Abstract
Experimental and clinical data support a role for estrogens in the development and growth of breast cancer, and lowered estrogen exposure reduces breast cancer recurrence and new diagnoses in high-risk women. There is varied evidence that increased physical activity is associated with breast cancer risk reduction in both pre- and postmenopausal women, perhaps via lowered estrogen levels. The purpose of this study was to assess whether exercise intervention in premenopausal women at increased breast cancer risk reduces estrogen or progesterone levels. Seven healthy premenopausal women at high risk for breast cancer completed a seven-menstrual-cycle study. The study began with two preintervention cycles of baseline measurement of hormone levels via daily first-morning urine collection, allowing calculation of average area under the curve (AUC) hormone exposure across the menstrual cycle. Participants then began five cycles of exercise training to a maintenance level of 300 min per week at 80–85% of maximal aerobic capacity. During the last two exercise cycles, urinary estradiol and progesterone levels were again measured daily. Total estrogen exposure declined by 18.9% and total progesterone exposure by 23.7%. The declines were mostly due to decreased luteal phase levels, although menstrual cycle and luteal phase lengths were unchanged. The study demonstrated the feasibility of daily urine samples and AUC measurement to assess hormone exposure in experimental studies of the impact of interventions on ovarian hormones. The results suggest value in exercise interventions to reduce hormone levels in high-risk women with few side effects and the potential for incremental benefits to surgical or pharmacologic interventions.
Keywords: menstrual cycle, BRCA1/2 mutation carriers
experimental and clinical data support a role for estrogens in the development and growth of breast cancer, with the primary hypothesis being that estrogens interact with receptors in a manner that increases cell proliferation rates (7, 70). Lowering endogenous estrogen levels via bilateral oopherectomy or blocking estrogen effects with selective estrogen receptor modulators such as tamoxifen and raloxifene reduce the rate of initial diagnoses among women at high risk of developing breast cancer (11, 29, 38, 34, 36, 55). Early onset of menarche, late first full-term pregnancy, and late menopause, factors that increase lifetime estrogen exposure, have all been associated with higher rates of breast cancer. On the other hand, there is conflicting epidemiologic evidence of a link between premenopausal endogenous estrogens and breast cancer risk. Of seven studies identified, four observed a significant inverse association of endogenous estrogens with breast cancer risk in premenopausal women (12, 28, 56, 34), while three others did not (21, 27, 69).
In contrast to estrogens, progesterone has long been thought to have an antiproliferative effect in the premenopausal breast, analogous to its opposition to estrogen-induced epithelial proliferation in the endometrium of the luteal phase of the menstrual cycle (16). Epidemiologic data are both supportive (27, 48, 59) and not supportive (13, 21, 69) of progesterone's protective effects, and there are animal data that implicate progesterone in mammary endothelial proliferation (23). The conflicting findings may result from alteration of the relationship of progesterone to mammary gland morphogenesis and function that occurs with full-term pregnancy, which may explain the protective effect of early age at first full-term pregnancy (45). In any case, however, breast mitotic activity is in fact highest during the luteal phase of the menstrual cycle, when progesterone levels are also highest.
Just as there is considerable support for a role of estrogens in the pathogenesis of breast cancer, there is wide-ranging evidence, including prospective cohort investigations in breast (39), colorectal (38), endometrial (16), lung (58a), ovarian (40), and prostate cancer (26), that increased levels of physical activity are associated with a reduction in cancer risk, including breast cancer risk (33, 35, 60, 65). The hypothesized mechanisms by which exercise reduces breast cancer risk are varied and complex, but evidence is accumulating that aerobic activity results in a negative energy balance (41) and lowers estrogen levels in both pre- and postmenopausal women (5, 9, 66). At the extreme in premenopausal women, exercise of significant frequency and intensity that is associated with a negative energy balance can lead to longer menstrual cycles and menstrual dysfunction [e.g., luteal phase deficiency (8), anovulatory cycles (5), and amenorrhea (66, 67)], but moderate exercise [e.g., household and/or recreational activities (39, 62)] where energy balance is maintained has been linked to a variety of health benefits without disrupted menstrual or reproductive function (42, 65).
Evidence of the presence, direction, and magnitude of effects of estrogen and progesterone on breast cancer pathogenesis has been limited by reliance on a single or small number of measurements of hormone levels as a surrogate marker for overall long-term hormonal exposure. The variability in hormone levels among premenopausal women over the course of the menstrual cycle makes the results of studies that use a single or only several measurements less accurate than would daily measures taken over an entire menstrual cycle (47, 50, 61). Use of single measurements may, as a consequence, be a source of conflicting findings about the relationship between both estrogen and progesterone levels, on the one hand, and breast cancer risk, on the other (13). The hypothesis regarding estrogens and breast cancer risk postulates that overall exposure increases mitotic activity. Therefore, daily measures over the entire menstrual cycle that permit calculation of the total area under the curve (AUC) provide a more accurate measure of overall exposure. Figure 1 depicts two hypothetical estradiol exposure curves across a single menstrual cycle. The area under the solid line is greater than the area under the dotted line, denoting greater overall estradiol exposure across the entire menstrual cycle. The AUC method of assessing total hormone exposure has become the standard in studies of the effect of exercise training on hormone levels and reproductive function (2, 68), but it has not been used to study the effects of exercise on hormone exposure in women at high risk of developing breast cancer.
Fig. 1.
