Abstract
Objectives
Vaccination during pregnancy significantly reduces the risk of influenza illness among pregnant women and their infants up to 6 months of age; however, many women do not get vaccinated. We examined disparities in vaccination coverage among women who delivered a live-born infant during the 2009–2010 influenza season, when two separate influenza vaccinations were recommended.
Methods
Pregnancy Risk Assessment Monitoring System (PRAMS) data from 29 states and New York City, collected during the 2009–2010 influenza season, were used to examine uptake of seasonal (unweighted n=27,153) and pandemic influenza A(H1N1)pdm09 (pH1N1) (n=27,372) vaccination by racially/ethnically diverse women who delivered a live-born infant from September 1, 2009, through May 31, 2010.
Results
PRAMS data showed variation in seasonal and pH1N1 influenza vaccination coverage among women with live-born infants by racial/ethnic group. For seasonal influenza vaccination, coverage was 50.5% for non-Hispanic white, 30.2% for non-Hispanic black, 42.1% for Hispanic, and 48.2% for non-Hispanic other women. For pH1N1, vaccination coverage was 41.4% for non-Hispanic white, 25.5% for non-Hispanic black, 41.1% for Hispanic, and 43.3% for non-Hispanic other women. Compared with non-Hispanic white women, non-Hispanic black women had lower seasonal (crude prevalence ratio [cPR] = 0.60, 95% confidence interval [CI] 0.55, 0.64) and pH1N1 (cPR=0.62, 95% CI 0.57, 0.67) vaccination coverage; these disparities diminished but remained after adjusting for provider recommendation or offer for influenza vaccination, insurance status, and demographic factors (seasonal vaccine: adjusted PR [aPR] = 0.80, 95% CI 0.74, 0.86; and pH1N1 vaccine: aPR=0.75, 95% CI 0.68, 0.82).
Conclusion
To reduce disparities in influenza vaccination uptake by pregnant women, targeted efforts toward providers and interventions focusing on pregnant and postpartum women may be needed.
Pregnant women are at increased risk for complications from influenza and are more likely than the general population to be hospitalized due to respiratory illness during influenza season.1–4 Seasonal vaccination can reduce morbidity and mortality associated with seasonal influenza.1,2 While the influenza vaccine recommendations for pregnant women date back to the 1960s, it was in 2004 that the American College of Obstetricians and Gynecologists (ACOG) and the Advisory Committee on Immunization Practices (ACIP) recommended that women be vaccinated with the inactivated influenza vaccine any time during pregnancy.5–7 Historically, the national estimates showed that, of the adult groups recommended to receive seasonal vaccination, pregnant women had the lowest coverage prior to the 2009–2010 influenza season.7–9 Research has shown that there are racial/ethnic and economic disparities in vaccination coverage among adults. In general, vaccination coverage is lower among non-Hispanic black and Hispanic women than among non-Hispanic white women and women of lower socioeconomic status.10–16 For pregnant women in particular, coverage prior to the 2009–2010 influenza season was less than 30%.8,9
Because influenza can be especially severe during pregnancy, pregnant women in particular were at increased risk of severe disease and mortality from pandemic influenza A(H1N1)pdm09 (pH1N1) virus infection.17 During the 2009–2010 influenza season, both the inactivated trivalent seasonal and monovalent pH1N1 vaccinations were recommended for pregnant women.17,18 Pregnant women were deemed a priority group for the pH1N1 vaccine due to high morbidity and mortality associated with pH1N1 infection within this group. Monovalent pH1N1 vaccine was purchased by the federal government and made available to the public at no cost. Additionally, the monovalent vaccine was made available later than the trivalent seasonal influenza vaccine.
Given the recommendation of vaccination for pregnant women and the importance of preventing morbidity and mortality from influenza, we examined disparities in vaccination uptake by pregnant women with recent live-born infants who participated in the Pregnancy Risk Assessment Monitoring System (PRAMS) survey. The rationale for examining data from the 2009–2010 influenza season was to learn about vaccination coverage of a vulnerable population (i.e., pregnant women) during the pandemic.19,20 Research questions guiding the analysis included (1) What are the differences in vaccination coverage among racial/ethnic groups of pregnant women? and (2) Are there differences by race/ethnicity in the patterns of vaccination uptake for the two influenza vaccinations offered during the 2009–2010 influenza season?
