Abstract
Comparing biological processes in closely related species with divergent life spans is a powerful approach to study mechanisms of aging. The oxidative stress hypothesis of aging predicts that longer-lived species would have lower reactive oxygen species (ROS) generation and/or an increased antioxidant capacity, resulting in reduced oxidative damage with age than in shorter-lived species. In this study, we measured ROS generation in the young adult animals of the long-lived white-footed mouse, Peromyscus leucopus (maximal life span potential, MLSP = 8 yr) and the common laboratory mouse, Mus musculus (C57BL/6J strain; MLSP = 3.5 yr). Consistent with the hypothesis, our results show that skeletal muscle mitochondria from adult P. leucopus produce less ROS (superoxide and hydrogen peroxide) compared with M. musculus. Additionally, P. leucopus has an increase in the activity of antioxidant enzymes superoxide dismutase 1, catalase, and glutathione peroxidase 1 at young age. P. leucopus compared with M. musculus display low levels of lipid peroxidation (isoprostanes) throughout life; however, P. leucopus although having elevated protein carbonyls at a young age, the accrual of protein oxidation with age is minimal in contrast to the linear increase in M. musculus. Altogether, the results from young animals are in agreement with the predictions of the oxidative stress hypothesis of aging with the exception of protein carbonyls. Nonetheless, the age-dependent increase in protein carbonyls is more pronounced in short-lived M. musculus, which supports enhanced protein homeostasis in long-lived P. leucopus.
Keywords: Peromyscus leucopus, mitochondria, reactive oxygen species, skeletal muscle, oxidative damage, comparative biology of aging
mammalian life span ranges a hundred-fold, and comparative studies on long-lived animals can offer insights into key cellular mechanism(s) that may contribute to successful aging and longevity. The white-footed mouse, Peromyscus leucopus (Rodentia: Cricetidae), is one of the most common rodent species residing in North America. Both P. leucopus and the commonly used laboratory mouse, Mus musculus, belong to the superfamily Muridae and closely resemble each other in both body size and physical appearance. Yet P. leucopus can live more than 8 yr in captivity (35, 54), while the C57BL/6J strain of M. musculus lives up to 3.5 yr under standard laboratory conditions (Jax.org).
In comparative aging studies, it is important to account for the effect of body size on species longevity, and it is calculated using the longevity quotient (LQ), which is the ratio of observed maximum life span as a function of that predicted allometrically using the equation (y = 3.34 Mkg0.193) from Ref. 23 for nonvolant and nonaquatic mammals. On the basis of both the body size of P. leucopus (23 g for average adult weight; Ref. 22) and M. musculus (20.5 g for average adult weight; Ref. 22), P. leucopus lives 1.3 times as long as expected, whereas mice are notoriously short-lived and only live half as long as predicted by the longevity quotient (LQ = 0.58).
Long-lived P. leucopus has caught the attention of biogerontologists since the initial proposals over two decades ago (13, 57). In the brain and heart, P. leucopus compared with short-lived M. musculus exhibit lower mitochondrial reactive oxygen species (ROS) production (58), diminished protein carbonyl (32, 58), and elevated activity of antioxidant enzymes, catalase, and glutathione peroxidase (21, 58). Vascular systems of P. leucopus are also resistant to various exogenous stressors (21, 66). We are particularly interested in testing the oxidative stress hypothesis in the skeletal muscle, a postmitotic tissue type, which is sensitive to oxidative damage with age. The impact of oxidative stress is particularly important in the development of loss in muscle mass and integrity with age, which can significantly contribute to reduced function and quality of life in the elderly (5, 34, 38).
We took advantage of the fact that P. leucopus lives twice as long as M. musculus (two-fold difference in LQ) to compare several features of the skeletal muscle tissues pertinent to aging between these two species. On the basis of predictions of the oxidative stress theory of aging, we hypothesized that P. leucopus compared with M. musculus would exhibit lower levels of mitochondrial ROS production, enhanced antioxidant activity, and reduced oxidative damage accumulation with age in the skeletal muscle.
