Abstract
Extramammary Paget’s disease (EMPD) is a rare intraepithelial malignancy arising in areas rich in apocrine glands, such as the perineum, vulva, axilla, scrotum, and penis. We describe the case of a man in his 50s who initially presented with a small eczematous lesion on his right groin, treated with topical ointments for eczema, until excisional biopsy of lesion unequivocally revealed invasive EMPD. Despite aggressive surgical interventions, his disease progressed to involve the scrotum and penis. Deemed unresectable, the patient was treated with systemic chemotherapy with minimal response. The rarity of EMPD, especially of the penis and scrotum, warrants an educated eye and heightened index of suspicion when dealing with eczematous lesions in the groin in any person. Early biopsy and histological examination is crucial for early surgical intervention of the lesions. There are no guidelines available to treat locally advanced unresectable disease. In addition, further studies are needed to identify genetic defects underlying the pathogenesis of this rare disease, to help improve treatment strategies and decrease morbidity.
Keywords: Extramammary Paget’s disease (EMPD), eczematous lesion, intraepithelial malignancy
Introduction
Extramammary Paget’s disease (EMPD) is a rare intraepithelial malignancy arising in areas rich in apocrine glands, such as the perineum, vulva, axilla, scrotum, and penis. EMPD of the scrotum and penis was first described by Crocker in 18891; however, the vulva remains the most common site involved with 65% of EMPD located in this area, followed by perianal EMPD which accounts for 20% of cases, and male genitalia, that is scrotum and penis, accounting for 14% of cases.2
EMPD typically presents as non-specific erythematous, erosive, eczematous, or circinate lesions of the skin, and often multiple topical therapies are tried before a diagnosis is made. A median delay of 2 years has been reported since symptoms first appear to the definite diagnosis of the disease.3 Sometimes, the EMPD has been present for 10–15 years before evidence of cancer or metastases appear. Penoscrotal lesions may start in the groin and spread to the scrotum and penis. Lesions may be associated with pruritus, but burning tenderness and edema may be experienced as well. Inguinal lymphadenopathy may be present in some cases and occasionally associated with edema of the legs.4
The precise pathogenesis of EMPD is not yet entirely elucidated. Current evidence suggests that EMPD is heterogeneous, encompassing at least two different forms: primary and secondary EMPD. The origin of primary EMPD is in skin, specifically the epidermis or the underlying apocrine sweat gland. This form, is not associated with distant adenocarcinoma, is initially limited to the epithelium, but may slowly progress to an invasive tumor, spreading to the dermis, blood, and lymphatic vessels; in advanced stages, it may produce lymph node or visceral, potentially lethal metastases.2 The secondary form of EMPD is associated with epidermotropic spread of malignant cells from an underlying adenocarcinoma from dermal adnexal glands or within contiguous epithelium, usually of genitourinary or gastrointestinal tract.2 Published reports suggest that up to 42% of patients have associated underlying malignancy; however, there is a low incidence of internal malignancy with penoscrotal EMPD.5
We describe a case of primary, locally advanced EMPD that presented initially in the groin with eventual spread to the contralateral groin, penis, and scrotum. We discuss the various treatments and efforts made to eradicate the disease in this situation, all of which have been unsuccessful. We performed literature review to emphasize the need for a high index of suspicion in the diagnosis of this rare neoplasm. The patient and his family have a rather extensive history of cancer, and we discuss the potential benefit of genetic testing in these cases.