METHODS
We examined data from the 2009–2010 PRAMS cycle from 29 states and New York City (NYC) to assess pH1N1 and seasonal influenza vaccination coverage among women who delivered a live-born infant. PRAMS is an ongoing, population-based survey that collects data on a wide range of maternal behaviors and experiences before, during, and after pregnancy. PRAMS surveys are currently administered by 40 states and NYC. Every month, stratified random samples of 100–300 women with recent live births are selected using the state's birth certificate registry. The selected mothers are mailed a questionnaire after delivery, and those who do not respond by mail within two months are contacted by telephone; 15 attempts are made to reach the respondents with viable phone numbers. The PRAMS data are weighted to account for survey design and nonresponse.
During the 2009–2010 influenza season, 29 states and NYC agreed to add a supplemental influenza vaccination module to their PRAMS survey and two sets of questions regarding seasonal and pH1N1 vaccination, and women's reporting of provider recommendation or offer of vaccinations was included in the supplement. The state median response rate was 69.1% (range: 53.7%–85.0%). For the purposes of this assessment, we started with women who delivered their babies from September 1, 2009, through May 31, 2010, and whose vaccination status for the 2009–2010 influenza season was known for seasonal (n=27,153) and pH1N1 (n=27,372) influenza vaccine. Women with unknown race/ethnicity were excluded from further analyses (n=205). The percentage of missing values for variables considered in the analysis ranged from <0.01% to 4%. While the majority of the women in the analytic sample were vaccinated during pregnancy, others may have been vaccinated during the early postpartum period. Therefore, we included both periods in the analysis due to the importance of protecting the baby after birth, also known as cocooning.1,2,18
