Abstract
The sense of limb position is crucial for movement control and environmental interactions. Our understanding of this fundamental proprioceptive process, however, is limited. For example, little is known about the accuracy of arm proprioception: Does it vary with changes in arm configuration, since some peripheral receptors are engaged only when joints move toward extreme angles? Are these variations consistent across different tasks? Does proprioceptive ability change depending on what we try to localize (e.g., fingertip position vs. elbow angle)? We used a robot exoskeleton to study proprioception in 14 arm configurations across three tasks, asking healthy subjects to 1) match a pointer to elbow angles after passive movements, 2) match a pointer to fingertip positions after passive movements, and 3) actively match their elbow angle to a pointer. Across all three tasks, subjects overestimated more extreme joint positions; this may be due to peripheral sensory signals biasing estimates as a safety mechanism to prevent injury. We also found that elbow angle estimates were more precise when used to judge fingertip position versus directly reported, suggesting that the brain has better access to limb endpoint position than joint angles. Finally, precision of elbow angle estimates improved in active versus passive movements, corroborating work showing that efference copies of motor commands and alpha-gamma motor neuron coactivation contribute to proprioceptive estimates. In sum, we have uncovered fundamental aspects of normal proprioceptive processing, demonstrating not only predictable biases that are dependent on joint configuration and independent of task but also improved precision when integrating information across joints.
INTRODUCTION
Proprioception—the sense of position and movement of the parts of the body in the absence of vision—plays a crucial role in daily life, contributing to motor skills and the general ability to successfully interact with the environment. It is largely agreed that muscle spindles are the major contributors to proprioception (see Gandevia et al. 1992; Matthews 1988); these receptors increase and decrease activity as a muscle stretches and contracts, respectively, thus providing signals relating to muscle length and velocity. Studies have shown that muscle spindles have preferred sensory directions and weighting individual responses based on each spindle's preferred direction results in a population code that accurately represents a limb's movement direction (Bergenheim et al. 2000; Jones et al. 2001; Roll et al. 2000, 2004) and static position (Ribot-Ciscar et al. 2003). Other studies, however, have implicated a role of joint receptors (Ferrell et al. 1987; Macefield et al. 1990) and cutaneous receptors (Collins and Prochazka 1996; Collins et al. 2005; Edin and Johansson 1995) in proprioception; how signals from these additional receptors influence proprioception remains unclear.
A number of behavioral studies have explored proprioceptive position estimates. Studies have tested fingertip position sense across limited areas of Cartesian space (Crowe et al. 1987; van Beers et al. 1996, 1998, 1999) and elbow angle sense across limited ranges of joint space (Darling 1991; Gritsenko et al. 2007; Soechting 1982; Zia et al. 2002). Variations in these estimates, however, have not been systematically explored across joint space. Since joint angles alter the activity of peripheral sensory signals, exploring variations across joint space rather than Cartesian space may provide fundamental insight into proprioceptive processing. Moreover, although it has been hypothesized that elbow angle sense should be more precise in tasks in which elbow angle estimates are integrated into the more behaviorally relevant estimates of fingertip position (van Beers et al. 1998), this hypothesis has never been directly tested or compared with hypotheses involving efferent information in addition to afferent information. Comparing the accuracy and precision of proprioceptive position estimates across tasks can provide unique insight into proprioceptive processing.
Here we tested whether there are any patterns in the accuracy and precision of position sense across limb configurations and, if so, whether these patterns are consistent across different tasks. Because responses of different sensory signals vary depending on joint angles, we hypothesized that these signals would differentially influence position sense depending on how the arm is configured. We also tested whether the accuracy and precision of position estimates vary depending on whether a subject tries to estimate joint angle versus estimate the endpoint position of the limb and whether the limb is moved actively versus passively. Based on evidence suggesting that the CNS primarily represents limb position in terms of endpoint position (Kalaska et al. 1990; Prud'homme and Kalaska 1994; Tillery et al. 1996), we hypothesized that behavioral estimates of fingertip position would produce more precise elbow angle estimate than would direct estimates of elbow angle.
METHODS
Subjects
We tested ten right-handed adults (six female, four male; ages 18–30 yr, mean 23.6). Two individuals were unable to be tested in Task 3, leaving eight participants in this task. Each subject gave written informed consent. All subjects were neurologically healthy and had normal or corrected-to-normal vision. Protocols were approved by the Johns Hopkins Institutional Review Board.