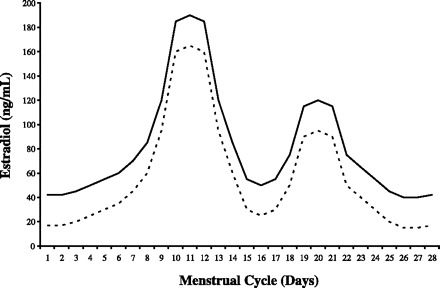
Hypothetical graph comparing higher (solid line) vs. lower (dashed line) total hormone exposure across a menstrual cycle as measured by the area under each curve.
The primary aim of the study reported here was to assess whether an exercise intervention in premenopausal women at increased risk for breast cancer reduces AUC estrogen levels overall and, more specifically, within the follicular, the luteal, or both phases of the menstrual cycle. The study is innovative in its 1) focus on women at substantially increased risk of breast cancer, and 2) application of daily measurement of urinary hormone levels developed for studies of the effects of exercise training on reproductive function to the study of breast cancer risk reduction. The significance of the study lies in its suggestion of a preventive intervention to reduce breast cancer rates in high-risk premenopausal women that may be more acceptable than currently available surgical [bilateral mastectomy and oophorectomy (10)] and drug therapy techniques (51), all of which have serious, long-term side effects (12, 64). While exercise is not likely to replace other interventions in patients at high risk of breast cancer (e.g., strong family history and BRCA mutation carriers) given persuasive evidence of their value (11, 29, 30, 34, 36, 55), it is important to ask what value exercise alone might have in women with a family history of breast cancer despite the absence of known genetic markers.
MATERIALS AND METHODS
Participants.
Healthy premenopausal eumenorrheic (menstrual cycles 25–32 days in length) women ages 25–35 yr (eligibility 18–35) at high risk of breast cancer [as assessed by either BRCA1/2-positive status (if tested) or, if BRCA1/2 status is unknown or negative, have a ≥16.5% lifetime risk of breast cancer, using the Claus model based on autosomal dominant inheritance (6)] were recruited as a convenience sample from women in the Cancer Risk Evaluation Program of the Abramson Cancer Center at the Hospital of the University of Pennsylvania after Institutional Review Board approval of the study. Women were eligible if they had intact ovaries and a uterus and had not had prophylactic mastectomy; gynecologic age of ≥4 yr (gynecologic age = current age − age of menarche) to increase the likelihood of relatively stable menstrual cycle lengths; no history of physician-diagnosed gynecological problems (e.g., fibroids, endometriosis, and polycystic ovary syndrome); prior tubal ligation or willingness to use a nonhormonal form of contraception during study participation and no hormonal contraception use with the past 3 mo; no medical conditions or medications that would prohibit participation in aerobic exercise; no history of cancer, excepting nonmelanoma skin cancer and in situ cervical cancers; body mass index (BMI) of at least 21 but not more than 50; no current diagnosis of eating disorders; not currently participating in any weight loss programs; not currently or recently (past 6 mo) pregnant and not planning to become pregnant during the study period; nonsmoker for at least the past year; no more than seven alcoholic beverages per week; leisure-time exercise energy expenditure of ≤500 kcals/wk over the past 6 mo; and not planning to move away from area during the period of the study. Demographic data were collected only at study entry and included age, race/ethnicity including Ashkenazi Jewish heritage [associated with a higher risk of BRCA1/2 mutations (12)], education, employment, breast cancer risk status, parity, self-reported medication use, and self-reported menstrual cycle length.
A total of 10 women were recruited to the study and embarked on the study protocol described in Fig. 2 lasting 7 menstrual cycles. Three women did not remain in the study to completion: one suffered a burst ovarian cyst, one due to an unusually long menstrual cycle upon study entry, and one for failure to collect urine samples in a way that resulted in usable data, resulting in analysis of seven participants.
Fig. 2.
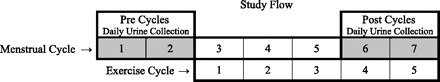
Flow diagram of study protocol. First morning urine was collected every day for the first 2 menstrual cycles (Pre Cycles) before initiation of the exercise intervention. At the beginning of menstrual cycle 3, the exercise intervention started and continued through the end of menstrual cycle 7. First morning urine was again collected every day for menstrual cycles 6 and 7 (Post Cycles; cycles 4 and 5 of the exercise intervention).
Anthropometry, body composition, and fitness.
Body height was assessed at baseline; body weight was assessed at both baseline and at study end. Percentage of body fat was calculated based on measurement of skin folds using a standard conversion formula (58). BMI was calculated from weight and height at each time point. Maximal aerobic fitness was predicted based on results from a treadmill test to exhaustion using the Bruce Protocol (4). Heart rate during this test is measured using 12-lead ECG monitoring. Maximal oxygen consumption (V̇o2max) was predicted based on the maximal workload achieved using a standard conversion formula (15).
Exercise intervention and exercise logs.