Women were classified as non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other. Covariates examined included maternal age, maternal education, marital status, prenatal care (defined as entry in the first trimester vs. later or no prenatal care), insurance coverage during prenatal care visits, health-care provider recommended or offered vaccinations, and state where data were collected. We used SUDAAN® version 11.0 for analyses.21 We conducted bivariate and multiple logistic regression analyses, taking into account selected sociodemographic variables found to be significant in the bivariate analyses at p<0.05. We constructed separate logistic regression models for seasonal influenza and pH1N1 immunization coverage, and we constructed additional models for each vaccination type to examine the influence of provider recommendation/offer of vaccination in closing the disparities in vaccination coverage among different groups of women. We generated predicated marginal estimates from logistic regression models to examine adjusted vaccination coverage prevalence. We examined potential interactions between provider recommendation or offer of vaccination and race/ethnicity in each of the full models due to differences in vaccination uptake of pH1N1 and seasonal influenza by whether or not there was receipt of a recommendation or offer. For the pH1N1 model, the interaction was not significant, and for seasonal influenza, the overall model estimates with interaction were not stable; therefore, we report the main effects.
RESULTS
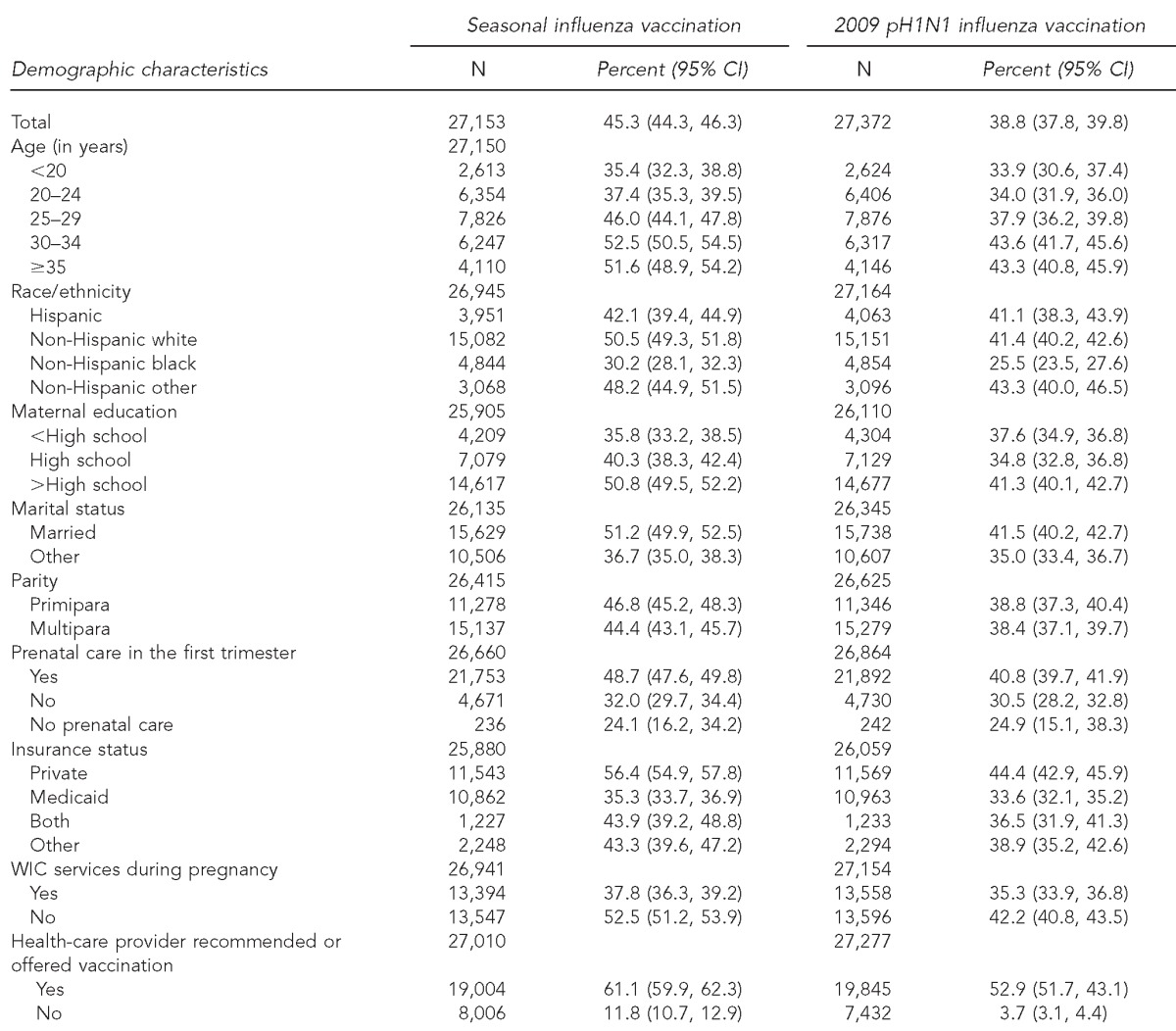
During the 2009–2010 influenza season, the overall influenza vaccination coverage among women with recent live-born deliveries was 45.3% for seasonal influenza vaccine, 38.8% for pH1N1 influenza vaccine (Table 1), and 27.3% for both (data not shown). Seasonal influenza vaccination coverage varied by age; women <20 years of age had the lowest coverage (35.4%) and coverage generally increased with age. We observed a similar pattern for pH1N1 influenza vaccination, with coverage of 33.9% for those <20 years of age and ≥43.3% for those aged ≥30 years.
Table 1.