Procedure
Experiments were performed on separate days using the KINARM (Kinesiological Instrument for Normal and Altered Reaching Movement, BKIN Technologies, Kingston, Ontario, Canada), an exoskeletal robotic arm that allows for individual application of torques to the elbow and shoulder joints. For all tasks, subjects sat with their right arm in the KINARM, and the robot was calibrated so that the right arm moved in a shoulder-level horizontal plane. The left arm remained out of the robot. Using a reflected rear-projection system, subjects viewed images that appeared to be in the same plane as their right arm, and during test blocks vision of their right arm was blocked by a screen (Fig. 1A). Throughout each experiment subjects saw a dot projected over their elbow joint and a line over their upper arm. At the start of each trial the elbow dot and upper arm line were red, indicating that the KINARM was moving the subject's right arm into a new configuration (movements were made along a bell-shaped trajectory profile with a maximum fingertip velocity of 0.5 m/s). Once in position for that trial, the KINARM held the elbow and shoulder in place (only the shoulder in Task 3). The elbow dot and upper arm line then turned green, and an additional element appeared on the screen depending on the task (Fig. 1B).
Fig. 1.

Setup and tasks. A: positioning in the KINARM. Subjects looked down into a horizontal mirror at chin level and saw visual elements reflected from a horizontal rear-projection screen above the mirror. Not shown: in all tasks subjects had electromyographs on their right arm, and for the passive elbow angle task and the passive fingertip task subjects held a joystick in their left hand. B: in all tasks subjects' arms were blocked from view with a screen, and only the white elements were visible on the display. In Task 1 subjects used a joystick to rotate a line until it matched the orientation of their forearm. In Task 2 subjects moved a dot until it matched the position of their fingertip. In Task 3 subjects rotated their forearm to match the orientation of a line.
Task 1: passive elbow angle matching.
A line was projected out of the elbow dot at a random angle at least ±15° from the forearm but no farther than ±30°. Subjects used a joystick in their left hand to rotate the second line about the elbow dot until they perceived that it was aligned over their forearm, with the angle of the upper arm line and the rotated line matching their elbow angle. Trials were all timed at 12 s so that if subjects finished early they were instructed to wait. At completion of the 12 s, the rotating line disappeared and the elbow dot and upper arm line again turned red as the arm was passively moved into the next trial's configuration. In all, 15 arm configurations were tested: shoulder angles of 60, 75, and 90° each paired with elbow angles of 30, 45, 60, 75, and 90° (see Fig. 2 for angle definitions). Each configuration was presented twice in a pseudorandom order within a block. Subjects completed four blocks of 30 test trials, for a total of 8 test trials per arm configuration.
Fig. 2.
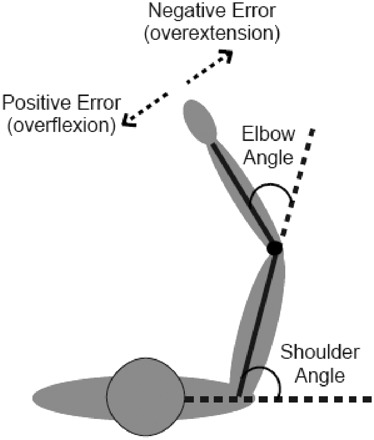
Angle definitions.
Task 2: passive fingertip matching.
A dot appeared at a random position between 8.5 and 17 cm from the subject's right index finger. Subjects used a joystick in their left hand (in a fashion similar to that in Task 1) to move the dot until they perceived that it was positioned over their index fingertip. Trials were timed at 18 s, which was slightly longer than the Task 1 trials since the response decision involved two dimensions (i.e., x and y positions of the fingertip) rather than one (i.e., elbow angle). At completion of the 18 s, the fingertip dot disappeared and the elbow dot and upper arm line again turned red as the arm was passively moved into the next trial's configuration. The same 15 arm configurations were tested and presented in the same order within each block as in Task 1. Due to the increased length of trials, subjects completed three rather than four blocks of 30 test trials (for a total of 6 test trials per arm configuration) to keep the total time of the experimental session comparable to that of the other two tasks.
Task 3: active elbow angle matching.
A line was projected out of the elbow dot as in Task 1, but instead of rotating the line to match the location of their fixed forearm subjects rotated their forearm about the elbow to match the location of the fixed line. Trials were set to 10 s each. The same 15 configurations were presented as were used in the passive tasks, and both the presentation order and the experimental protocol were the same as those in Task 1 (i.e., four blocks of 30 trials without vision of the right arm).
The order of Tasks 1 and 2 was randomized across subjects, but all subjects performed Task 3 last. This design ensured that subjects' responses in Tasks 1 and 2 were not influenced by remembered images from Task 3.
Control trials.
Each experiment ended with one block of 30 trials in which subjects could see their arm (i.e., two control trials per configuration). This final block with vision was performed as a control for potential errors not due to proprioception.
EMG recordings.
Across all three tasks, electromyographic (EMG) recordings were collected from five muscles in the right arm: brachialis, biceps brachii, triceps brachii, posterior deltoid, and pectoralis major. Amplifier gains were set to 10,000, and signals were sampled at a rate of 1,000 Hz. During Tasks 1 and 2, in which subjects were instructed to keep their right arms passive throughout testing, EMG signals were monitored on-line to ensure that no muscle activity could contribute to proprioceptive estimates. Trials in which activity was noted were discarded from later analyses.