Case Presentation
A man in his 50s with locally advanced EMPD involving the skin of groin, scrotum, and penis was admitted to our center for his third cycle of cisplatin/doxorubicin chemotherapy in August 2011. The patient’s medical history includes “numerous” squamous and basal cell carcinomas removed in the 1990s from his face and upper extremities, but no melanomas were reported. In addition, the patient has an extensive family history of skin cancers and breast cancer. Both the patient’s parents developed skin cancers at early ages, none of which were reported as melanoma. The patient’s two maternal aunts developed breast cancer at 35 and 70 years of age, and all four of the patient’s sisters developed breast cancers at 35, 40, 45, and 47 years of age. The patient’s history of EMPD began in 2002, when he observed a small eczematous lesion which appeared on his right groin which he initially dismissed it as a transient “rash”. It was not until 2008 that the lesion began to “itch, ooze, and become angrier,” at which time the patient consulted his dermatologist. It was diagnosed as eczema, and different ointments were administered, none of which provided any relief or improvement. In October 2009, the patient underwent punch biopsy of the right groin lesion which was unremarkable; however, excisional biopsy of the same lesion unequivocally revealed invasive apocrine adenocarcinoma and EMPD. Extensive resection and wide excision was performed at this time, but positive margins and focal lymphovascular invasion were observed. In January 2010, the patient developed similar lesions in his left groin and subsequent PET/CT showed diffused areas of high avidity in both left and right groin with bilaterally enlarged lymph nodes. In April 2010, the patient underwent bilateral wide excision and deep dissection of the groin lesions and lymph node dissection. Pathology showed metastatic carcinoma in 3 of the 20 lymph nodes in the right groin (Fig. 1). The left groin surgical specimen showed superficially invasive EMPD with no lymph node involvement. Immunohistochemistry stains for estrogen receptor (ER), progesterone receptor (PR), and Her2/neu were all negative. Yet another groin dissection was performed in December 2010 as well as a punch biopsy of a suspicious suprapubic lesion which was found to be EMPD. Despite all surgical interventions, the disease progressed to involve both the scrotum and penis. It was at this time that the tumor was considered unresectable and the patient was started on systemic chemotherapy in February 2011. He was initially treated with carboplatin and paclitaxel; however, after two cycles, he developed atrial fibrillation and his chemotherapy was changed to carboplatin/gemcitabine.
Figure 1.
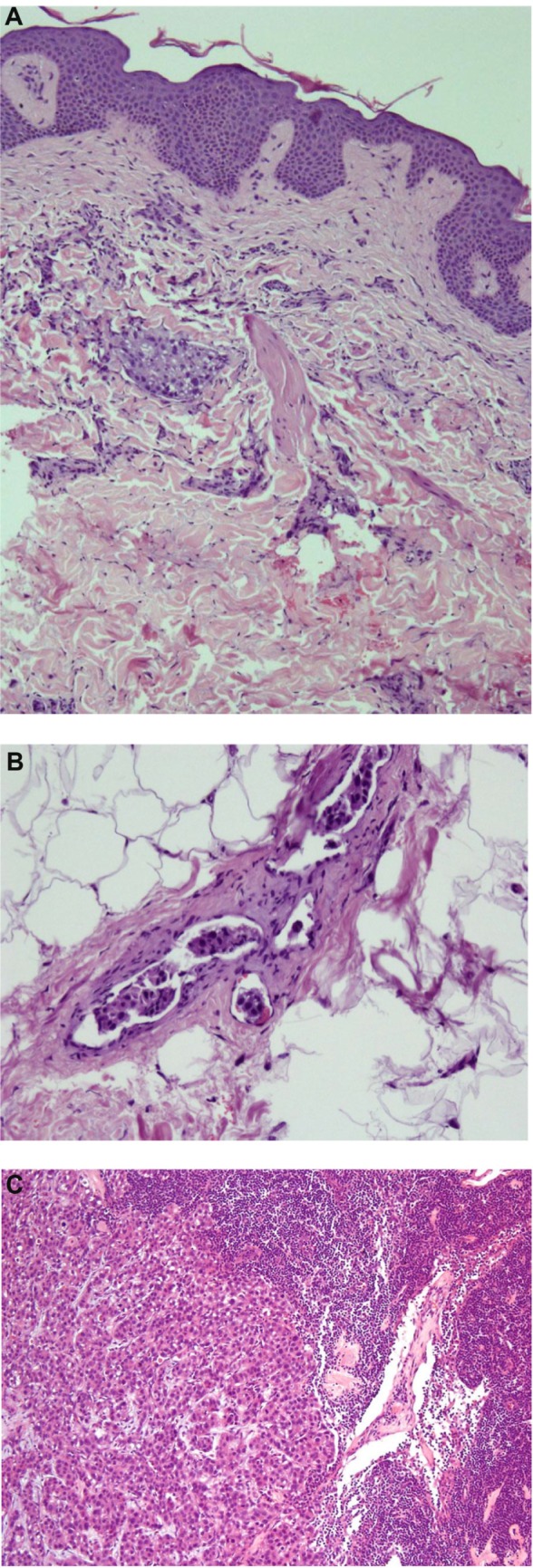
(A) Suprapubic skin: The epidermis is normal. Within the dermis are groups of atypical cells with abundant amphophilic cytoplasm. Nuclei are pleomorphic and hyperchromatic. The cellular features are consistent with an apocrine morphology. (B) Atypical cells present in dermal lymphovascular spaces. (C) Lymph node showing metastatic apocrine carcinoma.