Each participant was provided with a treadmill (Smooth Fitness Model 5.65, King of Prussia, PA), Polar Heart Rate monitor (Polar Electro Oy, Oulu, Finland), and ongoing support from a certified fitness professional. As described in Fig. 3, prescribed exercise consisted of 5 menstrual cycles of exercise with a 12-wk progression to 300 min/wk at 80–85% of maximum heart rate (25, 68). This is the level of aerobic activity recommended by the US Department of Health and Human Services for increased health benefits (62). Participants were instructed to work toward the weekly target minutes in no fewer than three exercise sessions per week, with sessions lasting at least 15 min but no more than 100 min. Exercise training sessions took place at the homes of the participants and included use of the downloadable Polar Heart Rate monitor and instructions not to exceed 85% of age predicted maximal heart rate during exercise sessions. A certified fitness professional visited participants' homes for the first exercise session of each of the first 5 wk (for a total of 5 visits per participant across the exercise intervention) to help individualize the duration and intensity of the exercise session and to instruct participants in the use of exercise logs and injury prevention. Thereafter, participants were invited to attend weekly exercise sessions at participating YMCAs. The Polar Heart Rate monitors were downloaded onto a laptop every 2 wk during a research staff person's visit to the participant's home. The certified fitness professional reviewed heart rate files with the participant and discussed the need for revising workout schedule or intensity based on the objectively monitored heart rate response, not based on absolute work on a specific treadmill. Adherence to prescribed exercise was assessed by calculating the ratio of minutes actually spent on the treadmill (summed across all exercise weeks) to the minutes prescribed for each participant.
Fig. 3.
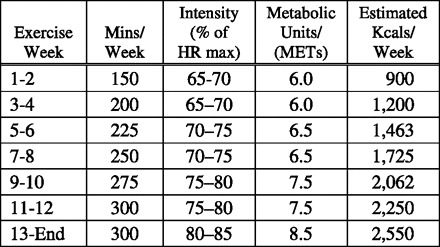
Prescribed exercise with a build in both duration (minutes/week) and intensity [percent of heart rate (HR) maximum] of exercise to a maintenance level after week 12 of 300 minutes per week at between 80 and 85% of maximum target heart rate. MET, metabolic equivalent.
Urine collection and measurement and analysis of hormone levels.
Participants performed urine collections by saturating a sponge that was placed in an air-tight vial at the first urine void every morning during two consecutive menstrual cycles. This method was validated by O'Connor et al. (52) and has been associated with a 96% compliance rate, higher than that seen with standard urine collection cups. Participants stored the sponge collection devices in the freezer section of their home refrigerator (−4°C) for weekly pickup and delivery on dry ice to the University of Pennsylvania. Samples were stored in a −80°C freezer before shipping to Pennsylvania State University and then stored at −20°C until analysis. Samples were unfrozen and aliquoted into 2-ml tubes (6 aliquots per daily sample and 1,800 μl per tube). Any samples containing sediment were centrifuged at 3,000 rpm for 15 min before aliquoting. Microtiter plate competitive enzyme immunoassays were used to measure the urinary metabolites of estrogen [estrone-1-glucuronide (E1G)] and progesterone [pregnanediol-3-glucuronide (PdG)]. The secretion of these urinary metabolites parallels serum concentrations of the parent hormones (43); daily urine collection is more practical than daily blood draws and favored over salivary samples because it is easier with urine samples to collect sufficient biospecimens and adjust for hydration status (18–20, 54). Each assay uses a polyclonal capture antibody from Coralie Monroe at the University of California (R522–2 for E1G and R12904 for PdG). The competitors for each assay are each urinary metabolite conjugated to horseradish peroxidase (E1G-HRP and PdG-HRP). An endpoint substrate color reaction is developed with azino-bis-ethylbenzthiazoline sulfonic acid and peroxidase. E1G and PdG standards from Sigma are used for the standard curve, and high and low internal controls are in-house samples. The interassay coefficients of variation for high and low internal controls, respectively, are 14.7 and 13.1% for E1G and 15.6 and 12.9% for PdG. All urine samples were corrected for specific gravity using a hand refractometer (NSG Precision Cells, Farmingdale, NY) to account for hydration status. All hormone determinations were assayed in duplicate; all samples from a given subject were tested in the same assay reagent batch.
The average values across the two menstrual cycles at each measurement (pre and post) were used in the analysis. The data were quantified using the AUC method (trapezoidal rule) and by comparing average levels over two cycles before the start of the exercise intervention and the last two cycles of the five-cycle exercise intervention (8).
Menstrual logs and cycle characteristics.
Participants were given calendars on which to record menstrual cycle information for all seven cycles of the study. Total menstrual cycle length was defined as the number of days from day 1 of menses to the day before the next menses. Estrogen and progesterone exposure, confirmation of ovulatory status, the presence or absence of luteal phase defects, and the lengths of the follicular and luteal phases were determined by analysis of daily urinary metabolites of E1G, PdG, and midcycle luteinizing hormone after completion of the study using previously published methods (9).
Statistical analyses.
Study results are reported as means, SDs, and percentages. Differences between baseline vs. end and pre- vs. postmeans were assessed using two-sided paired t-tests. The difference between pre- and postpercentages of cycles with luteal phase deficiency was assessed nonparametrically. Data were analyzed using Microsoft Excel (Microsoft, Redmond, WA); P < 0.05 was considered to be statistically significant.
RESULTS
The baseline characteristics of the study population are summarized in Table 1. Of the three participants tested for BRCA1/BRCA2 mutations, all were positive (42.9% overall); of the four not tested, three (42.9% overall) had a prior probability of a BRCA mutation of 50% and one (14.3% overall) had a prior probability of 25%. Claus model scores among the study participants range from 16.5 to 39.9%, with a mean of 23.5%.
Table 1.
