding to membrane receptors, as well as indirect effects mediated through conversion to more active androgenic or estrogenic metabolites, DHEA metabolites that are not on the pathway to active androgens also have significant effects on endothelial cells (Fig. 1). For example, 3β-androstenediol can inhibit inflammatory responses induced by tumor necrosis factor-α and lipopolysaccharide in human endothelial cells (71). Androsterone was reported to be a ligand for farnesoid X receptor, a nuclear receptor that modulates lung endothelial cell homeostasis (7, 40, 100). Notably, oxidized metabolites of DHEA activated ERβ to almost the same extent as 17β-estradiol (101). Unpublished data from our laboratory demonstrated that human endothelial cells freshly isolated from benign and malignant human prostate tissue expressed all of the enzymes necessary to produce the complete diversity of DHEA metabolites. This suggests that the adrenal androgen DHEA and its metabolites can directly modulate through autocrine mechanisms and prostate endothelial cell homeostasis (Godoy AS and Smith GJ, unpublished observations).
Although serum levels of testosterone fall to undetectable during androgen deprivation therapy (ADT), there is a continuous presence of circulating DHEA, suggesting that DHEA remains a key source of androgen precursors within the prostate tissue microenvironment (80). Since endothelial cells within the prostate are sensitive to the effects of both testicular and adrenal androgens, these cells represent a high-value, but unexplored, therapeutic cellular target for therapies targeted at the androgen axis. Hence, further understanding of AR signaling in these cells may improve the outcome of ADT.
Expression and Activity of VDR in Tumor Endothelium
Calcitriol (1,25-D3), the active metabolite of vitamin D, is a central factor in regulation of bone and mineral metabolism (6, 79). Studies have shown that calcitriol affects the endothelium of various tissues by regulation of endothelial cell proliferation and differentiation (14, 43, 72). Most of the biological actions of calcitriol are believed to be mediated by a high-affinity nuclear receptor, the VDR (39). VDR expression in endothelial cells was first demonstrated in capillary and venule endothelial cells of normal human skin and endothelial cells isolated from human umbilical vein and bovine aorta (62, 63). More recently, VDR expression was observed in tumor endothelium (5, 14) as well as in circulating endothelial progenitor cells (15).
Despite substantial interest in targeting the VDR signaling axis in the treatment of prostate cancer (97), very little is known about the effects of calcitriol on the vasculature within prostate tumors. There is no direct evidence that endothelial cells in human prostate cancer express VDR and respond to calcitriol's antiproliferative effects. However, CYP24, a well-known target of activated VDR, was expressed in the endothelium of human prostate cancers. This suggests that the VDR pathway may be activated and have a role in governing the biology of prostate endothelium (17).
Studies from others demonstrated that treatment of calcitriol can decrease vessel density VEGF expression in retinoblastoma and colon tumors in vivo (47, 86). However, it was unclear whether such antiangiogenic effects occurred by direct activation of VDR by calcitriol in endothelial cells, via indirect effects on angiogenic signaling mediated through other cells in the prostate tissue microenvironment, or by a combination of the two mechanisms. Evidence obtained from endothelial cells isolated freshly from murine models of squamous cell carcinoma and radiation-induced fibrosarcoma indicated that VDR was expressed in tumor endothelium and that exposure of endothelial cells to calcitriol markedly inhibited cell growth (5). This suggests that calcitriol may exert an antiproliferative effect via direct VDR activation in prostate tumor endothelial cells.
Interestingly, VDR activation in endothelium may not necessarily result in growth inhibition. VDR was also activated in nontumor microvascular endothelial cells that migrated into implanted Matrigel plugs (Matrigel-derived); however, calcitriol treatment did not result in growth inhibition in these cells (14). Comparing tumor-derived and Matrigel-derived endothelial cells, VDR protein appeared to be induced to a similar level, and there was no difference found in the coding region of the DNA for the VDR gene between these cells (14). However, receptor binding assays showed that the total VDR protein content was different; there was higher ligand-binding capacity in tumor endothelial cells than in Matrigel-derived endothelial cells (14). Despite different ligand binding characteristics, VDR in both cell types was phosphorylated and accumulated in the nucleus upon treatment with calcitriol. At the molecular level, VDR from the two types of endothelial cells was able to activate a target 24-hydroxylase (CYP24) promoter at similar rates (14), indicating that the VDR signaling in these cells is intact and functional. Hence, these studies suggested that although VDR signaling was activated in both cells, calcitriol exhibited a very different effect in endothelial cells, depending on the surrounding microenvironment (tumor vs. benign). Furthermore, VDR expression and promoter activity alone were not sufficient to determine the extent of the antiproliferative effects of vitamin D on endothelial cells.
