Abstract
Exposure to narrowband violet-blue light around 405 nm wavelength can induce lethal oxidative damage to bacteria and fungi, however effects on viruses are unknown. As photosensitive porphyrin molecules are involved in the microbicidal inactivation mechanism, and since porphyrins are absent in viruses, then any damaging effects of 405 nm light on viruses might appear unlikely. This study used the bacteriophage ɸC31, as a surrogate for non-enveloped double-stranded DNA viruses, to establish whether 405 nm light can induce virucidal effects. Exposure of ɸC31 suspended in minimal media, nutrient-rich media, and porphyrin solution, demonstrated differing sensitivity of the phage. Significant reductions in phage titer occurred when exposed in nutrient-rich media, with ~3-, 5- and 7-log10 reductions achieved after exposure to doses of 0.3, 0.5 and 1.4 kJ/cm2, respectively. When suspended in minimal media a 0.3-log10 reduction (P = 0.012) occurred after exposure to 306 J/cm2: much lower than the 2.7- and > 2.5-log10 reductions achieved with the same dose in nutrient-rich, and porphyrin-supplemented media, suggesting inactivation is accelerated by the photo-activation of light-sensitive components in the media. This study provides the first evidence of the interaction of narrowband 405 nm light with viruses, and demonstrates that viral susceptibility to 405 nm light can be significantly enhanced by involvement of exogenous photosensitive components. The reduced susceptibility of viruses in minimal media, compared with that of other microorganisms, provides further evidence that the antimicrobial action of 405 nm light is predominantly due to the photo-excitation of endogenous photosensitive molecules such as porphyrins within susceptible microorganisms.
Keywords: 405 nm light, bacteriophage, inactivation, photosensitizers, virus, ɸC31
Introduction
Visible violet-blue light in the region of 405 nm has antimicrobial effects, with germicidal activity recorded against a range of Gram-positive and Gram-negative bacteria, yeast, filamentous fungi, and even bacterial and fungal spores.1-9
Traditional methods of visible light microbial inactivation are associated with photodynamic inactivation (PDI) using exogenous photosensitizer molecules. PDI involves the addition of a photosensitizer in vitro which becomes excited by specific wavelengths of visible light, in the presence of oxygen, and reacts to produce reactive oxygen species (ROS), ultimately causing cell damage.10 This was demonstrated by Clifton11 who established the necessary requirement of light and air in conjunction with photosensitive dyes such as methylene blue for the inactivation of Staphylococcus bacteriophages.
More recent studies have been performed to identify alternative photosensitizers for viral PDI. Schagen et al.12 demonstrated a range of photosensitizers that can be used for inactivation of adenovirus including methylene blue, rose bengal, uroporphyrin or aluminum phthalocynine tetrasulphonate (AlPcS4), and advances have also been made on the production of new photosensitizers such as synthetic tetraaryl-porphyrins.13 An up-to-date summary of the many different photosensitizers used for photodynamic inactivation of mammalian viruses and bacteriophages has been detailed by Costa et al.14 Importantly, the efficacy of photodynamic inactivation of bacteriophages is not only dependent on the photosensitizer and its concentration, but also the dose, fluence rate and light source.15
The use of violet-blue light for microbial inactivation eliminates the necessity for exogenous photosensitizers. This narrow band of visible light between 400–420 nm, peaking at 405 nm, inactivates microorganisms without the need for exogenous photosensitizers and instead utilizes photosensitive porphyrin molecules present within the microbial cells.3 Similar to exogenous photosensitizers, when excited by absorption of photons, there is an energy transfer resulting in the production of the non-specific oxidising agent, singlet oxygen and other ROS. These toxic species induce an accumulation of oxidative damage and ultimately cause cell death.8,16,17
Growing evidence of the antimicrobial activity of violet-blue light has led to the development of this technology toward a range of decontamination applications. Numerous studies have suggested the potential of this antimicrobial light for wound decontamination, and the increased sensitivity of bacterial cells compared with mammalian cells should permit selective inactivation of wound contaminants.18-20 The use of 405 nm light for environmental decontamination has also been demonstrated. Trials in hospital burns and intensive care units demonstrated that levels of bacterial contamination on environmental surfaces around occupied isolation rooms could be reduced by up to 86% over and above reductions achieved by traditional cleaning alone.21-23
Although 405 nm light has anti-bacterial and anti-fungal efficacy, antiviral activity has yet to be determined. As 405 nm light inactivation is thought to rely on the photo-excitation of endogenous porphyrins, that are absent from virions,24 inactivation of viruses by this method, when suspended in a simple buffer solution, is thought to be unlikely. To investigate this, the bacteriophage ɸC31, a non-enveloped double stranded DNA phage, was used as a surrogate to study the effect of 405 nm light on viruses. This study provides the first evidence of the interaction of narrowband 405 nm light with viruses, and demonstrates the influence of the suspending media on phage susceptibility. As such, this study provides further evidence of the antimicrobial mechanism of action of 405 nm light.