Analysis
For all three tasks, the average error for each configuration from the two trials with vision was taken as a measure of error not due to proprioception. This error was subtracted from each test trial's error, and analyses were conducted on these purely proprioceptive errors.
Task 1.
For each trial, the final angle of the rotated line relative to the upper arm line was taken as measurement of a subject's perceived angle of his/her elbow. This perceived angle was compared with the actual elbow angle for each trial for a measure of accuracy, and the SD of the perceived angle was used as a measure of precision. For each subject an average elbow angle error was calculated for each of the 15 arm configurations.
Task 2.
The final x and y positions of the fingertip dot on each trial were taken as measurement of a subject's perceived position of his/her fingertip. This perceived position was compared with the actual fingertip position for each trial. We then calculated the corresponding elbow angle error, forearm length error, and absolute endpoint error based on the known length of the forearm. Because the fingertip was at the edge of the visual display for arm configuration shoulder 90°–elbow 90°, the fingertip dot frequently went off the screen when subjects were responding; this configuration was therefore dropped from this task, leaving 14 configurations.
Task 3.
As in Task 1, the angular difference between the displayed forearm line and the actual elbow angle (with the same sign convention as that of Task 1) provided a measure of accuracy for each trial, whereas the SD of the perceived angle provided a measure of precision. Because the target forearm line was along the edge of the visual display for arm configuration shoulder 90°–elbow 90°, subjects frequently rotated their arms off the screen; this configuration was therefore dropped for this task, leaving 14 configurations.
Stepwise regression was used for each subject performing each task to assess which variables best accounted for variability in average elbow angle errors (i.e., accuracy) and SD of elbow angle estimates (i.e., precision). Included variables were actual elbow angle, shoulder angle, change in elbow angle between the previous trial and the current trial, and distance from fingertip to shoulder. Stepwise regression with the same predictors was also performed for individual subjects for absolute endpoint errors on Task 2. Repeated-measures ANOVA (3 tasks × 3 shoulder angles × 4 elbow angles) was performed on group data for average errors and SD.
RESULTS
In the passive elbow angle task, the accuracy of one of the ten subjects correlated positively with time (i.e., performance degraded over time). This correlation, however, was small (r = 0.23) and performance in this subject was not related to time in the other two tasks. No other subjects (in any task) showed significantly degraded performance over time. We therefore included all subjects in all tasks since we are confident that degrading sensory signals and waning attention did not affect performance.
Elbow angle accuracy
Across tasks.
We found no difference in elbow angle accuracy across the three tasks—in all tasks average group accuracy was <2° (Fig. 3A). A repeated-measures ANOVA for elbow angle estimate error (3 tasks × 3 shoulder angles × 4 elbow angles) found no significant effect of task (P = 0.66; passive elbow angle task average error: −0.63 ± 1.85° SE; passive fingertip task: 1.81 ± 2.61°; active elbow angle task: 0.54 ± 3.39°) or shoulder angle (P = 0.09). The repeated-measures ANOVA, however, did find a significant effect of actual elbow angle (P < 0.01). This suggests that subjects' current elbow angles influence their accuracy, which was further investigated for each task.
Fig. 3.
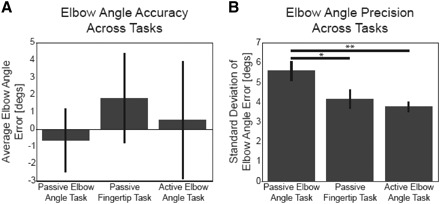
Group performance across tasks. A: there were no differences in group accuracy across tasks. B: compared with the passive elbow task (Task 1), subjects were slightly more precise at identifying the angle of their elbow in the fingertip position task (Task 2, *P = 0.046, planned comparison) and strongly more precise in the active elbow task (Task 3, **P = 0.004). Error bars represent SEs.
Within tasks.
Within each task a pattern in accuracy was observed across joint space: as elbow angles approached the limits of their range, estimates were biased toward the respective limit. An example of this behavior is demonstrated in Fig. 4A, which shows an individual subject's responses to two extreme elbow angles in the passive elbow angle task. Figure 4B shows average group accuracy for each configuration. Subjects were most accurate at intermediate elbow angles (60°). As the elbow became more extended (30–45°) estimates were biased toward being overly extended, and as the elbow became more flexed (75–90°) estimates were biased toward being overly flexed. Interpolating between the tested arm configurations, the heat plots in Fig. 4C demonstrate the global pattern in biases across joint space: biases strongly depend on elbow angle but not shoulder angle or task [there was a significant relationship between elbow angle estimates and shoulder angle for only the 60 vs. 90° comparison (post hoc Fisher's least significant difference, P = 0.03; see Fig. 4B)].
Fig. 4.