MATERIALS AND METHODS
Animals.
Young female animals (within 10% of MLSP) were used for mitochondrial experiments and enzymatic activity assays. The age ranges for young animals are 4–8 mo for M. musculus (C57BL/6J mice) and 4–9 mo for P. leucopus. Animals of P. leucopus were obtained from Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC). Both M. musculus and P. leucopus continue to gain weight until much older age. The postnatal growth rate is 0.0298 g/day and 0.0456 g/day for M. musculus and P. leucopus, respectively (based on Gompertz function from AnAge database). Studies by Guetzow and Judd (31) and Derrickson (25) have both shown that postnatal growth of P. leucopus is rapid and largely completed within 6 wk after birth. The female sex maturity is reached at about 42 days of age for M. musculus and 73 days for P. leucopus. Another closely related species of Peromyscus (P. maniculatus, commonly known as deer mice) has been shown to increase in body mass up to 5 yr of age in a study by Chappell et al. (18), although the growth rate declined slightly at around 485 days of age. The discrepancy between the actual animal body weight and reported average number could be due to the specific strain and sex. Skeletal muscle tissues from animals of different age groups (5, 12, 20, 30 mo for M. musculus and 7, 12, 26, 35, and 48 mo for P. Leucopus) were used for determination of F2-isoprostanes and protein carbonyl. Both animals were fed a standard NIH-31 mouse/rat diet (Harlan Teklad, Madison, WI) ad libitum. The animal rooms were maintained at 23°C and 40% relative humidity under artificial illumination with a 12:12-h light-dark cycle. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center San Antonio.
Measurement of body composition and respirometry.
Quantitative magnetic resonance imaging (qMRI) was used to determine the percentages of body fat and lean mass in unanesthetized animals, as described previously (42).
Oxygen consumption (V̇o2), CO2 production (V̇co2), and total animal activity were measured using the MARS respirometry system (Sable Systems International, Las Vegas, NV) with combined MAD-1 activity detector (same manufacturer). Animals were housed individually in normal live-in cages with their standard food and water. The temperature (Ta) was maintained at 23°C. The resting metabolic rate (ml O2·h−1·g body wt−1) was determined from averaged values of five consecutive lowest V̇o2 readings in the light cycle.
Muscle histology, fiber typing and transmission electron microscopy.
Hind limb muscles, including gastrocnemius, plantaris, and soleus, were dissected together and rapidly frozen in liquid nitrogen-cooled isopentane. Six-micrometer-thick cryosections were then processed using a cryostat. Muscle fiber types were determined by indirect immunofluorescence labeling using antibodies against different myosin heavy-chain isotypes (55) and Alexa-flurophore-conjugated secondary antibodies (Life Technologies, Grand Island, NY). The antibodies used are anti-myosin slow (Sigma NOQ 7.5 AD, 1:4,000), anti-myosin fast IIb [BF-F3, undiluted supernatant of hybridoma cell line; American Type Culture Collection (ATCC), Manassas, VA], and antimyosin fast IIa (SC-71, undiluted supernatant, ATCC). Sequential sections of the same muscle slides were assayed for succinate dehydrogenase activity and cytochrome-c oxidase activity, as previously described (44). Images were captured by using Nikon TE-2000 inverted microscope with 12-bit monochrome camera, pseudocolored and merged in NIS-Element imaging software. Fiber-type composition for gastrocnemius muscle was expressed as an averaged percentage of each fiber type from total quantifications of at least 1,000 nonoverlapping individual fibers from each animal (n = 4).
Gastrocnemius muscles were freshly dissected and fixed in 2% paraformaldehyde and 2.5% glutaraldehyde buffer and then postfixed with 1% osmium tetroxide followed by 1% uranyl acetate. The blocks were dehydrated and embedded in resin. Blocks were cut in ultrathin (90 nm) sections on a Reichert Ultracut UCT (Reichert, Vienna, Austria), stained with uranyl acetate followed by lead citrate and viewed on a Jeol 1230 EX transmission electron microscope (Jeol, Akishima, Japan) at 80 kV.