Results and Discussion
In order to determine the effect of 405 nm light (Fig. 1) on ɸC31, bacteriophages were suspended in NB and exposed to 405 nm light at an irradiance of 56.7 mW/cm2 (Fig. 2). Successful inactivation was achieved, with the general trend showing relatively linear kinetics, with an increasing dose resulting in decreasing bacteriophage population. In the case of the 103 PFU/ml population, significant inactivation was achieved after a dose of 153.1 J/cm2 (P = 0.016) and 2.7-log10 reduction achieved after exposure to 306.2 J/cm2 compared with the equivalent controls.
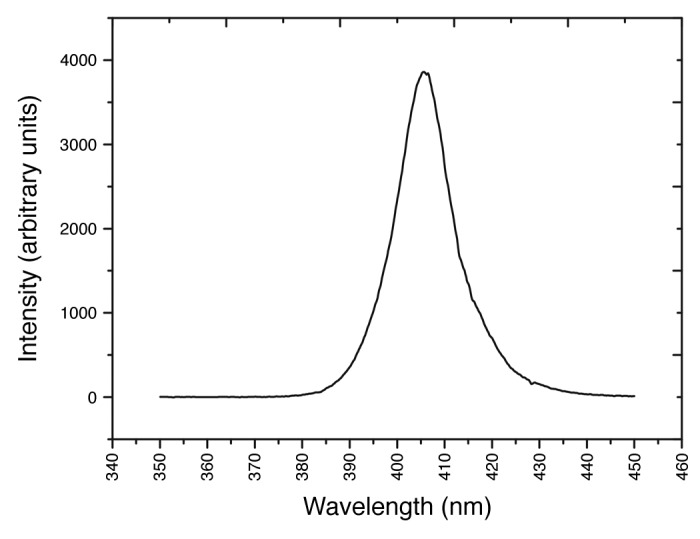
Figure 1. Emission spectrum of the 405 nm LED array, measured using a high resolution spectrometer (Ocean Optics, USA)
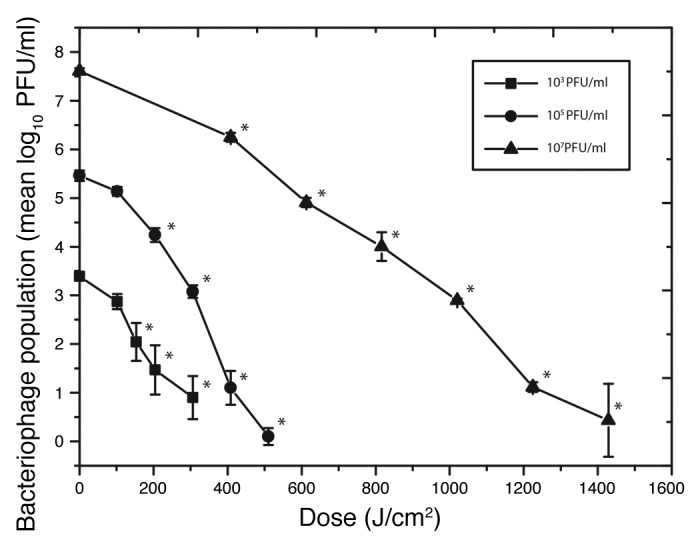
Figure 2. 405 nm light inactivation of bacteriophage ɸC31 suspended in nutrient broth at a range of population densities. The light irradiance used was 56.7 mW/cm2. “*” indicates light-exposed samples that were significantly different to the equivalent non-exposed control samples (P ≤ 0.05). No significant decrease was observed in the final control populations (P ≥ 0.05).