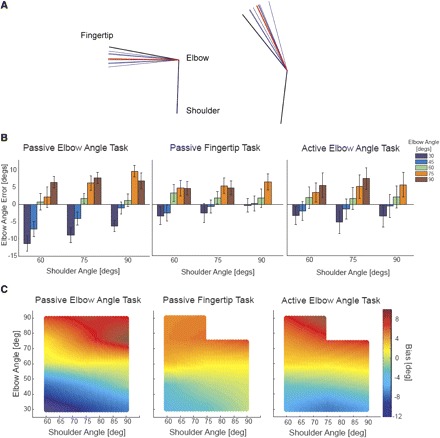
Accuracy across joint space. A: raw traces from a subject in Task 1. Black lines represent average actual arm position (for the particular configuration), blue lines represent perceived positions on individual trials, and red lines represent average perceived position. When the elbow was flexed (left), the subject perceived the angle as even more flexed, and when the elbow was extended (right), the subject perceived the angle as even more extended. B: the bar graphs show group errors for each arm configuration tested, for each task (error bars represent SEs). Consistent across shoulder angles, elbow angle estimates were overextended as the elbow became more extended and overflexed as the elbow became more flexed. This pattern was consistent across the 3 tasks. C: the heat plots show average group errors across joint space interpolated from the tested configurations. Hotter colors represent flexion errors and colder colors represent extension errors. Elbow estimate biases are dependent on elbow angle (i.e., colors change along the y-axis) but not shoulder angle or task.
For each subject four variables were entered as predictors of elbow angle estimate errors in stepwise multiple regressions for each task: actual elbow angle, shoulder angle, change in elbow angle between previous and current trials (i.e., movement amount and direction), and distance from fingertip to shoulder. Across-subject averages for each task reveal that these variables were significant predictors of elbow angle estimate error (passive elbow angle task average: R2 = 0.53; passive fingertip task: R2 = 0.39; active elbow angle task: R2 = 0.36). In all tasks, actual elbow angle was the best predictor of errors: across tasks it was the strongest predictor of error in the regression for 16/28 subjects and a significant contributor to the regression for 20/28 subjects. No other variable contributed as strongly or as frequently to the regression (shoulder angle: strongest predictor for 1/28 subjects and a significant contributor for 9/28 subjects; change in elbow angle: strongest predictor for 4/28 and a significant contributor for 11/28; distance from fingertip to shoulder: strongest predictor for 6/28 and a significant contributor for 9/28). Of the tested variables, actual elbow angle has the strongest influence on the accuracy of elbow angle estimates, with estimates biased toward the extremes.
Elbow angle precision
Across tasks.
On average, subjects were able to precisely identify their elbow angle: across-task precision averages were <5.5° (Fig. 3B). Although our direct elbow angle matching tasks differ from previous elbow angle tasks, which had subjects match their two elbow angles, our precision values are within the range previously reported (3 to 9°, single elbow values derived by dividing two-elbow estimates by the square root of two) (Clark et al. 1995; Darling 1991; Soechting 1982; Soechting and Ross 1984).
Our data show, however, that precision depends on task; a repeated-measures ANOVA for SD of elbow angle estimates (3 tasks × 3 shoulder angles × 4 elbow angles) found a significant effect of task (P = 0.01). Figure 3B shows that subjects were the least precise at identifying their elbow angle when their arms were moved passively and they reported their angle with the visual display (passive elbow angle task). Subjects were more precise at identifying elbow angle when their arms were passively moved into configurations but they were required to identify their fingertip position (passive fingertip task) or actively rotate their forearms about the elbow to match a particularly displayed angle (active elbow angle task). Averaging over all arm configurations, the group SD of elbow angle estimates in the passive elbow angle task was 5.61 ± 0.49° SE, which is higher than that in the passive fingertip task (average 4.19 ± 0.48°; planned comparison P = 0.046) and the active elbow angle task (average 3.79 ± 0.26°; planned comparison P = 0.004). There was no significant difference between group SDs of elbow angle estimates in the passive fingertip task and the active elbow angle task (planned comparison test P = 0.51). Across our three conditions, elbow angle precision was worst in the passive elbow angle task.
Within tasks.
There were no significant within-task differences in precision across joint space (repeated-measures ANOVA: effect of shoulder angle; P = 0.32, effect of elbow angle, P = 0.86). For each subject, actual elbow angle, shoulder angle, prior movement direction and amount, and distance from fingertip to shoulder were tested as predictors of elbow angle judgment precision. No parameters significantly predicted precision of elbow angle estimates in any task. On average, explained variance was low (passive elbow angle task average: R2 = 0.07; passive fingertip task: R2 = 0.11; and active elbow angle task: R2 = 0.16). Although we cannot definitively rule out the possibility that an effect was missed due to a low number of repetitions per configuration, given the low average explained variance it appears unlikely that elbow angle precision is dependent on arm configuration.