Effects of VDR Signaling Activation in Tumor Endothelium in Vitro and in Vivo
Calcitriol-mediated VDR activation led to varied responses in different endothelial cell populations. Calcitriol at nanomolar concentrations inhibited cell growth of isolated tumor endothelium (5, 14, 22); however, the growth of microvascular endothelial cells isolated from Matrigel plugs was only minimally affected by similar treatment. Resistance to calcitriol's effects was also observed in endothelial cells of other normal tissues, including those isolated from mouse brain, lung, and yolk sac (5, 14). Calcitriol elicited cell cycle arrest in tumor endothelial cells by inducing expression of p21 and p27, which was not observed in calcitriol-treated normal endothelial cells (14). The induction of p21 and p27 was accompanied by a reduction in DNA synthesis and a significant increase in apoptosis in tumor endothelial cells but not endothelial cells from benign tissue. Furthermore, there was a distinct difference in modulation of key survival and apoptotic signaling molecules by calcitriol treatment; there was a significant reduction in phospho-Erk, phospho-Akt, and the antiapoptotic protein Bcl-2 in tumor endothelial cells (Fig. 2). Also, an increase in caspase-3 expression and poly(ADP)-ribose polymerase cleavage was observed in tumor endothelial cells but not in normal endothelial cells (14).
There would be significant clinical implications if potent antiangiogenic agents that are candidates for cancer therapy are found to exert comparable effects on normal vasculature, such as impeding physiologically important normal angiogenic processes, wound healing, or menstruation. However, the selective growth inhibitory effects of calcitriol observed only for tumor endothelium could translate into a clinically significant difference in therapeutic efficacy. Treatment of tumor-bearing mice with calcitriol significantly reduced the mean tumor microvessel density marked by CD31 staining of endothelial cells, with little effect observed in the vasculature at nontumor sites such as Matrigel plugs (48). The reduction in mean vessel density in tumors correlated with an increased number of apoptotic endothelial cells and caspase-3 activation in double-staining experiments. It is important to note that the tumor and Matrigel plugs were generated on opposing flanks of the same mouse; therefore, the microvasculatures established at the two sites were comparable, albeit induced by different microenvironments.
The role of VDR in calcitriol-mediated growth inhibitory effects in tumor endothelium was evident when tumor endothelial cells isolated from a prostate tumor xenograft implanted in VDR-knockout (VDR-KO) mice failed to respond to calcitriol in vitro (12). In this model, murine prostate adenocarcinoma (TRAMP)-C2 prostate adenocarcinoma tumors were generated in VDR wild-type hosts and VDR knockout mouse hosts, and tumor endothelial cells were isolated from tumors in both hosts for analysis. Since formation of blood vessels within the tumor requires participation from host cells (24), the isolated endothelial cells portray the genetic makeup of the host. Tumor endothelial cells isolated from VDR wild-type mice showed an increase in VDR protein upon treatment with calcitriol and trans-activation of the CYP24 promoter (12). This led to an induction of G0/G1 cell cycle arrest and a decrease in S phase cells. In contrast, upregulation of VDR and induction of CYP24 were not observed in endothelial cells derived from tumors growing in VDR-KO mice.
The absence of VDR in endothelial cells of KO animals resulted in aberrant tumor vasculature; the tumor microvasculature was enlarged and was associated with fewer pericytes compared with the tumor vasculature in wild-type animals (12). Structural and functional deficiencies in tumor vasculature could result in limited delivery of chemotherapeutic agents, which would enhance resistance to chemotherapy (64). The increased abnormalities found in the tumor vasculature of VDR-KO mice implied that VDR could modulate differentiation and functionality of the vasculature in addition to mediating calcitriol's antiproliferative effects. In the tumors grown in VDR-KO background, upregulation of angiogenic factors such as VEGF, angiopoietin-1, HIF-1α, and PDGF was observed. The correlation of VDR loss with increased levels of proangiogenic factors suggested that VDR had direct and/or indirect suppressive effects on the transcription of these factors. Such inhibitory effects were also observed in other cell models, including human annulus and neutrophil cells and Kaposi sarcoma cells (33, 42, 62). Interestingly, VDR was also shown to improve the angiogenic properties of endothelial progenitor cells by inducing various factors, including VEGF (34).