More densely populated ɸC31 suspensions of 105 and 107 PFU/ml were also successfully inactivated by exposure to 405 nm light, with 5.4-log10 and 7.1-log10 reductions observed with applied doses of 510.3 J/cm2 and 1.43 kJ/cm2, respectively. No significant decrease was observed in the non-exposed control populations: P = 0.28, 0.65 and 0.31 for 103, 105 and 107 PFU/ml titers, respectively.
In contrast to the linear inactivation of ɸC31 in NB, very little inactivation occurred when ɸC31 was suspended in PBS. Data in Figure 3 demonstrates that when in PBS, only 0.3-log10 reduction of ɸC31 was achieved after a dose of 306.2 J/cm2. Although this inactivation was statistically significant compared with the non-exposed control population (P = 0.012), it is considerably lower than the 2.7-log10 reduction achieved when ɸC31 was suspended in NB after the same dose of 405 nm light.
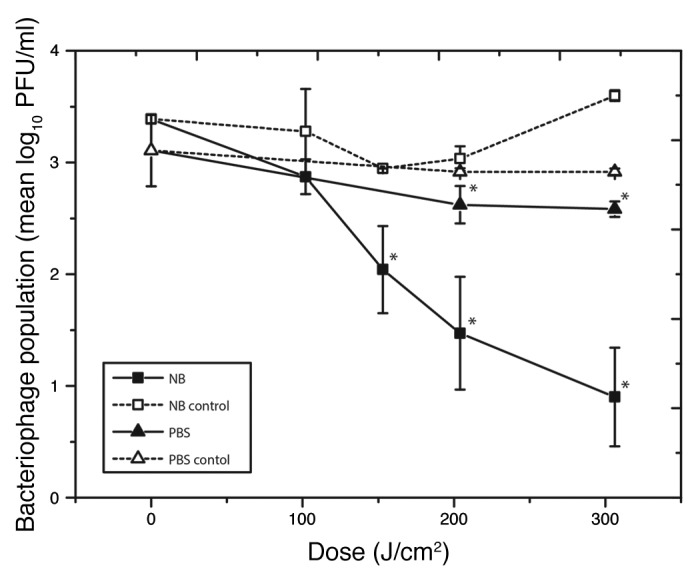
Figure 3. Comparison of inactivation of bacteriophage ɸC31 when suspended in either nutrient broth or phosphate buffer saline, upon exposure to 405 nm light at an irradiance of 56.7mW/cm2, “*” indicates light -exposed samples that were significantly different to equivalent controls (P ≤ 0.05).
The difference in inactivation of ɸC31 when suspended in NB vs. PBS is likely to reflect the complex protein and amino acid rich composition of NB in comparison with the simple salts composition of PBS. It is likely that certain components of NB are photosensitive and can act as exogenous photosensitizers which, when exposed to 405 nm light in the presence of oxygen, will produce ROS or other toxic photoproducts that can impart oxidative damage to the phage. This has been observed in other studies in which media has been irradiated with light and inhibited the growth of bacteria due to presence of ROS such as H2O2.25 This effect was not seen in the PBS solution; presumably due to the lack of photosensitive components, and because of the absence of porphyrin molecules within the phage virion.