Patient characteristics
| Variable | Baseline |
|---|---|
| Age, yr (range) | 31 (25–35) |
| Race/ethnicity, Caucasian | 100% |
| Ashkenazi Jewish | |
| No | 43.9% (n = 3) |
| Yes | 57.1% (n = 4) |
| Education | |
| Postgraduate degree | 71.4% (n = 5) |
| 4-yr college graduate | 14.3% (n = 1) |
| Some college | 14.3% (n = 1) |
| Employment | |
| Professional/technical | 71.4% (n = 5) |
| Managerial/administrative | 14.3% (n = 1) |
| Clerical | 14.3% (n = 1) |
| Risk status | |
| BRCA1/2 testing | |
| Positive | 42.9% (n = 3) |
| Negative | 0.0% (n = 0) |
| Not tested | 57.1% (n = 4) |
| Prior probability | |
| 50% | 42.9% (n = 3) |
| 25% | 14.3% (n = 1) |
| Claus model score | 24% (16–40%) |
| Parity | |
| Nulliparous | 42.9% (n = 3) |
| Primiparous | 14.3% (n = 1) |
| Multiparous | 42.9% (n = 3) |
| Self-reported medications | |
| Yes | 42.9% (n = 3) |
| No | 57.1% (n = 4) |
| Self-reported menstrual cycle | |
| length at baseline, days (range) | 29.2 (27–32) |
Number (n) of subjects out of 7.
Anthropometry, body composition, and fitness.
Body composition and fitness results are summarized in Table 2. Participants showed statistically significant declines in body weight (P = 0.025), BMI (P = 0.025), and body fat percentage (P = 0.02). Five participants (71%) had healthy weights (BMI 18.5–24.9) at the start of the study, while two (29%) were obese (BMI ≥30.0); at the end of the exercise intervention, only one participant (14%) changed BMI categories, falling from obese BMI to overweight BMI (25.0–29.9). The exercise intervention was associated with a 13% increase in maximal aerobic fitness (P = 0.012).
Table 2.
Anthropometry, body composition, and fitness
| Variable | At Baseline | At End | Mean %Change | P Value |
|---|---|---|---|---|
| Weight, kg | 67.1 ± 10.0 | 65.2 ± 9.5 | −2.8 ± 2.5 | 0.025 |
| Height, cm | 165.0 ± 6.4 | NA | NA | NA |
| BMI, kg/m2 | 24.8 ± 4.6 | 24.1 ± 4.3 | −2.8 ± 2.5 | 0.025 |
| %Healthy (18.5–24.9) | 71% | 71% | 0 | NA |
| %Overweight (25.0–29.9) | 0% | 14% | +14% | NA |
| %Obese (30.0 or higher) | 29% | 15% | −14% | NA |
| Body fat percentage | 38.4 ± 10.5 | 32.1 ± 10.3 | −15.8 ± 14.1 | 0.02 |
| Maximal aerobic fitness/V̇o2max, ml·kg−1·min−1 | 31.4 ± 3.3 | 35.3 ± 3.7 | +12.8 ± 9.4 | 0.012 |
Values are means ± SD; n = 7 subjects. BMI, body mass index; NA, not applicable; V̇o2max, maximal oxygen consumption.
Intervention adherence.
Adherence to exercise was measured as the percentage of total minutes of prescribed exercise summed across all weeks of the exercise intervention that a participant actually completed. Average adherence to exercise across all participants was 85% of total prescribed minutes and ranged from a low of 70% of total prescribed minutes per participant to a high of 97%. Across all participants and all weeks of the exercise intervention, the number of exercise sessions per week ranged from 1 to 10; the average and modal number of weekly sessions was 5, with over half of the exercise weeks comprised of 4 to 6 exercise sessions. No exercise-related adverse events were reported. None of the participants attended any of the weekly group exercise sessions offered at the YMCA.
Menstrual cycle characteristics and hormonal exposure.
Neither average menstrual cycle length (P = 0.51) nor average luteal phase length (P = 0.83) changed with the exercise intervention, but both estrogen (P = 0.03) and progesterone (P = 0.05) levels, as assessed by the AUC of urinary E1G and PdG conjugates, respectively, declined significantly with the exercise intervention, as shown in Table 3. A post hoc power analysis was conducted (β = 80%, α = 0.05, two-sided) for total estrogen and progesterone AUC; their powers (1-β error probability, or sensitivity), respectively, were 0.42 and 0.32. When the AUC for E1G and PdG were examined for the follicular and luteal phases separately, the changes noted for the overall cycle were mostly reflective of effects on the luteal phase rather than the follicular phase. For example, E1G AUC declined by 6.8% (P = 0.20) in the follicular phase vs. 30.3% (P = 0.02) in the luteal phase, and PdG AUC declined by 21.6% (P = 0.85) in the follicular phase vs. 25.7% (P = 0.002) in the luteal phase. The percentage of cycles showing luteal phase deficiency was unchanged with intervention (21% pre and post; P = 1.00).
Table 3.