Fingertip task
Endpoint errors.
Task 2 was the only task that involved localizing the fingertip. Subjects' average absolute distance between their perceived fingertip endpoints and the actual positions of their index fingertip (i.e., fingertip accuracy) ranged from 3.3 to 12.3 cm, with a group average of 8.0 ± 1.0 cm SE. Subjects' average SD of perceived endpoint positions (i.e., precision) ranged from 1.3 to 3.9 cm, with a group average of 2.5 ± 0.2 cm SE.
To test whether subjects were worse at locating their fingertip when their hand was farther from them, errors for each arm configuration were normalized by arm length and collapsed across subjects. There was no significant relationship between endpoint precision and distance from fingertip to head (r = 0.14, P = 0.10) or fingertip to shoulder (r = 0.08, P = 0.35). There was a small but significant relationship between endpoint accuracy and distance from fingertip to head (r = 0.14, P < 0.01) and fingertip to shoulder (r = 0.10, P = 0.03). Accuracy, but not precision, of fingertip localization may depend on how far the finger is from the body.
Each subject significantly underestimated the length of his/her forearm across arm configurations (P values of t-tests for each subject were all <0.01). Length errors were normalized by each subject's elbow-to-fingertip length and then averaged across the group; on average, subjects underestimated their forearm length by 11.4% of the total distance from the elbow to index fingertip (P < 0.01).
DISCUSSION
A number of factors influence one's sense of limb position. Here we found that elbow angle estimates are more precise when integrated into estimates of fingertip position or based on both sensory and motor information. These results imply that the CNS integrates different information in different tasks to estimate limb position. Across all tasks, we found that elbow angle estimates are biased toward extreme positions, suggesting that peripheral signals affect certain aspects of proprioception systematically across tasks.
Elbow angle estimates are biased toward extremes across tasks
Across our three tasks we found that position estimates of elbow angle were biased toward the elbow's extremes. There are several possible explanations for this finding. First, forward models, which predict the next state of a limb given the current state and a motor command (Davidson and Wolpert 2005), could bias position estimates based on movement direction—e.g., if the elbow is moved from extension to flexion, estimates may be biased in the direction of continued movement (Wolpert et al. 1995). We found biases, however, in both of our passive tasks, and even within the active task the direction of the preceding movement was not a strong predictor of elbow angle error in seven of eight subjects. Thus forward models cannot explain the across-task biases in elbow angle estimates.
A second potential explanation of our observed biases could involve the state of muscle spindles. Within intrafusal fibers in spindles, residual cross bridges between the actin and myosin after muscle contraction can disproportionally increase spindle activity when the muscle is subsequently stretched; this activity can lead to overestimations of position toward extremes (Ansems et al. 2006; Gregory et al. 1988; Proske et al. 1993). However, this hypothesis is unable to account for our results because residual spindle activity is observed only after held muscle contractions and in our passive tasks muscles remained inactive throughout the 0.5-h testing session. Furthermore, change in position between trials was not a strong predictor of elbow angle error, which demonstrates that subjects' errors were not influenced by residual activity related to their previous arm configuration.
A third possibility is that proprioceptive estimates are biased toward frequently held positions as reported by Gritsenko et al. (2007), who proposed that the CNS uses a Bayesian inference process to estimate elbow position, taking afferent and efferent input to form a current estimate and combining this estimate with a “kinesthetic prior” based on experienced states, which are predominantly central positions. This hypothesis could account for their results in conditions under which elbow estimates were made during movements. Yet, when subjects reported stationary postures after active movements, as they did in our active elbow angle task, elbow angle estimates were not biased toward central position. Instead they found biases at static flexed postures similar to ours, but no biases in extension toward overextension. We speculate that during static postures afferent signals are more stable than when they are sampled during movements, which may result in perception of static position being more greatly influenced by peripheral sensory signals than priors.
We propose that our results are best explained on a peripheral level by the proprioceptive contributions of joint and cutaneous receptors. Joint receptors increase activity as joint angles approach extremes (Burgess and Clark 1969; Burke et al. 1988; Clark and Burgess 1975). Studies have found that when muscle spindles can be activated, proprioception at the knee and finger joints is largely unaffected by anesthetizing joint receptors (Clark et al. 1979, 1985, 1986), although these studies examined the detection of only very small movements and differences in position within midrange joint angles. The effects of blocking joint receptors when examining proprioception closer to extreme angles remain unknown. Moreover, activity in single-joint afferents can lead to the perception of movement, whereas for muscle spindles to affect perception there must be population activity (Macefield et al. 1990). This suggests that when joint receptors are active—mainly toward extreme angles—they have the potential to strongly influence proprioceptive perception. Our data suggest that as joints approach extremes, joint receptors may bias perception of joint angle.