This inactivation mechanism is quite distinct from ultraviolet (UV) light mediated damage, which directly targets the DNA/RNA of illuminated phage and virions.26,27 Nucleic acid mutations which result from absorption of UV wavelengths can however be overcome by some bacteriophages, including phage T4, which have been found to carry their own repair genes, including denV for DNA excision repair.28-30 With regards to the present study, further evaluation of the survivors of the 405 nm light-exposed phage population was out-with the scope of the study, however PDI and 405 nm light inactivation of viruses is thought to be due to Type I and Type II photoreactions, resulting in non-specific oxidative damage to structures such as the capsid,31 therefore the potential for resistance development in exposed viruses, or other microorganisms, is unlikely.20,32 However further research in this area is required.
Comparison of the inactivation kinetics for bacteriophage suspended in PBS with those of bacteria and fungi highlight the greater susceptibility of bacteria and fungi compared with the phage. Previous studies detailing the antimicrobial efficacy of 405 nm light against yeast and bacteria including Saccharomyces cerevisiae, Staphylococcus aureus, Escherichia coli, Shigella sonnei and Listeria monocytogenes, demonstrated 5-log10 CFU/ml reductions of PBS-suspended populations with doses ranging from 36 to 300 J/cm2 respectively.5,7,9 Conversely, exposure of ɸC31 suspended in PBS at doses as high as 300 J/cm2 resulted in only a 0.3-log10 reduction in phage titer, highlighting the relative resilience of the phage to 405 nm light. This comparison further demonstrates that without porphyrins, or other photosensitive molecules, little inactivation occurs, indicating they are a necessary requirement for increasing susceptibility of microorganisms to 405 nm light.
Although 405 nm light had a lesser effect on the phage in comparison with other microorganisms it is interesting that some, albeit a low level, of phage inactivation was achieved in exposure experiments. It is possible that this decrease in population is due to general oxidative damage resulting from exposure to the LED emission spectrum. From Figure 1 it is evident that the tail of the spectral output includes a very small amount of UV-A photons (380–390 nm), and over extended exposure periods these wavelengths could have caused slight oxidative damage to proteins, such as those in the phage capsid, thus contributing to the slight inactivation observed at these dose levels.33
To further investigate if photosensitive molecules play a role in the 405 nm light induced ɸC31 inactivation mechanism, porphyrins were added to the PBS bacteriophage suspension, immediately before exposure to 405 nm light. The results in Figure 4 show that the addition of porphyrins increased the susceptibility of ɸC31 suspended in PBS, with a 3-log10 reduction observed after exposure to a dose of 612.4 J/cm2. Results also demonstrate that an equivalent 3-log10 reduction occurred with samples which were incubated for the same period of time in laboratory light, albeit at a significantly slower rate (P = 0.003 at 204.1 J/cm2; P = 0.01 at 408.2 J/cm2), highlighting that broadband laboratory lighting can also induce photo-excitation of porphyrins for phage inactivation; although less efficiently than that found with high irradiance 405 nm light.
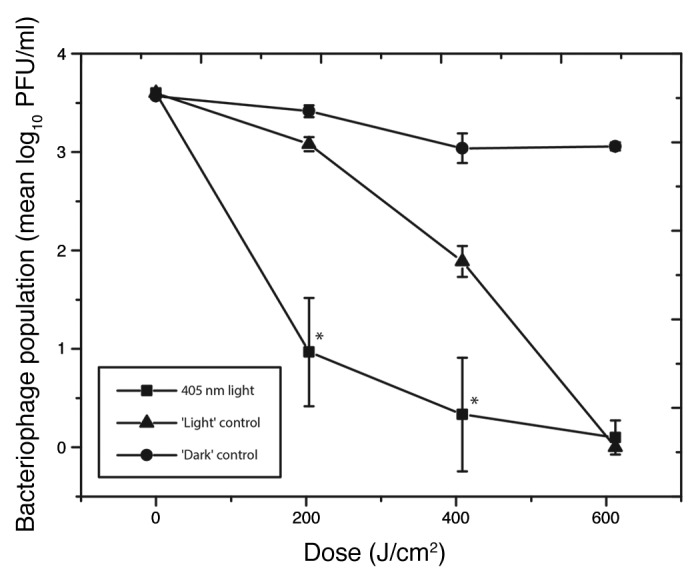
Figure 4. Inactivation of bacteriophage ɸC31 suspended in phosphate buffer saline supplemented with 5 ppm porphyrins upon exposure to 405 nm light, normal laboratory light (‘Light’ control) or complete darkness (‘Dark’ control). “*” indicates 405nm light-exposed samples that were significantly different to light control samples (P ≤ 0.05).