Enzymatic activity.
Activity of hexokinase (HK) is assayed on the basis of the reduction of NAD+ through a coupled reaction with glucose-6-phosphate dehydrogenase (G6PDH) and was determined spectrophotometrically by measuring the increase of NADH at λ 340 nm, as described previously (1). The activity of pyruvate kinase (PK) was determined in a lactate dehydrogenase (LDH)-coupled assay system by measuring the decrease in absorbance at λ 340 nm, resulting from the oxidation of NADH, as described in detail previously (67). GAPDH catalyzes the oxidation and phosphorylation of glyceraldehype-3-phosphate to 1,3 diphosphoglycerate and reduction of NAD+ to NADH. The activity was measured spectrophotometrically at λ 340 nm as a result of NAD+ reduction, as described previously (51). Activities of citrate synthase (CS) and cytochrome-c oxidase (COX) were performed spectrophotometrically, as described in detail previously (65).The enzymatic activity of each protein was determined and normalized to the value of M. musculus.
Mitochondria isolation from hind limb skeletal muscle.
Mitochondria were purified by using the modified method of Chappell and Perry (17), which has been previously described (46). Briefly, whole hind-limb skeletal muscle was excised and digested in a buffer containing 0.015% protease for 10 min at room temperature. Then, the tissue pieces were homogenized and centrifuged at 600 g for 10 min at 4°C. The supernatant, which contains mitochondria was filtered through a cheese cloth and centrifuged at 14,000 g for 10 min at 4°C. The pellet was resuspended and washed two more times. The final mitochondrial pellet was resuspended, and the protein concentration was measured using the Bradford method.
Mitochondrial ROS production and electron paramagnetic resonance spectroscopy.
The rate of mitochondrial H2O2 production rate was determined using Amplex Red (Life Technologies, Grand Island, NY), as described previously (44). Isolated mitochondria (30 μg) were incubated with either NADH-linked substrates, glutamate+malate (5 mM each) or FADH2-linked substrates, succinate (10 mM) plus rotenone (1 μM). Rotenone was added to block reverse electron transfer in the presence of succinate. In addition, rotenone (1 μM) and antimycin A (1 μM) were included to evaluate maximal H2O2 production. The slope was then converted into the rate of H2O2 production with a standard curve. Extramitochondrial superoxide release was measured by electron paramagnetic resonance spectroscopy (EPR) with the aid of a spin trap, 5-diisopropoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DIPPMPO; Enzo Life Sciences, Farmingdale, NY). DIPPMPO forms an adduct with O2·−, resulting in the generation of DIPPMPO-OOH, which then decays to the DIPPMPO-OH adduct by the action of glutathione peroxidases. EPR measurements were performed using an X-band MS200 spectrometer (Magnetech, Berlin, Germany), as previously described (44). Results of O2·− release were expressed as AU per 20 μg of mitochondrial protein.
Aconitase activity.
Mitochondrial matrix O2·− production was also indirectly measured by aconitase inactivation. The presence of a liable iron in [Fe-S]4 cluster in the active center of aconitase renders it susceptible for inactivation by O2·− (30). Therefore, the inactivation of matrix aconitase reflects indirectly the amount of O2·− production. Aconitase activity was measured following previously published protocol (49). Four conditions were assayed: no substrate, succinate alone, succinate+rotenone, and succinate+rotenone+antimycin A. The concentrations for succinate and rotenone are the same as EPR experiments above. The activity in each condition was normalized to the level of control condition (without substrate).
Measuring membrane potential (ΔΨ) with safranine O.
Membrane potential was monitored by fluorescence of the quench-dye safranine O, as described by Votyakova and Reynolds (69), based originally on the spectroscopic method of Akerman and Wikstrom (2). Safranine O fluorescence was followed using a Fluroskan-FL Ascent type 374 multiwell plate reader. Membrane potential was expressed as change of fluorescence from baseline per milligram of mitochondrial protein.