Seasonal and pH1N1 vaccination coverage by demographic characteristics among women with live-born infants in 29 states and New York City: 2009–2010 influenza season, PRAMS
pH1N1 = pandemic influenza A(H1N1)pdm09
PRAMS = Pregnancy Risk Assessment Monitoring System
CI = confidence interval
WIC = Special Supplemental Nutrition Program for Women, Infants, and Children
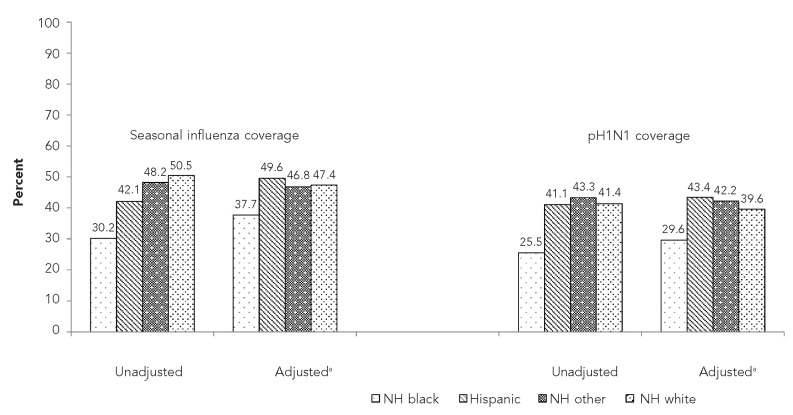
Vaccination coverage also varied by type of health insurance, with a lower prevalence of seasonal influenza vaccination among those on Medicaid (35.3%) than among those with private health insurance (56.4%). For pH1N1 influenza vaccine, coverage was highest for those with private health insurance (44.4%) and ranged from 33.6% for those with Medicaid to 38.9% for those with other types of health insurance. Women who reported participating in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) had lower coverage for each type of influenza vaccination than women not participating in WIC. For both seasonal and pH1N1 vaccination, a substantially higher prevalence of those whose provider recommended/offered a vaccination reported having been vaccinated (61.1% vs. 11.8% for seasonal vaccine and 52.9% vs. 3.7% for pH1N1 vaccine). Non-Hispanic black women had lower coverage than all other racial/ethnic groups for both seasonal (30.2%) and pH1N1 (25.5%) vaccines (Table 1, Figure).
Figure.
Unadjusted and adjusted prevalence of seasonal and pH1N1 influenza vaccination coverage among women with live-born infants, by racial/ethnic group: 2009–2010 influenza season, PRAMS, 29 states and New York City
aPrevalence estimates were generated using predicted marginals and adjusted for the following characteristics: maternal age; education; parity; prenatal care initiation; insurance status; participation in the Special Supplemental Nutrition Program for Women, Infants, and Children; vaccination recommendation or offer from a health-care provider; and the state of maternal residence.
pH1N1 = pandemic influenza A(H1N1)pdm09
PRAMS = Pregnancy Risk Assessment Monitoring System
NH = non-Hispanic
Overall provider recommendations for each vaccination differed by about eight percentage points for non-Hispanic white vs. non-Hispanic black and Hispanic women, respectively; no significant differences were observed between non-Hispanic white and non-Hispanic other women. For pH1N1, the percentage point differences for vaccine uptake was 15.9 between non-Hispanic white and non-Hispanic black women and 0.3 between non-Hispanic white and Hispanic women. When we examined the vaccination coverage by provider offer or recommendation, data showed that the biggest difference was between non-Hispanic white and non-Hispanic black women for both pH1N1 and seasonal influenza vaccinations (Table 2). Adjusting for sociodemographic and provider recommendation/offer reduced the disparity in vaccination coverage between non-Hispanic black and non-Hispanic white women from 20.3 to 9.7 percentage points for seasonal influenza vaccine and from 15.9 to 10.0 percentage points for pH1N1 influenza vaccine (Figure).
Table 2.