As previously mentioned, the combined use of photosensitive molecules and light to inactivate bacteriophage was established by Clifton11 who described the inactivation of Staphylococcus bacteriophage using methylene blue and sunlight. More recent studies have demonstrated the use of porphyrins and broadband visible light for viral inactivation. Egyeki et al.31 demonstrated that the addition of a tetraphenyl porphyrin derivative (TPFP), to suspensions of the Escherichia coli bacteriophage T7, caused phage inactivation with exposure to broadband visible light between 400–650 nm. As with the current study, the T7 phage used was a non-enveloped double-stranded DNA virus, however there are considerable differences between the structure of these phage, with Siphoviridae ɸC31 having a polyhedral capsid, and long (100 nm) tail, compared with the icosahedral capsid and short (29 nm) tail of Podoviridae T7.34-36 These differences aside, successful inactivation was achieved in both studies. Use of TPFP and broadband visible light achieved up to an approximate 2.6-log10 (-6 ln(N/N0)) reduction in T7 phage population with a dose of 200 J/cm2.31 The efficacy of this PDI treatment was similar to that observed in the current study with ɸC31 exposed to 405 nm light when suspended in both NB and porphyrin solution (2.7-log10 reduction with 306.2 J/cm2, and 2.4-log10 reduction with 204.1 J/cm2, respectively). This data taken with our study suggest that PDI and 405 nm light inactivation of bacteriophages is a universal feature, given the phylogenetic differences between ɸC31 and T7, suggesting that 405 nm light has broad application as an antiviral treatment.
Materials and Methods
Microorganisms
The bacteriophage and bacterium used in this study were ɸC31cΔ25 and Streptomyces coelicolor A3(2) ΔpglW.37,38 To cultivate S. coelicolor spores, the bacterium was spread onto soya flour mannitol agar plates (20 g/l soya flour [Holland & Barrett, UK]; 20 g/l mannitol [Fisher Scientific, UK]; 20 g/l agar bacteriological [Oxoid, UK]) and incubated at 30 °C for 7 d. Spores were harvested by adding 10 ml sterile water to the plates and scraping with an L-shaped spreader. This suspension was centrifuged at 3939 × g and the resultant pellet was re-suspended in 20% (w/v) glycerol (Fisher Scientific, UK). The suspension was stored at −20 °C, and defrosted when required.
To cultivate a stock population of bacteriophage ɸC31, the phage was diluted in nutrient broth (NB [Oxoid, UK]), and 100 µl of each dilution was pipetted onto enriched nutrient agar (28 g/l nutrient agar [Oxoid, UK]; 0.5% glucose, 10 mM magnesium sulfate (MgSO4), 8 mM calcium nitrate (Ca(NO3)2) [Fisher Scientific, UK]). A thin layer of molten soft agar (13 g/l NB; 0.3% agar bacteriological; 0.5% glucose; 10 mM MgSO4; 8 mM Ca(NO3)2) containing 0.1% S. coelicolor spores was poured onto the plates and swirled to ensure even distribution of ɸC31 across the plate. Plates were incubated at 28 °C overnight and the resultant plaques enumerated. To create a high-titer bacteriophage stock suspension, 10 ml NB was added to the plates belonging to the first dilution to cause complete bacterial clearance and was left for 3 h. The 10 ml liquid was then removed and filtered using a 0.45 µm filter and the resultant phage suspension was stored at 4 °C for experimental use according to the method by Kieser et al.39
Experimental Arrangement
A 99-DIE 405 nm light-emitting diode (LED) array (OptoDiode Corp, USA) was used for bacteriophage exposure. The LED array had maximal output at approximately 405 nm, and a bandwidth of approximately 14 nm (Fig. 1). The LED array was bonded to a heatsink and fan for thermal management, ensuring samples were not overheated. The LED array system was mounted on a polyvinylchloride housing designed to fit onto a 12-well microplate with the lid removed, with the array positioned directly above a single sample well. The array was powered by a DC supply (1.5 ± 0.05 A and 13.1 ± 0.1 V).