Cutaneous receptors also play a role in proprioception. Mechanoreceptors in the hairy nonglabrous skin of the hand (Edin 1992; Edin and Abbs 1991), ankle (Aimonetti et al. 2007), and thigh (Edin 2001) are activated by movements at nearby joints that result in skin stretch. Cells in the primary somatosensory cortex of monkeys discharge in meaningful patterns, depending on arm movement direction and posture, and the sensitivity of these cortical cells to skin stretch is correlated with how well they encode position information (Cohen et al. 1994). Experiments also show that different skin stretch patterns affect the perception of finger, elbow, and knee position and movement (Collins and Prochazka 1996; Collins et al. 2005; Edin and Johansson 1995). Cutaneous afferent activity shows a predominantly linear relationship to skin stretch (Edin 1992, 2001; Edin and Abbs 1991), suggesting that for many cutaneous receptors activity increases as a joint approaches an extreme position (i.e., where the skin is most stretched).
By biasing the perception of joint angles toward extremes, these signals could help prevent movements beyond a joint's range of motion. It follows from this hypothesis that subjects would sense their elbow as more extended when their shoulder is abducted versus adducted: as the arm moves farther away from the central visual workspace it becomes harder to judge elbow angle extensions. In other words, there is a greater risk of misperceiving elbow angle when the shoulder is abducted, and thus at these shoulder angles the system should protectively bias estimates even more to prevent dangerous extensions. This could explain the only elbow angle bias we saw that depended on shoulder angle: for extended elbow angles, subjects perceived their elbow as significantly more extended when the shoulder was configured at 60 versus 90° (Fig. 4B).
If these biases indeed reflect a protective mechanism, their strength in these experiments may appear surprising considering that the most extended elbow angle tested was 30° and the most flexed was 90°. However, under natural conditions individuals do not perceive their limbs to be in configurations outside a joint's range of motion. In other words, unless illusory effects are induced (Craske 1977), we do not perceive our elbow as flexed to the point of hitting us or extended to a negative angle. The minimum and maximum angles tested in our experiments thus provided ample room to observe biases toward extremes. Future studies should test joint angle perception near extremes while skin and joint receptors are anesthetized; if no bias toward extremes is observed under these conditions then this would support the proposed role of joint and skin receptors in protectively biasing joint angle perception.
Precision of elbow angle estimates depends on task conditions
We found that the precision of elbow angle estimates varies across tasks. First, we observed that elbow angle estimates were more precise in our fingertip localization task than they were in our passive elbow angle task, supporting the hypothesis that the CNS can more precisely estimate joint angles when those estimates are integrated into limb endpoint position. A previous study derived elbow angle precision from fingertip matching data and reported elbow angle SDs in the range of 0.6 to 1.1° (van Beers et al. 1998), which is smaller than the range we observed in our fingertip task (2.1 to 6.1°). In their analysis, however, subjects' elbow positions and arm lengths were based on the recorded positions of the shoulder, elbow, and hand of one subject and one degree of freedom was assumed for the shoulder, although it could move in three. In our fingertip localization study all variables were known for each subject, for each trial, and we controlled arm configurations, allowing us to directly measure elbow angle estimates.
Given our greater behavioral need to estimate hand position rather than joint angles, the CNS may directly calculate hand position from peripheral sensory signals, whereas isolated joint angle estimates may need to be extracted from these representations. Indeed, along the dorsal column–medial lemniscal pathway—the pathway most consider to be the primary means by which proprioceptive information reaches the cerebral cortex (cf. Wall and Noordenbos 1977)—most proprioceptive neurons in the primary somatosensory cortex are tuned to arm movement direction (Prud'homme and Kalaska 1994) and activity is related best to linear combinations of joint and segment angles rather than to single-joint angles (Tillery et al. 1996). Along the dorsal spinocerebellar tract, the other pathway by which proprioceptive information reaches the CNS, cells also encode endpoint position of limbs (Bosco et al. 2000). It therefore appears that the CNS optimizes estimates of limb endpoint positions rather than conscious estimates of joint angles, a design that likely contributes to our behavioral results.
Subjects' strategies may have also contributed to our finding that elbow angle estimates are more precise in the fingertip versus passive elbow angle task. After the passive elbow angle task some subjects reported that they had been trying to point the response line toward their fingertip. Thus despite encouraging a focus on elbow angle rather than fingertip position, identifying fingertip position may have been the goal in both tasks for some subjects. In this case one would predict better precision in the task directly requiring identification of finger position versus the task requiring the distant pointing to finger position.