Therefore, in consideration of targeting VDR in cancer therapy, it would be important to investigate whether VDR inhibitory effects on the activity of angiogenic factors are applicable to the tumor vasculature across different tumor types, including CaP. Studies such as those that use cross-breeding of VDR-KO mice (56) or mice expressing VDR-KO under the influence of the endothelial-specific promoter Tie-1 (46), with TRAMP (28) or LPB-Tag (50) transgenic mouse prostate cancer models, will allow mechanistic studies of the role of VDR in tumor endothelium in spontaneously arising prostate adenocarcinomas. In fact, LPB-Tag tumors progressed more rapidly in VDR-KO mice compared with wild-type animals (67); however, the role of VDR in the tumor vasculature was not examined. Molecular evidence obtained from such studies will provide invaluable insight into the role of VDR activity in regulation of the neovasculature during tumor angiogenesis.
AR and VDR Cross-Talk
In prostate cancer cells, activation of AR signaling led to suppression of VDR trans-activation (96), which was probably due to competition for the same pool of coregulators required for receptor-mediated trans-activation. Interestingly, calcitriol demonstrated limited growth inhibitory effects on castration-sensitive lymph node carcinoma of the prostate (LNCap) cells grown in medium lacking androgens and no effect on castration-resistant derivatives of LNCaP cells (69). Furthermore, the use of antiandrogens such as bicalutamide repressed calcitriol's anti-tumor effect by downregulating VDR-induced expression of AS3 (APRIN) (69). However, it was noted that such negative regulation of VDR by Casodex was limited to LNCaP-derived prostate cancer cell lines and that androgen deprivation therapy should not obviate the concurrent use of VDR agonists (69). The interdependence of AR and VDR may vary between different prostate cancer cell lines, and the cross-talk between AR and VDR has not been investigated thoroughly in prostate tumor endothelial cells, especially those from castration-resistant prostate tumors. Therefore, the effects of varying AR and VDR agonists or analogs that activate these receptors may result in differential modulation of nuclear receptor cross-talk and provide new approaches for selective inhibition of growth of prostate cancers (66).
Clinical Effects of Modulating AR and VDR Signaling on Prostate Cancer Vasculature
The historic paradigm of ADT as a monofunctional treatment of CaP and BPH is founded on the hypothesis that the prostate secretory epithelial cell/cancer epithelial cell is the only androgen-sensitive cell type within in the complex and highly interactive prostate tissue microenvironment and that AR functions independent of other nuclear receptors. However, several pieces of evidence suggest strongly that human prostate endothelial cells demonstrate cell type-specific responses to ADT, including 1) the empirical observation that ADT induced a reduction of hematuria due to BPH, 2) treatment with AR antagonists (e.g., flutamide or bicalutamide) and steroid metabolism inhibitors (e.g., finasteride or dutasteride) reducing bleeding during radical prostatectomy surgery, and 3) treatment of patients with gonadotrophin-releasing hormone agonist (bicalutamide/goserelin), or dutasteride, producing a profound vascular collapse in the prostate without collateral systemic vascular perturbation. Consequently, human prostate endothelial cells represent an unexplored target for androgens and ADT. In accord with these observations, our group demonstrated for endothelial cells from both human CaP tissue and human benign prostate tissue that 1) prostate endothelial cells express AR (30), 2) ADT induces apoptosis in a prostate endothelial cell that preceded the peak of apoptosis in the cancer epithelial cell compartment by several days (29), and 3) ADT-induced cell death results in acute loss of prostate vascular integrity that allows leakage of serum components (including androgens/steroids) into the interstitial tissue space (29).
ADT-induced transient destabilization of the human prostate endothelial cell compartment may present a “therapeutic window” for delivery of chemotherapeutic agents. Therefore, the study of the regulatory role of androgens in the prostate microvasculature may provide the molecular basis for development of new therapeutic modalities. This paradigm-shifting approach would change the monolithic paradigm of ADT as a first-line therapy that is focused on induction of apoptotic death in CaP cells to a dynamic paradigm where ADT is employed in a neoadjuvant setting to improve therapeutic efficacy of conventional and new treatment modalities. This new approach would capitalize on the “therapeutic window” opened by the acute apoptotic death of prostate endothelial cells to allow access to the interstitial tissue space for chemotherapeutic agents that are usually blocked by the intact endothelial barrier. Moreover, these studies could provide new biomarkers and potential therapeutic targets for agents to inhibit neoangiogenesis more effectively after ADT, blocking and/or delaying recurrence and making ADT curative instead of palliative.