For light exposure, phage were diluted to the appropriate starting population in NB. One-ml samples were held in the well of a 12-well microplate, with a depth of 4 mm, and the LED housing placed above. The plate was placed on a 1 cm high stand to allow adequate air flow below the sample plate during light exposure. The distance between the sample surface and LED array was approximately 2 cm, and at this distance, a constant irradiance of 56.7 mW/cm2 was maintained.
ɸC31 populations of 103, 105 and 107 PFU/ml were exposed to increasing doses of 405 nm light. Control samples were also held under identical conditions but exposed to normal laboratory lighting conditions. Post exposure, the number of active phage particles was quantified using the double-agar layer method,40 with samples (100, 200 and 500 µl volumes) pipetted onto nutrient agar plates, and soft agar containing 0.1% S. coelicolor spores thinly poured on top. The plates were left to set and then co-incubated overnight at 28 °C. Post-incubation, the surviving ɸC31 were enumerated and results expressed as plaque-forming units per milliliter (PFU/ml). Exposures of 103 PFU/ml phage populations were also repeated with ɸC31 suspended in phosphate buffer saline (PBS [Oxoid, UK]), and PBS supplemented with 5 ppm meso-Tetra (N-methyl-4-pyridyl) porphine tetra tosylate (Frontier Science, USA). For this, stock bacteriophage was serially diluted to the desired concentration in PBS, with the final dilution being into either PBS or porphyrin-supplemented PBS, respectively.
Inactivation results are reported as bacteriophage population (log10 PFU/ml) as a function of dose, J/cm2 (irradiance × exposure time), and are presented as mean values from a minimum of triplicate samples ± standard deviations. Significant differences in phage population were calculated at the 95% confidence interval using analysis of variance (one-way) with Minitab, version 16, statistical software.
Conclusion
The focus of the present study was to establish whether 405 nm light can induce virucidal effects, with the bacteriophage ɸC31 being used as a model virus. The results provide the first evidence of the susceptibility of a bacteriophage to inactivation by narrowband 405 nm light and the influence that the suspending media has on phage susceptibility. These findings are of interest as they highlight that bacteriophage and possibly other viruses can be inactivated by 405 nm light if they are suspended in liquids or substrates that contain appropriate photosensitive components. Further studies are needed to elucidate the nature of the photosensitive components in the nutrient media (NB) that are activated by high-intensity 405 nm light. Additional information of this kind could help to elucidate the environmental and chemical conditions that would be most conducive to viral inactivation when exposed to high intensity 405 nm light.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Karen McKenzie for technical support, and The University of Strathclyde for funding support.