Electron transport chain complexes (I, II, III, and IV) and ATPase activity.
The activities of individual electron transport chain (ETC) complex were measured spectrophotometrically using enriched membrane fraction of mitochondrial extract, which has been treated with dodecyl-maltoside (1%), as described previously (46). The activity was measured by the extinction rate of electron donors or the production rate of electron acceptors for each ETC complex. After the initial rate was established, specific inhibitors were administered to determine the inhibitor-sensitive activity, as described in detail previously (20, 45). Individual electron transport chain complexes were separated by blue native gel (BN-PAGE) based on the size difference and visualized by scanning the gel (detailed methods refer to Ref. 71). The intensity of each band that corresponds to the abundance of each complex was quantified using National Institutes of Health (NIH) ImageJ software. After BN-PAGE separation, a band of ATPase was excised and incubated overnight with reaction buffer containing 35 mM Tris, 270 mM glycine, 14 mM MgSO4, 0.2% Pb(NO3)2, and 8 mM ATP. The intensity of the white band, which represents ATPase activity, was quantified using NIH ImageJ software.
Mitochondrial ATP production.
The rate of ATP synthesis was measured using the luciferin/luciferase assay kit (ATP bioluminescence assay kit HS II, Roche, Indianapolis, IN). Luciferin chemiluminescence was kinetically followed using a Fluoroskan-FL Ascent type 374 multiwell plate reader. Mitochondria (4 μg) were incubated with substrates (glutamate+malate or succinate+rotenone), and the measurement was started by the addition of luciferin/luciferase buffer containing 0.6 mM ADP. The initial slope was converted into nanomoles of ATP using the standards.
Western blot analysis.
Mitochondrial extracts were separated by SDS-PAGE and electroblotted onto PVDF membranes. Standard Western blot protocol was followed to examine uncoupling protein 3 (UCP3) and ATP synthase protein level. Antibodies used are anti-UCP3 (U7757; Sigma) and anti-ATP synthase β subunit (A21351; Molecular Probes), both of which cross-react with proteins from many other species.
Antioxidant enzyme activity.
Manganese superoxide dismutase (MnSOD) and copper zinc superoxide dismutase (CuZnSOD) activity in skeletal muscle homogenates from M. musculus and P. leucopus was measured using native gels, as previously described (7). Tissue homogenates were separated on 10% polyacrylamide gels in a phosphate buffer at pH 8.9. The gel was then incubated in a solution containing nitro blue tetrazolium (NBT), riboflavin, and tetramethylethylenediamine (TEMED). SOD prevents the reduction of NBT by superoxide, which forms the blue formazan, so that achromatic regions represent SOD activity. In another identical gel, KCN was included in the reaction buffer that inhibits CuZnSOD activity, so that it confirms the specificity. The activity of catalase was measured using Amplex red catalase assay kit (Life Technologies, Grand Island, NY) following the manufacturer's manual. Activity of catalase was converted to milliunits per microgram of skeletal muscle homogenate from a standard curve. The activity of glutathione peroxidase was measured by kinetically following the distinction of NADPH fluorescence (excitation at λ355 nm and emission at λ460 nm), as described previously (70). The activity was expressed as fluorescence unit per microgram of skeletal muscle homogenate.
F2-isoprostanes assay.
The levels of F2-isoprostanes in skeletal muscle tissue were determined using gas chromatography/negative-ion chemical ionization/mass spectrometry (GC-NICI-MS), as previously described (48). Briefly, skeletal muscles (∼100 mg) from animals of various ages were homogenized in ice-cold Folch solution (chloroform/methanol 2:1) containing 5 mg/100 ml butylatedhydroxytoluene. Lipids were then extracted and hydrolyzed with 15% KOH. After acidification with HCl, the F2-isoprostanes were extracted with a C18 Sep-Pak and a silica Sep-Pak column, converted to pentafluororobenzyl esters, and purified by thin-layer chromatography. The purified F2-isoprostanes were derivatized to trimethylsilyl ether derivatives and quantified by GC/MS using [2H4]8-Iso-PGF2α as an internal standard. The amounts of F2-isoprostanes are expressed as nanograms of 8-Iso-PGF2α per gram of tissue.