VDR, on the other hand, can be activated by calcitriol in both normal and tumor vasculature of the prostate. However, as described above, VDR activation results in an antiproliferative effect only on tumor endothelium, without the normal vasculature being affected. Hence, calcitriol may potentially affect prostate tumor angiogenesis without toxicity to the normal vasculature. Clinically, the maximum tolerated dose (MTD) of calcitriol alone and in combination with a number of cytotoxic agents and/or glucocorticoids was determined in phase I studies (68, 97, 103). Furthermore, in a phase II trial in patients with androgen-independent prostate cancer, the combination of dexamethasone and calcitriol resulted in an enhanced but still limited anti-tumor effect (98). However, these studies were hampered by limited drug bioavailability to the tumor because drug dosage was constrained by hypercalcemia, a confounding adverse effect associated with calcitriol treatment (102).
Bioavailability of calcitriol is also limited by endogenous 25-hydroxylase (CYP24) activity. CYP24 is the key enzyme responsible for initiation of the vitamin D degradation pathway by directing the side chain metabolism of vitamin D metabolites, including calcitriol (75). CYP24 is expressed in most vitamin D target tissues and can be induced by calcitriol (78), providing a negative feedback regulation for attenuation of the effects of excessive calcitriol levels. Interestingly, CYP24 was found to be epigenetically silenced in endothelium of multiple preclinical tumor models, including TRAMP, murine radiation-induced fibrosarcoma, and murine squamous cell carcinoma, and the loss of CYP24 activity may explain the enhanced cytotoxic effects of calcitriol in these cells (13). A different study observed that CYP24 was hypermethylated in the endothelium of human prostate cancer tissues (17), further supporting the clinical use of vitamin D against tumor vasculature. Hence, CYP24 expression may provide the basis for the selective VDR-mediated growth inhibitory effects in tumor endothelium without affecting normal vasculature.
Since most clinical studies with calcitriol were designed primarily to determine the anti-tumor effects of calcitriol on tumor cells, it is unclear whether the anti-tumor effects observed were also attributable to effects of calcitriol on tumor endothelium or whether calcitriol at the MTD demonstrated a selective growth inhibition to tumor endothelium without affecting normal vasculature. More clinical studies are necessary to validate the use of calcitriol in targeting tumor endothelium.
Conclusion
Recent evidence demonstrated that AR and VDR have major roles in maintenance of homeostasis of human prostate endothelial cells, and both receptors are druggable targets that allow comparison of independent and concurrent therapies. However, there are several important questions related to the specific roles of AR and VDR in endothelial cells that remain unanswered. 1) Can AR and VDR expression levels in tumor-associated prostate endothelial cells predict the angiogenic capacity of prostate cancer and thus serve as a prognostic marker for CaP? 2) Do AR and VDR function similarly in benign prostate-associated endothelial cells as they do in tumor-associated prostate endothelial cells? And 3) What is the role of these receptors in the maintenance of homeostasis of prostate endothelial cells in benign tissue and/or in prostate cancer tissue? Considering that both AR and VDR modulate endothelial cell proliferation, can these two receptors differentially affect other endothelial cell processes, such as differentiation, migration, branching, and maturation into functional vasculature in normal and diseased prostate, and if so, do they have similar or opposite effects?
Evidence of expression of functional AR in human prostate endothelial cells in CaP tissue and of the acute effect of ADT on human prostate vascular integrity indicate that human prostate vasculature has a unique potential as a first-line target for ADT. Similarly, modulation of VDR-activated signaling with calcitriol or vitamin D analogs may be effective in selective targeting of tumor endothelium in the prostate without affecting the normal endothelium. Such selectivity is particularly important to avoid adverse effects commonly observed with current antiangiogenic agents. Understanding the molecular mechanisms involved in each signaling pathway could be beneficial for identification of common targets for prostate endothelial cells.
GRANTS
A. S. Godoy (W81XWH-08-1-0330), V. P. Montecinos (W81XWH-08-1-0299), and I. Chung (W81XWH-10-1-0241) were supported by Department of Defense grants. A. S. Godoy is supported by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Grant 1130051 and Department of Defense Grant W81XWH-12-1-0341. V. P. Montecinos is supported by the MECESUP Grant PUC-0703 and FONDEF grant CA12I10333. I. Chung is supported by the University of Malaya HIR-MOHE Grant UM.C/HIR/MOHE/MED-12. G. J. Smith is supported by NCIP01-CA77739.