Glossary
Abbreviations:
- PDI
photodynamic inactivation
- ROS
reactive oxygen species
- NB
nutrient broth
- PBS
phosphate buffered saline
- LED
light-emitting diode
- PFU
plaque forming units
- UV
ultraviolet
References
- 1.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–7. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guffey JS, Wilborn J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed Laser Surg. 2006;24:684–8. doi: 10.1089/pho.2006.24.684. [DOI] [PubMed] [Google Scholar]
- 3.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol Lett. 2008;285:227–32. doi: 10.1111/j.1574-6968.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- 4.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2008;40:734–7. doi: 10.1002/lsm.20724. [DOI] [PubMed] [Google Scholar]
- 5.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol. 2009;75:1932–7. doi: 10.1128/AEM.01892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdoch LE, Maclean M, MacGregor SJ, Anderson JG. Inactivation of Campylobacter jejuni by exposure to high-intensity 405-nm visible light. Foodborne Pathog Dis. 2010;7:1211–6. doi: 10.1089/fpd.2010.0561. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. ScientificWorldJournal. 2012;2012:137805. doi: 10.1100/2012/137805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maclean M, Murdoch LE, MacGregor SJ, Anderson JG. Sporicidal effects of high-intensity 405 nm visible light on endospore-forming bacteria. Photochem Photobiol. 2013;89:120–6. doi: 10.1111/j.1751-1097.2012.01202.x. [DOI] [PubMed] [Google Scholar]
- 9.Murdoch LE, McKenzie K, Maclean M, Macgregor SJ, Anderson JG. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biol. 2013;117:519–27. doi: 10.1016/j.funbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Yin R, Dai T, Avci P, Jorge AE, de Melo WC, Vecchio D, Huang Y-Y, Gupta A, Hamblin MR. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol. 2013;13:731–62. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifton CE. Photodynamic action of certain dyes on the inactivation of Staphylococcus bacteriophage. Exp Biol Med. 1931;28:745–6. doi: 10.3181/00379727-28-5512. [DOI] [Google Scholar]
- 12.Schagen FHE, Moor ACE, Cheong SC, Cramer SJ, van Ormondt H, van der Eb AJ, Dubbelman TMAR, Hoeben RC. Photodynamic treatment of adenoviral vectors with visible light: an easy and convenient method for viral inactivation. Gene Ther. 1999;6:873–81. doi: 10.1038/sj.gt.3300897. [DOI] [PubMed] [Google Scholar]
- 13.Banfi S, Caruso E, Buccafurni L, Battini V, Zazzaron S, Barbieri P, Orlandi V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: an in vitro study on Gram negative and Gram positive bacteria. J Photochem Photobiol B. 2006;85:28–38. doi: 10.1016/j.jphotobiol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Costa L, Faustino MAF, Neves MGPMS, Cunha A, Almeida A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses. 2012;4:1034–74. doi: 10.3390/v4071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa L, Carvalho CMB, Faustino MAF, Neves MGPMS, Tomé JPC, Tomé AC, Cavaleiro JAS, Cunha A, Almeida A. Sewage bacteriophage inactivation by cationic porphyrins: influence of light parameters. Photochem Photobiol Sci. 2010;9:1126–33. doi: 10.1039/c0pp00051e. [DOI] [PubMed] [Google Scholar]
- 16.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B. 2008;92:180–4. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, Redl H, van Griensven M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42:917–21. doi: 10.1016/j.injury.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 19.McDonald R, Macgregor SJ, Anderson JG, Maclean M, Grant MH. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: an in vitro model of wound healing. J Biomed Opt. 2011;16:048003. doi: 10.1117/1.3561903. [DOI] [PubMed] [Google Scholar]
- 20.Dai T, Gupta A, Huang Y-Y, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP, Hamblin MR. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013;57:1238–45. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclean M, Macgregor SJ, Anderson JG, Woolsey GA, Coia JE, Hamilton K, Taggart I, Watson SB, Thakker B, Gettinby G. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J Hosp Infect. 2010;76:247–51. doi: 10.1016/j.jhin.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Maclean M, Booth MG, Anderson JG, MacGregor SJ, Woolsey GA, Coia JE, Hamilton K, Gettinby G. Continuous decontamination of an intensive care isolation room during patient occupancy using 405 nm light technology. J Infect Prevent. 2013;14:176–81. doi: 10.1177/1757177413483646. [DOI] [Google Scholar]