Prevalence of provider recommendation/offer for seasonal and pH1N1 influenza vaccination and coverage, by provider recommendation/offer, among women of different racial/ethnic groups during the 2009–2010 influenza season in 29 states and New York City, PRAMS
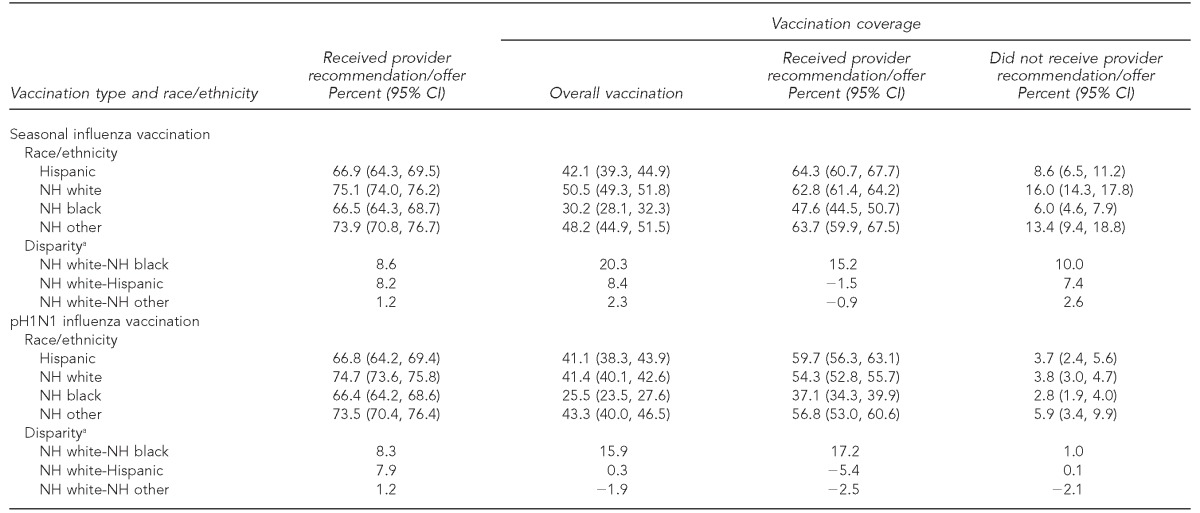
aPercentage-point difference between reference group (non-Hispanic white women) and the comparison group
pH1N1 = pandemic influenza A(H1N1)pdm09
PRAMS = Pregnancy Risk Assessment Monitoring System
CI = confidence interval
NH = non-Hispanic
Multivariable models showed that after adjusting for reported provider recommendation/offer of vaccination, the probability of reporting seasonal vaccination was 28% lower for non-Hispanic black than for non-Hispanic white women (Table 3, Model 2). After further adjusting for women's age, education, parity, prenatal care initiation, insurance status, WIC participation, and the state of maternal residence, the probability of having seasonal vaccination coverage was 20% lower for non-Hispanic black than for non-Hispanic white women (Table 3, Model 3). Similarly, after adjusting for provider recommendation/offer of vaccination, the probability of non-Hispanic black women reporting pH1N1 vaccination was 31% lower than among non-Hispanic white women (Table 3, Model 2). After further adjusting for demographic factors and state of maternal residence, the prevalence of pH1N1 vaccination was 25% lower for non-Hispanic black vs. non-Hispanic white women (Table 3, Model 3). Findings for the other racial/ethnic groups were similar to the reference group of non-Hispanic white women for both seasonal and pH1N1 vaccination (Table 3).
Table 3.
Models showing prevalence ratios for seasonal and pH1N1 vaccination coverage among women with live-born infants from 29 states and New York City: 2009–2010 influenza season, PRAMS
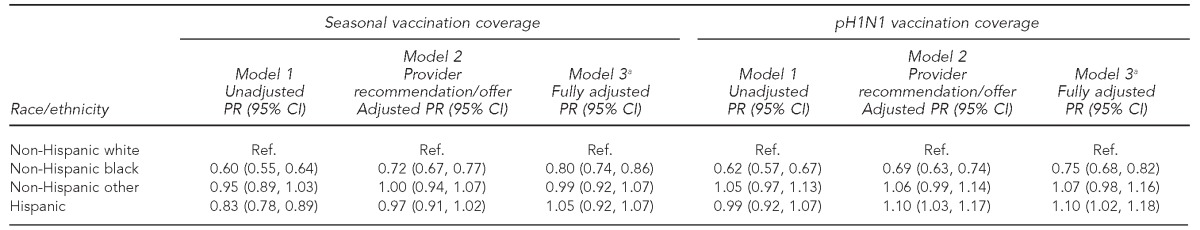
aModel 3 was adjusted for the following characteristics: maternal age; education; parity; prenatal care; insurance coverage; participation in the Special Supplemental Nutrition Program for Women, Infants, and Children; provider recommendation/offer; and state from which data were collected.
pH1N1 = pandemic influenza A(H1N1)pdm09
PRAMS = Pregnancy Risk Assessment Monitoring System
PR = prevalence ratio
CI = confidence interval
Ref. = reference group
Respondents who indicated that they had not received the seasonal or pH1N1 influenza vaccinations during the 2009–2010 influenza season were asked why they did not get vaccinated. Overall reasons for not getting vaccination included: my doctor didn't mention anything about a flu shot during my pregnancy (seasonal: 32.3%, pH1N1: 30.0%); I was worried about the side effects of the flu shot for me (seasonal: 46.6%, pH1N1: 63.4%); I was worried that the flu shot might harm my baby (seasonal: 49.5%, pH1N1: 64.6%); and I normally don't get a seasonal flu shot (seasonal: 70.8%, pH1N1: 59.7%) (data not shown). Significant differences by race/ethnicity were observed among women who reported not getting both vaccines for one of two reasons: the doctor did not mention anything about a flu shot during the woman's pregnancy and women not normally getting a flu shot (data available upon request).