In addition to proprioceptive differences driven by whether the fingertip or elbow angle was reported, we also observed differences driven by whether the arm was actively or passively moved. Many studies have found that proprioceptive estimates are more precise when subjects report positions after active rather than passive movements (Adamovich et al. 1998; Brouchon and Paillard 1966; Craske and Crawshaw 1975; Gritsenko et al. 2007; Gurfinkel' et al. 1985; Laufer et al. 2001; Zia et al. 2002), a trend thought to result from the additional position information available in active movements from efference copies of motor commands and alpha-gamma motor neuron coactivation. Direct effects of motor commands on proprioception have been demonstrated in studies showing that when movement of a limb is blocked, efforts to move still result in a perceived change in position, with the amplitude of the perceived position change dependent on the amount of movement effort (Gandevia et al. 2006; Smith et al. 2009).
There was no significant group difference between precision on the active elbow angle task and the passive fingertip task. Since the improved precision in these tasks appears to result from distinct mechanisms, these benefits could be additive.
Fingertip position estimates
In Task 2, where subjects reported the perceived x and y positions of their fingertip, additional variables other than elbow angle estimates could be explored. The range of our subjects' fingertip estimation precision across 14 arm configurations was 1.3 to 3.9 cm, which is within the range previously reported in other studies (Crowe et al. 1987; van Beers et al. 1996, 1998, 1999). Unlike our results, a previous study found a significant relationship between precision of fingertip estimates and distance between the hand and shoulder, with estimates becoming less precise the farther the finger was from the shoulder (van Beers et al. 1998). This study, however, had subjects report their perceived fingertip position by matching the position of their two index fingertips. The observed increase in variance with farther distances could therefore reflect less motor precision for larger movements rather than a decrease in the precision of the sensory estimate. Since subjects in our study reported the perceived position of their fingertip without making arm movements to that position, our measures are more likely to reflect precision of the position estimate. While no relationship between precision and distance from hand to shoulder was observed, it is possible that an effect was missed due to an insufficient number of repetitions.
Conclusion
Our results show that there are systematic biases in position sense that are independent of task demands and are thus likely explained by changes in peripheral proprioceptive signals. Other aspects, like the precision of estimates, are affected by task demands and thus likely depend on high-level integration of sensory and motor signals. In addition to furthering our knowledge of sensory processing, understanding the fundamental properties of proprioception will help us appreciate which specific aspects of this sense are impaired in different patient populations and how best to go about assisting these populations.
GRANTS
This research was supported by an “Autism Speaks” predoctoral fellowship.
ACKNOWLEDGMENTS
We thank all participants in this research study; S. Bensmaia, A. Okamura, S. Hsiao, and C. Connor for discussion regarding the methods and results; D. Grow for help with adapting the joystick used in Tasks 1 and 2; and N. Bhanpuri for general assistance in implementing the experimental design.
REFERENCES
- Adamovich SV, Berkinblit MB, Fookson O, Poizner H. Pointing in 3D space to remembered targets. I. Kinesthetic versus visual target presentation. J Neurophysiol 79: 2833–2846, 1998 [DOI] [PubMed] [Google Scholar]
- Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol 580: 649–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansems GE, Allen TJ, Proske U. Position sense at the human forearm in the horizontal plane during loading and vibration of elbow muscles. J Physiol 576: 445–455, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans: I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res 134: 301–310, 2000 [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE, Eian J. Reference frames for spinal proprioception: limb endpoint based or joint-level based? J Neurophysiol 83: 2931–2945, 2000 [DOI] [PubMed] [Google Scholar]
- Brouchon M, Paillard J. Influence of active or passive conditions of mobilization of a limb on the precision of the locating of its final position (in French). C R Seances Soc Biol Fil 160: 1281–1285, 1966 [PubMed] [Google Scholar]
- Burgess PR, Clark FJ. Characteristics of knee joint receptors in the cat. J Physiol 203: 317–335, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol 402: 347–361, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ, Burgess PR. Slowly adapting receptors in cat knee joint: can they signal joint angle? J Neurophysiol 38: 1448–1463, 1975 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW. Proprioception with the proximal interphalangeal joint of the index finger. Evidence for a movement sense without a static-position sense. Brain 109: 1195–1208, 1986 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol 54: 1529–1540, 1985 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Horch KW, Bach SM, Larson GF. Contributions of cutaneous and joint receptors to static knee-position sense in man. J Neurophysiol 42: 877–888, 1979 [DOI] [PubMed] [Google Scholar]
- Clark FJ, Larwood KJ, Davis ME, Deffenbacher KA. A metric for assessing acuity in positioning joints and limbs. Exp Brain Res 107: 73–79, 1995 [DOI] [PubMed] [Google Scholar]