- 23.Bache SE, Maclean M, MacGregor SJ, Anderson JG, Gettinby G, Coia JE, Taggart I. Clinical studies of the High-Intensity Narrow-Spectrum light Environmental Decontamination System (HINS-light EDS), for continuous disinfection in the burn unit inpatient and outpatient settings. Burns. 2012;38:69–76. doi: 10.1016/j.burns.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Mc Grath S, van Sinderen D. Bacteriophage: Genetics and Molecular Biology. Norfolk, UK: Caister Academic Press; 2007. Pages 71-72, 95-97 [Google Scholar]
- 25.Waterworth PM. The action of light on culture media. J Clin Pathol. 1969;22:273–7. doi: 10.1136/jcp.22.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merriam V, Gordon MP. Pyrimidine dimer formation in ultraviolet irradiated TMV-RNA. Photochem Photobiol. 1967;6:309–19. doi: 10.1111/j.1751-1097.1967.tb08879.x. [DOI] [PubMed] [Google Scholar]
- 27.Yasui A, McCready SJ. Alternative repair pathways for UV-induced DNA damage. Bioessays. 1998;20:291–7. doi: 10.1002/(SICI)1521-1878(199804)20:4<291::AID-BIES5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Valerie K, Henderson EE, de Riel JK. Expression of a cloned denV gene of bacteriophage T4 in Escherichia coli. Proc Natl Acad Sci U S A. 1985;82:4763–7. doi: 10.1073/pnas.82.14.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recinos A, 3rd, Augustine ML, Higgins KM, Lloyd RS. Expression of the bacteriophage T4 denV structural gene in Escherichia coli. J Bacteriol. 1986;168:1014–8. doi: 10.1128/jb.168.2.1014-1018.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banga SS, Boyd JB, Valerie K, Harris PV, Kurz EM, de Riel JK. denV gene of bacteriophage T4 restores DNA excision repair to mei-9 and mus201 mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1989;86:3227–31. doi: 10.1073/pnas.86.9.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egyeki M, Turóczy G, Majer Z, Tóth K, Fekete A, Maillard P, Csík G. Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: efficiency and mechanism of action. Biochim Biophys Acta. 2003;1624:115–24. doi: 10.1016/j.bbagen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Donnelly RF, McCarron PA, Tunney MM. Antifungal photodynamic therapy. Microbiol Res. 2008;163:1–12. doi: 10.1016/j.micres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Girard PM, Francesconi S, Pozzebon M, Graindorge D, Rochette P, Drouin R, Sage E. UVA-induced damage to DNA and Proteins: direct versus indirect photochemical processes. J Phys Conf Ser. 2011;261:1–10. doi: 10.1088/1742-6596/261/1/012002. [DOI] [Google Scholar]
- 34.Suárez JE, Caso JL, Rodriguez A, Hardisson C. Structural characteristics of the Streptomyces bacteriophage ɸC31. FEMS Microbiol Lett. 1984;22:113–7. [Google Scholar]
- 35.Ackermann H-W. 5500 Phages examined in the electron microscope. Arch Virol. 2007;152:227–43. doi: 10.1007/s00705-006-0849-1. [DOI] [PubMed] [Google Scholar]
- 36.Cuervo A, Pulido-Cid M, Chagoyen M, Arranz R, González-García VA, Garcia-Doval C, Castón JR, Valpuesta JM, van Raaij MJ, Martín-Benito J, et al. Structural characterization of the bacteriophage T7 tail machinery. J Biol Chem. 2013;288:26290–9. doi: 10.1074/jbc.M113.491209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinclair RB, Bibb MJ. The repressor gene (c) of the Streptomyces temperate phage phi c31: nucleotide sequence, analysis and functional cloning. Mol Gen Genet. 1988;213:269–77. doi: 10.1007/BF00339591. [DOI] [PubMed] [Google Scholar]
- 38.Khodakaramian G, Lissenden S, Gust B, Moir L, Hoskisson PA, Chater KF, Smith MCM. Expression of Cre recombinase during transient phage infection permits efficient marker removal in Streptomyces. Nucleic Acids Res. 2006;34:e20. doi: 10.1093/nar/gnj019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces genetics. Norfolk, UK: John Innes Foundation; 2000. [Google Scholar]
- 40.Davis LG, Didner MD, Battey JF. Basic Methods in Molecular Biology. New York, USA: Elsevier; 1986, Pages 333–335 [Google Scholar]