Menstrual and hormonal changes
| Variable | Average Pre | Average Post | Mean %Change | P Value |
|---|---|---|---|---|
| Menstrual cycle length, days | 28.9 ± 2.0 | 29.4 ± 1.7 | +3.6 ± 0.01 | 0.51 |
| Luteal phase length, days | 11.3 ± 2.5 | 11.1 ± 2.4 | +1.4 ± 0.25 | 0.83 |
| Luteal phase deficiency | ||||
| %Cycles, out of 14 cycles) | 21% | 21% | 0.0 | 1.00 |
| No. of subjects (out of 7) | 3 | 3 | 0.0 | |
| Estrogen AUC, E1G ng/ml | ||||
| Total cycle | 11,20.1 ± 309.1 | 908.2 ± 160.5 | −18.9 ± 15.2 | 0.03 |
| Follicular phase | 672.9 ± 181.3 | 610.3 ± 139.8 | −6.8 ± 16.9 | 0.20 |
| Luteal phase | 452.0 ± 174.9 | 291.5 ± 63.8 | −30.3 ± 19.0 | 0.02 |
| Progesterone AUC, PdG, ng/ml | ||||
| Total cycle | 88.2 ± 35.9 | 67.3 ± 20.4 | −23.7 ± 28.9 | 0.05 |
| Follicular phase | 20.6 ± 10.5 | 19.7 ± 4.1 | −21.6 ± 66.6 | 0.85 |
| Luteal phase | 67.7 ± 32.5 | 47.4 ± 19.9 | −25.7 ± 17.7 | 0.002 |
Values are means ± SD; n = 7 subjects. Pre, cycles 1 and 2; Post, cycles 6 and 7; AUC, area under the curve; E1G, estrone-1-glucuronide; PdG, pregnanediol-3-glucuronide.
DISCUSSION
The exercise intervention evaluated in this small study reduced total estrogen exposure by 18.9% and total progesterone exposure by 23.7% in premenopausal women at high risk of developing breast cancer. These changes are reflective of largely luteal phase effects: overall estrogen exposure during the follicular and luteal phases declined 6.8 and 30.3%, respectively, and overall progesterone exposure declined by 21.6 and 25.7%, respectively. Placing these changes into context is challenging, given that there is no established “safe” level of estrogen or progesterone exposure among premenopausal high-risk women. While this study looked solely at urine hormone levels rather than plasma hormone levels, current findings add to those from the Nurses' Health Study that women with a threefold increased risk of ER+/PR+ breast cancer had 35% higher luteal phase plasma estradiol levels than woman at lower breast cancer risk (13).
The small sample size and absence of a true control group are limitations of the study that require that the results be regarded as preliminary. Nonetheless, the current findings establish the merit of a larger trial of the study hypothesis. They also demonstrate the feasibility of using daily urine samples and AUC measurement to assess total exposure to ovarian hormones in experimental studies of the impact of interventions on ovarian hormones in high-risk patients. Because AUC measurement is a better indicator of total hormone exposure than are single or even several assessments per cycle, the measures used in this study are likely to be more reflective of overall hormonal exposure than what has been measured in prior epidemiologic studies of the relationship between prediagnostic hormone levels and subsequent risk for breast cancer in premenopausal women. By reducing a source of measurement error, this superior metric may help clarify conflicting findings on the relationship between hormone levels and breast cancer, including whether progesterone levels have a net proliferative or protective effect on breast cancer risk in premenopausal women.
It is worth noting that none of the women in the study chose to attend the weekly group exercise sessions at YMCAs. This finding is consistent with the hypothesis supporting at-home exercise for maximizing adherence both in studies of the impact of exercise on outcomes of interest as well as when exercise is recommended as a clinical intervention (24, 32, 53, 57).
There are, of course, many questions that must eventually be answered to provide sound evidence-based guidance about optimal exercise intervention for premenopausal women at high risk of developing breast cancer. For example, how do hormone levels vary with lower vs. higher exercise “dose,” that is, how much exercise is needed to have a beneficial effect on estrogen levels (and ultimately breast cancer risk reduction) and can that level be reached without compromising reproductive function or bone health? Will longer term and/or higher intensity exercise produce menstrual disturbances such as anovulatory cycles or amenorrhea? Was the caloric deficit produced in this study caused solely through exercise or were dietary changes an independent source of weight loss and hormonal changes? Although the current study is not able to address these questions, the hope is that reduction in endogenous estrogen levels in high-risk women will lower breast cancer risk and improve long-term outcomes.
Not all women at elevated risk develop breast cancer, and it is acknowledged that there are nongenetic, environmental aspects to neoplastic development among those with elevated risk. Nonetheless, the strongest basis for breast cancer prevention among women with elevated risk is through hormonal intervention. (46, 63, 64) However, both surgical and pharmacologic preventive techniques have serious negative long-term consequences that may be unacceptable to many women. The results of this study suggest potential value in exercise interventions that might address the same hormonal issues but with fewer serious side effects and less deleterious impact on quality of life. High-risk women who are highly motivated to reduce their risk may benefit from knowing the degree of risk reduction they can reasonably expect from exercise, even if the benefits consist only of delayed onset, lower stage at diagnosis, or reduced breast density that improves detection in early screening (1, 3, 22, 31, 43). Exercise may offer high-risk women a way to reduce hormone levels and would improve their overall fitness (reducing weight, BMI, and body fat percentage, and increasing maximal aerobic fitness), making them better prepared physically and psychologically for the rigors of therapy that would follow a breast cancer diagnosis.
GRANTS
This project was funded, in part, under a grant with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.A.K., N.I.W., and K.H.S. analyzed data; D.A.K., N.I.W., and K.H.S. interpreted results of experiments; D.A.K. and K.H.S. prepared figures; D.A.K. drafted manuscript; D.A.K., N.I.W., S.M.D., M.S.K., J.E.S., and K.H.S. edited and revised manuscript; D.A.K., N.I.W., S.M.D., M.S.K., J.E.S., and K.H.S. approved final version of manuscript; N.I.W., S.M.D., M.S.K., and K.H.S. conception and design of research; N.I.W. and K.H.S. performed experiments.