DISCUSSION
The PRAMS 2009–2010 influenza season data showed that there was significant variation in both seasonal and pH1N1 influenza vaccination coverage by race/ethnicity, even after adjusting for other sociodemographic factors. Despite the ACIP and ACOG recommendations for pregnant women to be vaccinated anytime during pregnancy, seasonal vaccination coverage for pregnant women was below the Healthy People 2020 target of 80% for pregnant women1,5,18,22 However, compared with previous seasons, when the coverage for pregnant women was <30%, the overall seasonal vaccination coverage for the 2009–2010 influenza season was higher (>45%) than what had been observed previously.6–9,23,24
The patterns in racial/ethnic disparities differed for pH1N1 and seasonal influenza vaccination coverage, and these patterns were similar to what had been observed previously.25–28 In all racial/ethnic groups, reported receipt of a provider recommendation/offer of influenza vaccination was highly associated with both seasonal and pH1N1 vaccination. For pH1N1, there was no difference between Hispanic women and non-Hispanic white women in vaccination coverage overall even before adjusting for provider recommendation/offer. The lower prevalence of provider recommendation/offer for Hispanic women was perhaps offset by higher vaccination coverage that was similar to non-Hispanic white women. The disparity in seasonal vaccination coverage was reduced to non-significant for Hispanic women compared with non-Hispanic white women after adjusting for provider advice or offer and demographic variables. One thing to note about the 2009–2010 pandemic was that earlier cases were reported as originating in Mexico, which might have influenced the Hispanic population's awareness and acceptance of the vaccinations, attitudes about vaccinations, cost as a potential barrier, perception of threat, and possibly adherence to the advice or vaccination offered by the provider, all potentially resulting in higher coverage in this population.29
Compared with non-Hispanic white women, there was a large disparity in seasonal and pH1N1 vaccination coverage for non-Hispanic black women, due in part to lower prevalence of provider recommendation/offer and lower vaccination coverage among non-Hispanic black women with a recommendation/offer compared with women of other races/ethnicities. In the multivariable analyses, disparities persisted for non-Hispanic black women in both sets of models, albeit at lower levels than the unadjusted models, indicating that the disparities gap can potentially be narrowed.
Heightened awareness about the importance of vaccinating pregnant women to prevent severe morbidity and mortality during the 2009–2010 influenza season, and outreach and encouragement from all health-care sectors, may have assisted in reducing disparities.1,2,5,17–23 The observed disparities for non-Hispanic black women compared with non-Hispanic white women for both influenza vaccines in 2009–2010 might be related to a number of factors, including a lower likelihood of receiving a provider recommendation or offer for influenza vaccination, a limited influence of recommendation/offer when received, and a lower likelihood of vaccination without any recommendation and offer from a provider. Research is needed to examine the influence of provider recommendation or offer among different racial/ethnic groups. According to existing studies, lower vaccination rates may be related to cultural norms, beliefs, and perceptions about vaccinations; timing of the recommendations; and other circumstances of provider visits, strength and effectiveness of the recommendation/offer, and quality of the patient-provider relationship.10–12,19,25–28
The PRAMS data revealed that among those who did not get seasonal or pH1N1 influenza vaccinations, many women worried about the side effects of vaccinations for themselves and their babies, indicating a general need to assure pregnant women about the safety of the vaccination, particularly for a new vaccine (pH1N1). These findings are consistent with other research, which notes that women, especially those from racial/ethnic minority groups, were worried about vaccines, and therefore were less likely to get vaccinated than white women.19,26,28 Among those who did not get the seasonal or pH1N1 influenza vaccine, evaluation of their reasons for not getting vaccinated revealed racial/ethnic differences in reported lack of recommendation/offer for seasonal or pH1N1 vaccination during pregnancy, and that they don't normally get a flu shot. These reasons may potentially be addressed by ensuring that coordinated efforts are made to increase provider awareness of the ACOG/ACIP guidelines about vaccinating pregnant women with seasonal influenza vaccine to protect both mother and baby.