- Cohen DA, Prud'homme MJ, Kalaska JF. Tactile activity in primate primary somatosensory cortex during active arm movements: correlation with receptive field properties. J Neurophysiol 71: 161–172, 1994 [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol 496: 857–871, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol 94: 1699–1706, 2005 [DOI] [PubMed] [Google Scholar]
- Craske B. Perception of impossible limb positions induced by tendon vibration. Science 196: 71–73, 1977 [DOI] [PubMed] [Google Scholar]
- Craske B, Crawshaw M. Shifts in kinesthesis through time and after active and passive movement. Percept Mot Skills 40: 755–761, 1975 [DOI] [PubMed] [Google Scholar]
- Crowe A, Keessen W, Kuus W, van Vliet R, Zegeling A. Proprioceptive accuracy in two dimensions. Percept Mot Skills 64: 831–846, 1987 [DOI] [PubMed] [Google Scholar]
- Darling WG. Perception of forearm angles in 3-dimensional space. Exp Brain Res 87: 445–456, 1991 [DOI] [PubMed] [Google Scholar]
- Davidson PR, Wolpert DM. Widespread access to predictive models in the motor system: a short review. J Neural Eng 2: S313–S319, 2005 [DOI] [PubMed] [Google Scholar]
- Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol 531: 289–297, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol 67: 1105–1113, 1992 [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol 65: 657–670, 1991 [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol 487: 243–251, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Gandevia SC, McCloskey DI. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. J Physiol 386: 63–71, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Burke D. Kinaesthetic signals and muscle contraction. Trends Neurosci 15: 62–65, 1992 [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Smith JL, Crawford M, Proske U, Taylor JL. Motor commands contribute to human position sense. J Physiol 571: 703–710, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol 59: 1220–1230, 1988 [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Krouchev NI, Kalaska JF. Afferent input, efference copy, signal noise, and biases in perception of joint angle during active versus passive elbow movements. J Neurophysiol 98: 1140–1154, 2007 [DOI] [PubMed] [Google Scholar]
- Gurfinkel' VS, Debreva EE, Levik Y. Relationship between perception of the position of parts of the body and movement (in Russian). Hum Physiol 11: 4–7, 1985 [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo AB. Directional tuning of human forearm muscle afferents during voluntary wrist movements. J Physiol 536: 635–647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Prud'homme M, Hyde ML. Parietal area 5 neuronal activity encodes movement kinematics, not movement dynamics. Exp Brain Res 80: 351–364, 1990 [DOI] [PubMed] [Google Scholar]
- Laufer Y, Hocherman S, Dickstein R. Accuracy of reproducing hand position when using active compared with passive movement. Physiother Res Int 6: 65–75, 2001 [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol 429: 113–129, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. Proprioceptors and their contribution to somatosensory mapping: complex messages require complex processing. Can J Physiol Pharmacol 66: 430–438, 1988 [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol 41: 705–721, 1993 [DOI] [PubMed] [Google Scholar]
- Prud'homme MJ, Kalaska JF. Proprioceptive activity in primate primary somatosensory cortex during active arm reaching movements. J Neurophysiol 72: 2280–2301, 1994 [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Bergenheim M, Albert F, Roll JP. Proprioceptive population coding of limb position in humans. Exp Brain Res 149: 512–519, 2003 [DOI] [PubMed] [Google Scholar]
- Roll JP, Albert F, Ribot-Ciscar E, Bergenheim M. “Proprioceptive signature” of cursive writing in humans: a multi-population coding. Exp Brain Res 157: 359–368, 2004 [DOI] [PubMed] [Google Scholar]
- Roll JP, Bergenheim M, Ribot-Ciscar E. Proprioceptive population coding of two-dimensional limb movements in humans: II. Muscle-spindle feedback during “drawing-like” movements. Exp Brain Res 134: 311–321, 2000 [DOI] [PubMed] [Google Scholar]
- Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC. Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. J Appl Physiol 106: 950–958, 2009 [DOI] [PubMed] [Google Scholar]
- Soechting JF. Does position sense at the elbow reflect a sense of elbow joint angle or one of limb orientation? Brain Res 248: 392–395, 1982 [DOI] [PubMed] [Google Scholar]
- Soechting JF, Ross B. Psychophysical determination of coordinate representation of human arm orientation. Neuroscience 13: 595–604, 1984 [DOI] [PubMed] [Google Scholar]
- Tillery SI, Soechting JF, Ebner TJ. Somatosensory cortical activity in relation to arm posture: nonuniform spatial tuning. J Neurophysiol 76: 2423–2438, 1996 [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. How humans combine simultaneous proprioceptive and visual position information. Exp Brain Res 111: 253–261, 1996 [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res 122: 367–377, 1998 [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. Localization of a seen finger is based exclusively on proprioception and on vision of the finger. Exp Brain Res 125: 43–49, 1999 [DOI] [PubMed] [Google Scholar]
- Wall PD, Noordenbos W. Sensory functions which remain in man after complete transection of dorsal columns. Brain 100: 641–653, 1977 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science 269: 1880–1882, 1995 [DOI] [PubMed] [Google Scholar]
- Zia S, Cody FW, O'Boyle DJ. Identification of unilateral elbow-joint position is impaired by Parkinson's disease. Clin Anat 15: 23–31, 2002 [DOI] [PubMed] [Google Scholar]