DISCLOSURES
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
A.S.G., I.C., V.P.M., R.B., C.S.J., and G.J.S. contributed to the conception and design of the research; A.S.G., I.C., V.P.M., R.B., C.S.J., and G.J.S. analyzed the data; A.S.G., I.C., R.B., C.S.J., and G.J.S. interpreted the results of the experiments; A.S.G. and I.C. prepared the figures; A.S.G., I.C., V.P.M., R.B., C.S.J., and G.J.S. drafted the manuscript; A.S.G., I.C., V.P.M., R.B., C.S.J., and G.J.S. edited and revised the manuscript; A.S.G., I.C., V.P.M., R.B., C.S.J., and G.J.S. approved the final version of the manuscript; I.C. and V.P.M. performed the experiments.
REFERENCES
- 1. Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82: 3493–3497, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117: 421–426, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Ali IU, Senger DR, Smith LE. Angiogenesis as a potential biomarker in prostate cancer chemoprevention trials. Urology 57: 143–147, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Alonzi R, Padhani AR, Taylor NJ, Collins DJ, D'Arcy JA, Stirling JJ, Saunders MI, Hoskin PJ. Antivascular effects of neoadjuvant androgen deprivation for prostate cancer: an in vivo human study using susceptibility and relaxivity dynamic MRI. Int J Radiat Oncol Biol Phys 80: 721–727, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL. Antiproliferative effects of 1alpha,25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology 143: 2508–2514, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocr Rev 14: 3–19, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA 101: 3668–3673, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blauer M, Vaalasti A, Pauli SL, Ylikomi T, Joensuu T, Tuohimaa P. Location of androgen receptor in human skin. J Invest Dermatol 97: 264–268, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Cai J, Hong Y, Weng C, Tan C, Imperato-McGinley J, Zhu YS. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am J Physiol Heart Circ Physiol 300: H1210–H1221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, Lin AS, Li Y, Reiter CE, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem 283: 29228–29238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choy M, Rafii S. Role of angiogenesis in the progression and treatment of prostate cancer. Cancer Invest 19: 181–191, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res 69: 967–975, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung I, Karpf AR, Muindi JR, Conroy JM, Nowak NJ, Johnson CS, Trump DL. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. J Biol Chem 282: 8704–8714, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Chung I, Wong MK, Flynn G, Yu WD, Johnson CS, Trump DL. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res 66: 8565–8573, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Cianciolo G, La Manna G, Cappuccilli ML, Lanci N, Della Bella E, Cuna V, Dormi A, Todeschini P, Donati G, Alviano F, Costa R, Bagnara GP, Stefoni S. VDR expression on circulating endothelial progenitor cells in dialysis patients is modulated by 25(OH)D serum levels and calcitriol therapy. Blood Purif 32: 161–173, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev 8: 338–362, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Deeb KK, Luo W, Karpf AR, Omilian AR, Bshara W, Tian L, Tangrea MA, Morrison CD, Johnson CS, Trump DL. Differential vitamin D 24-hydroxylase/CYP24A1 gene promoter methylation in endothelium from benign and malignant human prostate. Epigenetics 6: 994–1000, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dull P, Reagan RW, Jr, Bahnson RR. Managing benign prostatic hyperplasia. Am Fam Physician 66: 77–84, 2002 [PubMed] [Google Scholar]
- 19. Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8: 210–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Alfy M, Luu-The V, Huang XF, Berger L, Labrie F, Pelletier G. Localization of type 5 17beta-hydroxysteroid dehydrogenase, 3beta-hydroxysteroid dehydrogenase, and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology 140: 1481–1491, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Feldman D, Zhao XY, Krishnan AV. Vitamin D and prostate cancer. Endocrinology 141: 5–9, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Flynn G, Chung I, Yu WD, Romano M, Modzelewski RA, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces apoptosis. Oncology 70: 447–457, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Folkman J. Is tissue mass regulated by vascular endothelial cells? Prostate as the first evidence. Endocrinology 139: 441–442, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186, 1971 [DOI] [PubMed] [Google Scholar]