It appears that pregnant women's reactions to the 2009–2010 influenza season were different from previous years. In general, more women were vaccinated than in previous seasons,7,8 which may have been related to the seriousness of pH1N1, the availability of vaccine at no cost, and the heightened awareness about the need for pregnant women to be vaccinated for their own and their babies' protection. Vaccination coverage may be improved with increased access to prenatal care, frequency and quality of provider recommendations, and efforts to ensure that consistent and clear messages about the prevention of influenza are developed and delivered to audiences.25–29 These results reinforce the importance of provider recommendations and of reducing barriers to vaccination, such as making vaccination available in settings where pregnant women seek care and advice, responding to pregnant women's concerns, and promoting prevention messages.14–16,26–31
Limitations
The results of this study need to be considered with the following limitations. First, PRAMS data were available from 29 states and NYC and might not be generalizable to all women with live births in the U.S. Second, the cohort of women available for this analysis, with live births from September 2009 to May 2010, represents a subset of all women who were pregnant during the influenza season. Third, as two influenza vaccines were available during the 2009–2010 influenza season, a reporting bias may have occurred for seasonal estimates potentially due to misclassification of the vaccines. Fourth, the PRAMS data are self-reported several months post-partum; therefore, recall bias may have occurred in that those who did not receive the vaccination also did not recall receiving advice about it.
CONCLUSION
The results showed racial/ethnic disparity in vaccination coverage during 2009–2010 among women who had a recent live birth. Our findings are consistent with what others have found, in that non-Hispanic black women are less likely to report receiving seasonal influenza vaccination than non-Hispanic white women, and that reasons may include perceptions of vaccination effectiveness, distrust, and perhaps orientation to taking preventive actions (e.g., getting the flu vaccine).10–15,19,20,25–28 As Naleway et al. and others point out, it is important to promote influenza vaccination as a routine preventive measure for pregnant women to reduce morbidity and mortality due to influenza.6,7 The PRAMS data highlight the continued need to educate providers, as well as women, about the need for and safety of seasonal influenza vaccination for pregnant women and their infants to reduce disparities in vaccination coverage.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The Pregnancy Risk Assessment Monitoring System (PRAMS) protocol and data were approved by CDC and state Institutional Review Boards of the states collecting PRAMS data.
REFERENCES
- 1.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) [published errata appear in MMWR Recomm Rep 2010;59(31):993 and MMWR Recomm Rep 2010;59(35):1147] MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61(32):613–8. [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee opinion no. 468: influenza vaccination during pregnancy. Obstet Gynecol. 2011;116:1006–7. doi: 10.1097/AOG.0b013e3181fae845. [DOI] [PubMed] [Google Scholar]
- 6.Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev. 2006;28:47–53. doi: 10.1093/epirev/mxj002. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson DJ, Rasmussen SA. The safety of adjuvants in influenza vaccines: what do we know and why do we need them? Am J Obstet Gynecol. 2012;207:145–6. doi: 10.1016/j.ajog.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Ahluwalia IB, Jamieson DJ, Rasmussen SA, D'Angelo D, Goodman D, Kim H. Correlates of seasonal influenza vaccine among pregnant women in Georgia and Rhode Island. Obstet Gynecol. 2010;116:949–55. doi: 10.1097/AOG.0b013e3181f1039f. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy ED, Ahluwalia IB, Ding H, Lu PJ, Singleton JA, Bridges CB. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. Am J Obstet Gynecol. 2012;207(3 Suppl):S9–16. doi: 10.1016/j.ajog.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 10.Link MW, Ahluwalia IB, Euler GL, Bridges CB, Chu SY, Wortley PM. Racial and ethnic disparities in influenza vaccination coverage among adults during the 2004–2005 season. Am J Epidemiol. 2006;163:571–8. doi: 10.1093/aje/kwj086. [DOI] [PubMed] [Google Scholar]