ACKNOWLEDGMENTS
We thank the staff for the project for the effort, including Amy Rogerino, Lorita Grant, Cathy J. Bryan, Rachel Dubin, and Justin C. Brown.
REFERENCES
- 1. Aiello EJ, Tworoger SS, Yasui Y, Stanczyk FZ, Potter J, Ulrich CM, Irwin M, McTiernan A. Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev 14: 1411–1417, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Beitins IZ, McArthur JW, Turnbull BA, Skrinar GS, Bullen BA. Exercise induces two types of human luteal dysfunction: confirmation by urinary free progesterone. J Clin Endocrinol Metab 72: 1350–1358, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med 356: 227–236, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85: 546–562, 1973 [DOI] [PubMed] [Google Scholar]
- 5. Bullen BA, Skrinar GS, Beitins IZ, von Mering G, Turnbull BA, McArthur JW. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med 312: 1349–1353, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer 73: 643–651, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Clemens M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med 344: 276–285, 2001 [DOI] [PubMed] [Google Scholar]
- 8. DeSouza MJ. Menstrual disturbances in athletes: a focus on luteal phase defects. Med Sci Sports Exerc 35: 1553–1563, 2003 [DOI] [PubMed] [Google Scholar]
- 9. DeSouza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab 83: 4220–4232, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Domchek SM, Armstrong K, Weber BL. Clinical management of Brca1 and Brca2 mutation carriers. Nat Clin Pract Oncol 3: 2–3, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, Van t'veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Schildkraut J, Blum JL, Rebbeck TR. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 304: 967–975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eccles DM. Hereditary cancer: guidelines in clinical practice. Breast and ovarian cancer. Genetics Ann Oncol 15, Suppl 4: iv133–138, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA 296: 193–201, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst 98: 1406–1415, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Foster C, Jackson AS, Pollock ML, Taylor MM, Hare J, Sennett SM, Rod JL, Sarwar M, Schmidt DH. Generalized equations for predicting functional capacity from treadmill performance. Am Heart J 107: 1229–1234, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S, Linseisen J, Rohrmann S, Pischon T, Schulz M, Tjønneland A, Johnsen NF, Overvad K, Mendez M, Arguelles MV, Garcia CM, Larrañaga N, Chirlaque MD, Ardanaz E, Bingham S, Khaw KT, Allen N, Key T, Trichopoulou A, Dilis V, Trichopoulos D, Pala V, Palli D, Tumino R, Panico S, Vineis P, Bueno-de-Mesquita HB, Peeters PH, Monninkhof E, Berglund G, Manjer J, Slimani N, Ferrari P, Kaaks R, Riboli E. Physical activity and the risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer 121: 347–355, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Genuth SM. The endocrine system. In: Physiology, edited by Berne RM, Levy MN. Philadelphia, PA: Mosby Year Book, 1993, p. 813–1024 [Google Scholar]
- 18. Goldenberger B, Lowenthal B, Darwin W, Cone E. Intrasubject variation of creatinine and specific gravity measurements in consecutive urine specimens of heroin users. Clin Chem 41: 1136–1147, 1995 [PubMed] [Google Scholar]
- 19. Guthrie RM, Lott JA, Kriesel S, Miller IL. Does the dipstick meet medical needs for urine specific gravity? J Fam Pract 25: 512–514, 1987 [PubMed] [Google Scholar]
- 20. Haddow JE, Knight GJ, Palomaki GE, Neveux LM, Chilmonczyk BA. Replacing creatinine measurements with specific gravity values to adjust urine creatinine concentrations. Clin Chem 40: 562–564, 1994 [PubMed] [Google Scholar]
- 21. Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev 18: 79–85, 1994 [PubMed] [Google Scholar]
- 22. Irwin ML, Aiello EJ, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, Ballard-Barbash R. Pre-diagnosis physical activity and mammographic density in breast cancer survivors. Breast Cancer Res Treat 95: 171–178, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ismail PM, Amato P, Soyal SM, DeMayo FJ, Conneely OM, O'Malley BW, Lydon JP. Progesterone involvement in breast development and tumorigenesis—as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids 68: 779–787, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Jakicic JM, Winters C, Lang W, Wing RR. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA 282: 1554–1560, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 78: 684–689, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Johnsen NF, Tjønneland A, Thomsen BL, Christensen J, Loft S, Friedenreich C, Key TJ, Allen NE, Lahmann PH, Mejlvig L, Overvad K, Kaaks R, Rohrmann S, Boing H, Misirli G, Trichopoulou A, Zylis D, Tumino R, Pala V, Bueno-de-Mesquita HB, Kiemeney LA, Suárez LR, Gonzalez CA, Sánchez MJ, Huerta JM, Gurrea AB, Manjer J, Wirfält E, Khaw KT, Wareham N, Boffetta P, Egevad L, Rinaldi S, Riboli E. Physical activity and the risk of prostate cancer: the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer 125: 902–908, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, Bueno de Mesquita HB, Chang-Claude J, Clavel-Chapelon F, Fournier A, van Gils CH, Gonzalez CA, Gurrea AB, Critselis E, Khaw KT, Krogh V, Lahmann PH, Nagel G, Olsen A, Onland-Moret NC, Overvad K, Palli D, Panico S, Peeters P, Quirós JR, Roddam A, Thiebaut A, Tjønneland A, Chirlaque MD, Trichopoulou A, Trichopoulos D, Tumino R, Vineis P, Norat T, Ferrari P, Slimani N, Riboli E. Serum sex steroids in premenopausal women and breast cancer risk within the European prospective investigation into cancer and nutrition (Epic). J Natl Cancer Inst 97: 755–765, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiol Biomarkers Prev 9: 575–579, 2000 [PubMed] [Google Scholar]