- 25. Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol 20: 1153–1163, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Franck-Lissbrant I, Haggstrom S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology 139: 451–456, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem 279: 34741–34749, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res 56: 4096–4102, 1996 [PubMed] [Google Scholar]
- 29. Godoy A, Montecinos VP, Gray DR, Sotomayor P, Yau JM, Vethanayagam RR, Singh S, Mohler JL, Smith GJ. Androgen deprivation induces rapid involution and recovery of human prostate vasculature. Am J Physiol Endocrinol Metab 300: E263–E275, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Godoy A, Watts A, Sotomayor P, Montecinos VP, Huss WJ, Onate SA, Smith GJ. Androgen receptor is causally involved in the homeostasis of the human prostate endothelial cell. Endocrinology 149: 2959–2969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gormley GJ. Finasteride: a clinical review. Biomed Pharmacother 49: 319–324, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24: 7410–7425, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Gruber HE, Hoelscher G, Ingram JA, Chow Y, Loeffler B, Hanley EN., Jr 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine (Phila Pa 1976) 33: 755–765, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Grundmann M, Haidar M, Placzko S, Niendorf R, Darashchonak N, Hubel CA, von Versen-Höynck F. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol 303: C954–C962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol 37, Suppl 2: S2–S14, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Gustavsson H, Jennbacken K, Welen K, Damber JE. Altered expression of genes regulating angiogenesis in experimental androgen-independent prostate cancer. Prostate 68: 161–170, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Gustavsson H, Welen K, Damber JE. Transition of an androgen-dependent human prostate cancer cell line into an androgen-independent subline is associated with increased angiogenesis. Prostate 62: 364–373, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Haapala K, Kuukasjärvi T, Hyytinen E, Rantala I, Helin HJ, Koivisto PA. Androgen receptor amplification is associated with increased cell proliferation in prostate cancer. Hum Pathol 38: 474–478, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13: 325–349, 1998 [DOI] [PubMed] [Google Scholar]
- 40. He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, Pitt B, Xie W, Li S. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res 98: 192–199, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol 6: 395–404, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Hirsch D, Archer FE, Joshi-Kale M, Vetrano AM, Weinberger B. Decreased anti-inflammatory responses to vitamin D in neonatal neutrophils. Mediators Inflamm 2011: 598345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res 164: 3–28, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate 52: 213–235, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res 61: 2736–2743, 2001 [PubMed] [Google Scholar]
- 46. Iljin K, Petrova TV, Veikkola T, Kumar V, Poutanen M, Alitalo K. A fluorescent Tie1 reporter allows monitoring of vascular development and endothelial cell isolation from transgenic mouse embryos. FASEB J 16: 1764–1774, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, Ishiguro S. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer 81: 730–733, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Johnson CS, Chung I, Trump DL. Epigenetic silencing of CYP24 in the tumor microenvironment. J Steroid Biochem Mol Biol 121: 338–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol 7: 494–509, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML, Matusik RJ. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest 78: 319–333, 1998 [PubMed] [Google Scholar]
- 51. Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med (Berl) 77: 656–665, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Kravchick S, Cytron S, Mamonov A, Peled R, Linov L. Effect of short-term dutasteride therapy on prostate vascularity in patients with benign prostatic hyperplasia: a pilot study. Urology 73: 1274–1278, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Krill D, DeFlavia P, Dhir R, Luo J, Becich MJ, Lehman E, Getzenberg RH. Expression patterns of vitamin D receptor in human prostate. J Cell Biochem 82: 566–572, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Laine M, Blauer M, Ylikomi T, Tuohimaa P, Aitasalo K, Happonen RP, Tenovuo J. Immunohistochemical demonstration of androgen receptors in human salivary glands. Arch Oral Biol 38: 299–302, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Lekas E, Johansson M, Widmark A, Bergh A, Damber JE. Decrement of blood flow precedes the involution of the ventral prostate in the rat after castration. Urol Res 25: 309–314, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94: 9831–9835, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liang T, Hoyer S, Yu R, Soltani K, Lorincz AL, Hiipakka RA, Liao S. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J Invest Dermatol 100: 663–666, 1993 [DOI] [PubMed] [Google Scholar]