After two cycles of carboplatin/gemcitabine, he developed significant dyspnea on exertion and his chemotherapy was changed to cisplatin/doxorubicin. Since February 2011, the patient has received two cycles of carboplatin/paclitaxel, two cycles of carboplatin/gemcitabine, and three cycles of cisplatin/doxorubicin. Despite aggressive surgical and medical treatment, the last PET scan showed interval progression of disease involving the right inguinal region and scrotum.
Discussion
EMPD is an exceedingly rare form of adenocarcinoma that is typically found in apocrine gland containing cutaneous regions. The most common site of presentation is the vulva with more than 200 reported cases, followed by the perianal region with more than 86 cases reported.2 The disease usually occurs in older patients between the ages of 50 and 80 years with a predilection for Caucasian females of 1.4:1 versus males.2
Since the first reported case by Crocker, the true pathogenesis has yet to be elucidated and is somewhat controversial with several theories being proposed. Many authors believe that Paget’s disease is caused by an undetected underlying malignancy providing metastatic cells to the overlying epidermis.6,7 A second theory states that Paget’s disease results from multiple foci of malignant transformations from a population of cells with common embryological origin which accounts for the high incidence of “skip areas” between the subjacent invasive adenocarcinoma and the epidermal lesions. Yet another belief is that Paget’s cells derive from or differentiate toward exocrine and apocrine gland cells possibly explaining why EMPD occurs in areas containing sweat glands.3,8 Several case reports have been reported reviewing the histology for EMPD and images portraying the disease itself.9–11 Few, if any, appear to be as advanced as the case we report here.
Surgical intervention with wide local excision to eradicate the tumor mass is the primary treatment for EMPD. However, the invasion of epidermis makes it difficult to obtain complete resection which leads to a high frequency of recurrences.9 There are case reports on radiotherapy in a selected group of patients who were not surgical candidates or as adjuvant treatment to surgical excision.12 In locally advanced or metastatic EMPD, systemic chemotherapy (docetaxel, 5-FU, vincristine, mitomycin-C, carboplatin, etoposide) has been used.13 Although certain cases have had measurable responses, it is difficult to draw any conclusions.
As mentioned in the case report, our patient had an extensive family history of skin cancer and breast cancer. As EMPD is a neoplasm with potential of metastatic spread, it is likely to have defects in putative oncogenes and tumor suppressor genes as is the case with other human cancers. However, little is known about the genetic abnormality underlying EMPD. There have been few reports of overexpression of ras p21,14 c-erbB-2,15 and altered expression of p53 tumor suppressor protein on some cases of EMPD. However, in another small study from Japan, tumor cells of EMPD in 12 cases examined failed to show either p53 mutation or loss of heterozygosity at several loci commonly lost in other human cancers including 13q and 17p. Interestingly, c-erbB-2 expression was seen in 6 of the 12 cases.16 Evidence provided by several studies implicates pathogenetic germ-line mutations of BRCA2, with lower frequency, and of BRCA1 in male breast cancer. In fact, mutations in BRCA1 and BRCA2 were estimated to be responsible for 16–76% of the male breast cancers occurring in high-risk breast/ovarian cancer families.17 Whether this applies to Paget’s and EMPD needs further exploration. In our case, one of his sisters was tested for BRCA mutations and it was wild type. We are currently working on obtaining DNA samples from all available family members to confirm this fact. If there is no BRCA or other known cancer-gene involvement, some unknown abnormalities might lead to such a dense clustering of cancers with the family.