- 29. Kauff ND, Barakat RR. Risk-reducing salpingo-oophorectomy in patients with germline mutations in Brca1 or Brca2. J Clin Oncol 25: 2921–2927, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA, Boyd J, Borgen PI, Barakat RR, Norton L, Castiel M, Nafa K, Offit K. Risk-reducing salpingo-oophorectomy in women with a Brca1 or Brca2 mutation. N Engl J Med 346: 1609–1615, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Kerlikowski K. The mammogram that cried wolf. N Engl J Med 356: 297–300, 2007 [DOI] [PubMed] [Google Scholar]
- 32. King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group- vs. home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA 266: 1535–1542, 1991 [PubMed] [Google Scholar]
- 33. King MC, Marks JH, Mandell JB; New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in Brca1 and Brca2. Science 302: 643–646, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN. Chemoprevention of breast cancer: a summary of the evidence for the U.S Preventive Services Task Force. Ann Intern Med 137: 59–69, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Kotsopoulos J, Olopado OI, Ghadirian P, Lubinski J, Lynch HT, Isaacs C, Weber B, Kim-Sing C, Ainsworth P, Foulkes WD, Eisen A, Sun P, Narod SA. Changes in body weight and the risk of breast cancer in Brca1 and Brca2 mutation carriers. Breast Cancer Res 7: R833–843, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramer JL, Velazquez IA, Chen BE, Rosenberg PS, Struewing JP, Greene MH. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-up of Brca1 mutation carriers. J Clin Oncol 23: 8629–8635, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Lahmann PH, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman M, Johnsen NF, Tjønneland A, Overvad K, Mendez M, Quirós JR, Martinez C, Dorronsoro M, Navarro C, Gurrea AB, Bingham S, Khaw KT, Allen N, Key T, Trichopoulou A, Trichopoulos D, Orfanou N, Krogh V, Palli D, Tumino R, Panico S, Vineis P, Bueno-de-Mesquita HB, Peeters PH, Monninkhof E, Berglund G, Manjer J, Ferrari P, Slimani N, Kaaks R, Riboli E. Physical activity and risk of colon and rectal cancers: the European prospective investigation in to cancer and nutrition. Cancer Epidemiol Biomarkers Prev 15: 2398–2407, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Lahmann PH, Friedenreich C, Schuit AJ, Salvini S, Allen NE, Key TJ, Khaw KT, Bingham S, Peeters PH, Monninkhof E, Bueno-de-Mesquita HB, Wirfält E, Manjer J, Gonzales CA, Ardanaz E, Amiano P, Quirós JR, Navarro C, Martinez C, Berrino F, Palli D, Tumino R, Panico S, Vineis P, Trichopoulou A, Bamia C, Trichopoulos D, Boeing H, Schulz M, Linseisen J, Chang-Claude J, Chapelon FC, Fournier A, Boutron-Ruault MC, Tjønneland A, Føns Johnson N, Overvad K, Kaaks R, Riboli E. Physical activity and breast cancer risk: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 16: 36–42, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Lahmann PH, Friedenreich C, Schulz M, Cust AE, Lukanova A, Kaaks R, Tjønneland A, Johnsen NF, Overvad K, Fournier A, Boutron-Ruault MC, Clavel Chapelon F, Boeing H, Linseisen J, Rohrmann S, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Mattiello A, Sacerdote C, Agnoli C, Tumino R, Quirós JR, Larrañaga N, Agudo AT, Sánchez MJ, Berglund G, Manjer J, Monninkhof EM, Peeters PH, Bueno-de-Mesquita HB, May AM, Allen N, Khaw KT, Bingham S, Rinaldi S, Ferrari P, Riboli E. Physical activity and ovarian cancer risk: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 18: 351–354, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Loucks AB. Energy availability, not body fatness, regulates reproductive function in women. Exerc Sport Sci Rev 31: 144–148, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 88: 297–311, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Lundstrom E, Christow A, Kersemaekers W, Svane G, Azavedo E, Söderqvist G, Mol-Arts M, Barkfeldt J, von Schoultz B. Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am J Obstet Gynecol 186: 717–722, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. A prospective study of age-specific physical activity and premenopausal breast cancer. J Natl Cancer Inst 100: 728–737, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer 12: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Metcalfe KA, Goel V, Lickley L, Semple J, Narod SA. Prophylactic bilateral mastectomy: patterns of practice. Cancer 95: 236–242, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Michaud DS, Manson JE, Spiegelman D, Barbieri RL, Sepkovic DW, Bradlow HL, Hankinson SE. Reproducibility of plasma and urinary sex hormone levels in premenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev 8: 1059–1064, 1999 [PubMed] [Google Scholar]