- 11.Racial/ethnic disparities in influenza and pneumococcal vaccination levels among persons aged ≥65 years—United States, 1989–2001. MMWR Morb Mortal Wkly Rep. 2003;52(40):958–62. [PubMed] [Google Scholar]
- 12.Wortley P. Who's getting shots and who's not: racial/ethnic disparities in immunization coverage. Ethn Dis. 2005;15(2 Suppl 3):S3–6. [PubMed] [Google Scholar]
- 13.Singleton JA, Santibanez TA, Wortley PM. Influenza and pneumococcal vaccination of adults aged ≥65: racial/ethnic differences. Am J Prev Med. 2005;29:412–20. doi: 10.1016/j.amepre.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Linn ST, Guralnik JM, Patel KV. Disparities in influenza vaccine coverage in the United States, 2008. J Am Geriatr Soc. 2010;58:1333–40. doi: 10.1111/j.1532-5415.2010.02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ompad DC, Galea S, Vlahov D. Distribution of influenza vaccine to high-risk groups. Epidemiol Rev. 2006;28:54–70. doi: 10.1093/epirev/mxj004. [DOI] [PubMed] [Google Scholar]
- 16.Logan JL. Disparities in influenza immunization among US adults. J Natl Med Assoc. 2009;101:161–6. doi: 10.1016/s0027-9684(15)30830-0. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 18.Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-10):1–8. [PubMed] [Google Scholar]
- 19.Steelfisher GK, Blendon RJ, Bekheit MM, Mitchell EW, Williams J, Lubell K, et al. Novel pandemic A(H1N1) influenza vaccination among pregnant women: motivators and barriers. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S116–23. doi: 10.1016/j.ajog.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Goldfarb I, Panda B, Wylie B, Riley L. Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S112–5. doi: 10.1016/j.ajog.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Research Triangle Institute, Inc. Research Triangle Park (NC): Research Triangle Institute, Inc.; 2012. SUDAAN®: Version 11.0. [Google Scholar]
- 22.Department of Health and Human Services (US) Healthy people 2020 topics & objectives [cited 2013 Feb 11] Available from: URL: http://healthypeople.gov/2020/topicobjectives2020/pdfs/HP2020objectives.pdf.
- 23.Influenza vaccination coverage among pregnant women—United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep. 2011;60(32):1078–82. [PubMed] [Google Scholar]
- 24.Ding H, Santibanez TA, Jamieson DJ, Weinbaum CM, Euler GL, Grohskopf LA, et al. Influenza vaccination coverage among pregnant women—National 2009 H1N1 Flu Survey (NHFS) Am J Obstet Gynecol. 2011;204(6 Suppl 1):S96–106. doi: 10.1016/j.ajog.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Wray RJ, Buskirt TD, Jupka K, Lapka C, Jacobsen H, Pakpahan R, et al. Influenza vaccination concerns among older blacks: a randomized controlled trial. Am J Prev Med. 2009;36:429–34. doi: 10.1016/j.amepre.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Meharry PM, Colson ER, Grizas AP, Stiller R, Vaszquez M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern Child Health J. 2013;17:156–64. doi: 10.1007/s10995-012-0957-3. [DOI] [PubMed] [Google Scholar]
- 27.Eiser AR, Ellis G. Cultural competence and the African American experience with health care: the case for specific content in cross-cultural education. Acad Med. 2007;82:176–83. doi: 10.1097/ACM.0b013e31802d92ea. [DOI] [PubMed] [Google Scholar]
- 28.Frew PM, Painter JE, Hixson B, Kulb C, Moore K, del Rio C, et al. Factors mediating seasonal and influenza A (H1N1) vaccine acceptance among ethnically diverse populations in the urban south. Vaccine. 2012;30:4200–8. doi: 10.1016/j.vaccine.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (US) The 2009 H1N1 pandemic: summary highlights, April 2009–April 2010 [cited 2013 May 14] Available from: URL: http://www.cdc.gov/h1n1flu/cdcresponse.htm.
- 30.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol. 2011;204:146.e1–7. doi: 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (US) The guide to community preventive services: increasing appropriate vaccination [cited 2014 May 9] Available from: URL: http://www.thecommunity guide.org/vaccines/index.html.