- 58. Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). J Biol Chem 277: 21379–21388, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Lowe FC, Somers WJ. The use of ketoconazole in the emergency management of disseminated intravascular coagulation due to metastatic prostatic cancer. J Urol 137: 1000–1002, 1987 [DOI] [PubMed] [Google Scholar]
- 60. Mahajan K, Coppola D, Rawal B, Chen YA, Lawrence HR, Engelman RW, Lawrence NJ, Mahajan NP. Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer. J Biol Chem 287: 22112–22122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mantalaris A, Panoskaltsis N, Sakai Y, Bourne P, Chang C, Messing EM, Wu JH. Localization of androgen receptor expression in human bone marrow. J Pathol 193: 361–366, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Masood R, Nagpal S, Zheng T, Cai J, Tulpule A, Smith DL, Gill PS. Kaposi sarcoma is a therapeutic target for vitamin D(3) receptor agonist. Blood 96: 3188–3194, 2000 [PubMed] [Google Scholar]
- 63. Merke J, Milde P, Lewicka S, Hügel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest 83: 1903–1915, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mizukami Y, Sasajima J, Ashida T, Kohgo Y. Abnormal tumor vasculatures and bone marrow-derived pro-angiogenic cells in cancer. Int J Hematol 95: 125–130, 2012 [DOI] [PubMed] [Google Scholar]
- 65. Mohler JL. Ten years of progress in prostate cancer. J Natl Compr Canc Netw 10: 136–140, 2012 [DOI] [PubMed] [Google Scholar]
- 66. Mooso B, Madhav A, Johnson S, Roy M, Moore ME, Moy C, Loredo GA, Mehta RG, Vaughan AT, Ghosh PM. Androgen Receptor regulation of Vitamin D receptor in response of castration-resistant prostate cancer cells to 1alpha-Hydroxyvitamin D5 - a calcitriol analog. Genes Cancer 1: 927–940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mordan-McCombs S, Brown T, Wang WL, Gaupel AC, Welsh J, Tenniswood M. Tumor progression in the LPB-Tag transgenic model of prostate cancer is altered by vitamin D receptor and serum testosterone status. J Steroid Biochem Mol Biol 121: 368–371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Muindi JR, Peng Y, Potter DM, Hershberger PA, Tauch JS, Capozzoli MJ, Egorin MJ, Johnson CS, Trump DL. Pharmacokinetics of high-dose oral calcitriol: results from a phase 1 trial of calcitriol and paclitaxel. Clin Pharmacol Ther 72: 648–659, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Murthy S, Agoulnik IU, Weigel NL. Androgen receptor signaling and vitamin D receptor action in prostate cancer cells. Prostate 64: 362–372, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 32: 245–253, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Norata GD, Cattaneo P, Poletti A, Catapano AL. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits tumor necrosis factor alpha and lipopolysaccharide induced inflammatory response in human endothelial cells and in mice aorta. Atherosclerosis 212: 100–106, 2010 [DOI] [PubMed] [Google Scholar]
- 72. Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147: 5542–5548, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Oesterling JE. Endocrine therapies for symptomatic benign prostatic hyperplasia. Urology 43: 7–16, 1994 [DOI] [PubMed] [Google Scholar]
- 74. Oesterling JE, el Etreby MF, Gormley GJ, Imperato-McGinley JL, Roehrborn CG, Schröder FH, Tunn UW. Endocrine therapies for BPH: scientific rationale, clinical results, and patient selection. Prog Clin Biol Res 386: 231–250, 1994 [PubMed] [Google Scholar]
- 75. Omdahl J, May B. The 25-hydroxyvitamin D 24-hydroxylase. In: Vitamin D (2nd ed), edited by Feldman D, Pike JW, Glorieux FH. Burlington, MA: Elsevier Academic, 2005, p. 85–104 [Google Scholar]
- 76. Peng Y, Li CX, Chen F, Wang Z, Ligr M, Melamed J, Wei J, Gerald W, Pagano M, Garabedian MJ, Lee P. Stimulation of prostate cancer cellular proliferation and invasion by the androgen receptor co-activator ARA70. Am J Pathol 172: 225–235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology 129: 3187–3199, 1991 [DOI] [PubMed] [Google Scholar]
- 78. Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29: 664–673, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med 320: 980–991, 1989 [DOI] [PubMed] [Google Scholar]