Conclusion
The rarity of EMPD, especially of the penis and scrotum, warrant a heightened index of suspicion when dealing with eczematous lesions involving the groin in any person. Early biopsy and histological examination are crucial for early surgical intervention of the lesions. There are no guidelines available to treat locally advanced unresectable disease. Combination of systemic chemotherapy agents used in advanced disease is based on the treatment used for visceral adenocarcinomas. In addition, further studies are needed to identify genetic defects underlying the pathogenesis of this rare disease, to help improve treatment strategies and decrease morbidity.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: DI. Contributed to the writing of the manuscript: KO, OS. Agree with manuscript results and conclusions: DI, KO, OS, HG, CM, LF, JC. Jointly developed the structure and arguments for the paper: DI, KO, OS, HG, CM, LF, JC. Made critical revisions and approved final version: DI, KO, OS, HG, CM, LF, JC.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: JC discloses personal fees received from Roche, Novartis, Celgene and Eisai, outside the submitted work. Other authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Crocker HR. Paget’s disease affecting the scrotum and penis. Trans Pathol Soc Lond. 1889;40:187–91. [Google Scholar]
- 2.Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;2(5):581–90. doi: 10.1111/j.1468-3083.2007.02154.x. [DOI] [PubMed] [Google Scholar]
- 3.Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget’s disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2000;158(2):313–8. doi: 10.1111/j.1365-2133.2007.08314.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoch H. Adenocarcinoma of the scrotum (extramammary Paget’s disease): case report and review of literature. J Urol. 1984;132:137–9. doi: 10.1016/s0022-5347(17)49501-2. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Grossfeld GD, McAninch JW, Santucci R. Extramammary Paget’s disease of the penis and scrotum: excision, reconstruction and evaluation of occult malignancy. J Urol. 2001;16(6):2112–7. doi: 10.1016/s0022-5347(05)65516-4. [DOI] [PubMed] [Google Scholar]
- 6.Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009. doi: 10.1016/s0190-9622(85)70254-x. [DOI] [PubMed] [Google Scholar]
- 7.Mehta NJ, Torno R, Sorra T. Extramammary Paget’s disease. South Med J. 2000;93:713–5. [PubMed] [Google Scholar]
- 8.Shepard V, Davidson EJ. Mammary and extramammary Paget’s disease. BJOG. 2000;112:273–9. doi: 10.1111/j.1471-0528.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 9.Zollo JD, Zeitouni NC. The Roswell park cancer institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 10.Lai YL, Yang WG, Tsay PK, Swei H, Chuang SS, Wen CJ. Penoscrotal extramammary Paget’s disease: a review of 33 cases in a 20-year experience. Plast Reconstr Surg. 2003;112(4):1017–20. doi: 10.1097/01.PRS.0000076193.67701.6A. [DOI] [PubMed] [Google Scholar]
- 11.Ekwueme KC, Zakhour HD, Parr NJ. Extramammary Paget’s disease of the penis: a case report and review of the literature. J Med Case Reports. 2009;3:1–4. doi: 10.1186/1752-1947-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows NP, Jones DH, Hudson PM, et al. Treatment of extramammary Paget’s disease by radiotherapy. Br J Dermatol. 1995;132:970–2. doi: 10.1111/j.1365-2133.1995.tb16957.x. [DOI] [PubMed] [Google Scholar]
- 13.Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807–26. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Mori O, Hachisuka H, Sasai Y. Expression of ras p21 in mammary and extramammary Paget’s disease. Arch Pathol Lab Med. 1990;114:858–61. [PubMed] [Google Scholar]
- 15.Keatings L, Sinclair J, Wright C, et al. c-erbB-2 oncoprotein expression in mammary and extramammary Paget’s disease. An immunohistochemical study. Histopathology. 1990;17:243–7. doi: 10.1111/j.1365-2559.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 16.Takata M, Hatta N, Takehara K. Tumor cells of extramammary Paget’s disease do not show either p53 mutation or allelic loss at several selected loci implicated in other cancers. Br J Cancer. 1997;76(7):904–8. doi: 10.1038/bjc.1997.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch HT, Watson P, Narod SA. The genetic epidemiology of male breast cancer. Cancer. 1999;86:744–6. doi: 10.1002/(SICI)1097-0142(19990901)86:5<744::AID-CNCR4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]