- 48. Micheli A, Muti P, Secreto G, Krogh V, Meneghini E, Venturelli E, Sieri S, Pala V, Berrino F. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer 112: 312–318, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Munro C, Stbenfeldt G, Cragun J, Addiego LA, Overstreet JW, Lasley BL. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their majority urinary metabolites as measured by enzyme immunoassay and radioassay. Clin Chem 37: 838–844, 1991 [PubMed] [Google Scholar]
- 50. Muti P, Trevisan M, Micheli A, Krogh V, Bolelli G, Sciajno R, Berrino F. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev 5: 917–922, 1996 [PubMed] [Google Scholar]
- 51. Narod SA. Modifiers of risk of hereditary breast cancer. Oncogene 25: 5832–5836, 2006 [DOI] [PubMed] [Google Scholar]
- 52. O'Connor KA, Brindle E, Holman DJ, Klein NA, Soules MR, Campbell KL, Kohen F, Munro CJ, Shofer JB, Lasley BL, Wood JW. Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin Chem 49: 1139–1148, 2003 [DOI] [PubMed] [Google Scholar]
- 53. O'Dougherty M, Dallman A, Turcotte L, Patterson J, Napolitano MA, Schmitz KH. Barriers and motivators for strength training among women of color and Caucasian women. Women Health 47: 41–62, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Pettersson K, Alfthan H, Stenman UH, Turpeinen U, Suonpää M, Söderholm J, Larsen SO, Nørgaard-Pedersen B. Simultaneous assay of alpha-fetoprotein and free beta subunit of human chorionic gonadotropin by dual-label time-resolved immunofluorometric assay. Clin Chem 39: 2084–2089, 1993 [PubMed] [Google Scholar]
- 55. Rebbeck TR. Prophylactic oophorectomy in Brca 1 and Brca2 mutation carriers. Eur J Cancer 38, Suppl 6: S15–7, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Rosenberg CR, Pasternack BS, Shore RE, Koenig KL, Toniolo PG. Premenopausal estradiol levels and the risk of breast cancer: a new method of controlling for day of the menstrual cycle. Am J Epidemiol 140: 518–525, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annu Rev Nutr 20: 21–44, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Siri WE. The gross composition of the body. Adv Biol Med Physics 4: 239–280, 1956 [DOI] [PubMed] [Google Scholar]
- 58a. Steindorf K, Friedenreich C, Linseisen J, Rohrmann S, Rundle A, Veglia F, Vineis P, Johnsen NF, Tjønneland A, Overvad K, Raaschou-Nielsen O, Clavel-Chapelon F, Boutron-Ruault MC, Schulz M, Boeing H, Trichopoulou A, Kalapothaki V, Koliva M, Krogh V, Palli D, Tumino R, Panico S, Monninkhof E, Peeters PH, Boshuizen HC, Bueno-de-Mesquita HB, Chirlaque MD, Agudo A, Larrañaga N, Quirós JR, Martínez C, Barricarte A, Janzon L, Berglund G, Bingham S, Khaw KT, Key TJ, Norat T, Jenab M, Cust A, Riboli E. Physical activity and lung cancer risk: the European prospective investigation into cancer and nutrition cohort. Int J Cancer 119: 2387–2397, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Thomas H, Key T, Allen D, Moore JW, Dowsett M, Fentiman IS, Wang DY. Re: reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst 89: 396–398, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Thune I, Brenn T, Lund E, Gaard M. Physical activity and the risk of breast cancer. N Engl J Med 336: 1269–1275, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Toniolo P, Koenig KL, Pasternack BS, et al. Reliability of measurements of total, protein-bound, and unbound estradiol in serum. Cancer Epidemiol Biomarkers Prev 3: 47–50, 1994 [PubMed] [Google Scholar]
- 62. US Department of Health and Human Services. Physical Activity Guidelines for Adults 2008. http://www.health.gov/paguidelines/guidelines/chapter4.aspx [14 July 2011].
- 63. Uyei A, Peterson SK, Erlichman J, Broglio K, Yekell S, Schmeler K, Lu K, Meric-Bernstam F, Amos C, Strong L, Arun B. Association between clinical characteristics and risk-reduction interventions in women who underwent Brca1 and Brca2 testing: a single-institution study. Cancer 107: 2745–2751, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Wainberg S, Husted J. Utilization of screening and preventive surgery among unaffected carriers of a Brca1 or Brca2 gene mutation. Cancer Epidemiol Biomarkers Prev 13: 1989–1995, 2004 [PubMed] [Google Scholar]
- 65. Williams NI. Lessons from experimental disruptions of the menstrual cycle in humans and monkeys. Med Sci Sports Exerc 35: 1564–1572, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology 2: 2381–2389, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab 86: 5184–5193, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Winters KM, Adams WC, Meredith CN, Loan MD, Lasley BL. Density and cyclic ovarian function in trained runners and active controls. Med Sci Sports Exerc 28: 776–78, 1996 [DOI] [PubMed] [Google Scholar]
- 69. Wysowski DK, Comstock GW, Helsing KJ, Lau HL. Sex hormone levels in serum in relation to the development of breast cancer. Am J Epidemiol 125: 791–799, 1987 [DOI] [PubMed] [Google Scholar]
- 70. Yue W, Santen R, Wang J, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, Korach KS, Devanesan P, Todorovic R, Rogan EG, Cavalieri EL. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem 86: 477–86, 2003 [DOI] [PubMed] [Google Scholar]