- 80. Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res 13: 2030–2037, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Schultheiss D, Badalyan R, Pilatz A, Gabouev AI, Schlote N, Wefer J, von Wasielewski R, Mertsching H, Sohn M, Stief CG, Jonas U. Androgen and estrogen receptors in the human corpus cavernosum penis: immunohistochemical and cell culture results. World J Urol 21: 320–324, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Shabisgh A, Tanji N, D'Agati V, Burchardt M, Rubin M, Goluboff ET, Heitjan D, Kiss A, Buttyan R. Early effects of castration on the vascular system of the rat ventral prostate gland. Endocrinology 140: 1920–1926, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Shabsigh A, Chang DT, Heitjan DF, Kiss A, Olsson CA, Puchner PJ, Buttyan R. Rapid reduction in blood flow to the rat ventral prostate gland after castration: preliminary evidence that androgens influence prostate size by regulating blood flow to the prostate gland and prostatic endothelial cell survival. Prostate 36: 201–206, 1998 [DOI] [PubMed] [Google Scholar]
- 84. Shabsigh A, Ghafar MA, de la Taille A, Burchardt M, Kaplan SA, Anastasiadis AG, Buttyan R. Biomarker analysis demonstrates a hypoxic environment in the castrated rat ventral prostate gland. J Cell Biochem 81: 437–444, 2001 [PubMed] [Google Scholar]
- 85. Shabsigh A, Lee B, Buttyan R. Unique morphological aspects of the rat ventral prostate gland revealed by vascular corrosion casting. Prostate 39: 240–245, 1999 [DOI] [PubMed] [Google Scholar]
- 86. Shokravi MT, Marcus DM, Alroy J, Egan K, Saornil MA, Albert DM. Vitamin D inhibits angiogenesis in transgenic murine retinoblastoma. Invest Ophthalmol Vis Sci 36: 83–87, 1995 [PubMed] [Google Scholar]
- 87. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 62: 10–29, 2012 [DOI] [PubMed] [Google Scholar]
- 88. Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology 144: 3449–3455, 2003 [DOI] [PubMed] [Google Scholar]
- 89. Sinha AA, Quast BJ, Reddy PK, Lall V, Wilson MJ, Qian J, Bostwick DG. Microvessel density as a molecular marker for identifying high-grade prostatic intraepithelial neoplasia precursors to prostate cancer. Exp Mol Pathol 77: 153–159, 2004 [DOI] [PubMed] [Google Scholar]
- 90. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89: 5245–5255, 2004 [DOI] [PubMed] [Google Scholar]
- 91. Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132: 1952–1960, 1993 [DOI] [PubMed] [Google Scholar]
- 92. Somjen D, Kohen F, Gayer B, Kulik T, Knoll E, Stern N. Role of putative membrane receptors in the effect of androgens on human vascular cell growth. J Endocrinol 180: 97–106, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Somjen D, Kohen F, Jaffe A, Amir-Zaltsman Y, Knoll E, Stern N. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension 32: 39–45, 1998 [DOI] [PubMed] [Google Scholar]
- 94. Strohmeyer D, Rössing C, Strauss F, Bauerfeind A, Kaufmann O, Loening S. Tumor angiogenesis is associated with progression after radical prostatectomy in pT2/pT3 prostate cancer. Prostate 42: 26–33, 2000 [DOI] [PubMed] [Google Scholar]
- 95. Tempany CM, Partin AW, Zerhouni EA, Zinreich SJ, Walsh PC. The influence of finasteride on the volume of the peripheral and periurethral zones of the prostate in men with benign prostatic hyperplasia. Prostate 22: 39–42, 1993 [DOI] [PubMed] [Google Scholar]
- 96. Ting HJ, Bao BY, Hsu CL, Lee YF. Androgen-receptor coregulators mediate the suppressive effect of androgen signals on vitamin D receptor activity. Endocrine 26: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 97. Trump DL, Muindi J, Johnson CS, Hershberger PA. Clinical development of calcitriol and calcitriol analogs in oncology: progress and considerations for future development. In: Vitamin D (2nd ed). Burlington, MA: Elsevier Academic, 2005, p. 1741–1749 [Google Scholar]
- 98. Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 106: 2136–2142, 2006 [DOI] [PubMed] [Google Scholar]
- 99. Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380: 297–309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang S, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology 147: 4025–4033, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev 38: 89–116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Woloszynska-Read A, Johnson CS, Trump DL. Vitamin D and cancer: clinical aspects. Best Pract Res Clin Endocrinol Metab 25: 605–615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yu WD, McElwain MC, Modzelewski RA, Russell DM, Smith DC, Trump DL, Johnson CS. Enhancement of 1,25-dihydroxyvitamin D3-mediated antitumor activity with dexamethasone. J Natl Cancer Inst 90: 134–141, 1998 [DOI] [PubMed] [Google Scholar]
- 104. Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62: 1008–1013, 2002 [PubMed] [Google Scholar]
- 105. Zhuang SH, Burnstein KL. Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology 139: 1197–1207, 1998 [DOI] [PubMed] [Google Scholar]