Protein carbonyl assay.
The total protein carbonyl levels were measured by fluorescein-5-thiosemicarbazide (FTC) labeling of carbonyl groups in total muscle extract and separation by electrophoresis, as previously described (19). Briefly, the muscle from animals of different age was homogenized in deaerated buffer and centrifuged at 4°C for 1 h at 100,000 g. The resulting supernatant (cytosolic fraction) was treated with 1% streptomycin sulfate and incubated at 37°C for 10 min. The cytosolic extracts were diluted and then labeled with FTC (1 mM), and subsequently, unbound FTC was washed. The final protein pellets were then dissolved in a phosphate buffer pH 8.0 containing 0.5 mM MgCl2, 1 mM EDTA, and 8 M urea. Dissolved proteins were then subjected to SDS-PAGE. After electrophoresis, the image of the fluorescent proteins on the gel was captured with the Typhoon 9400 using FTC settings (GE Healthcare Biosciences, Pittsburgh, PA). The same gel was stained with Coomassie blue and scanned again with Typhoon scanner. The carbonyl content of the protein samples was expressed as the ratio of FTC fluorescent intensity (carbonyls) to Coomassie blue absorption (protein concentration).
Statistical analysis.
All results were expressed as means ± SE, unless otherwise mentioned. All data were analyzed using Student's t-test unless otherwise described. P < 0.05 was considered statistically significant. Data of isoprostanes and protein carbonyl with age were analyzed using Pearson correlation test (two-tailed).
RESULTS
Body composition and resting metabolic rate.
As shown in Table 1, female P. leucopus has a significantly smaller body mass than M. musculus at 8 mo of age. However, qMRI analysis revealed that there is no difference in percentage of lean mass and fat mass between P. leucopus and M. musculus (Table 1). The resting metabolic rates for the two species are comparable (2.71 ± 0.07 ml O2·h−1·g body wt−1 for M. musculus vs. 2.66 ± 0.19 ml O2·h−1·g−1 body wt P. leucopus) under standard rodent housing conditions, in accordance with previous reports (24, 33).
Table 1.
Body composition of young adult M. musculus and P. leucopus
| Age, mo | MLSP, yr# | LQ | Body Weight, g | Fat Mass, g | Fat % | Lean Mass, g | Lean % | |
|---|---|---|---|---|---|---|---|---|
| M. musculus (C57BL/6) | 8.5 | 3.5 | 0.58 | 25.1 ± 1.1 | 7.0 ± 0.8 | 27% ± 2% | 18.2 ± 0.5 | 73% ± 2% |
| P. leucopus | 8 | 8 | 1.31 | 20.5 ± 1.3* | 4.7 ± 0.9 | 22% ± 3% | 15.9 ± 0.5* | 78% ± 3% |
Data are expressed as means ± SE; n = 8. Whole body fat mass, lean body mass, total mass, percent body fat, and percentage lean mass were measured using quantitative MRI machine. LQ denotes the longevity quotient, which is the ratio of maximal life span to the estimated life span based on the body mass.
Maximal life span potential (MLSP) values are taken from jax.org for C57BL/6 mouse and Anage database for P. leucopus.
P < 0.05 compared with M. musculus.
Characterization of fiber-type composition in hind limb skeletal muscle of P. leucopus and M. musculus.
Skeletal muscle is a heterogeneous tissue that varies in terms of innervation, oxidative/glycolytic metabolism, and contractile properties. On the basis of these differences, skeletal muscle can be divided into type I (slow oxidative), type IIa (fast oxidative), type IIx (fast intermediate), and type IIb (fast glycolytic). It has been well documented that muscles of various fiber types are differentially affected by age, and fast-twitch fibers are the most vulnerable to age-related atrophy (29, 36, 37). Unlike humans, most muscles in mouse are composed of a mixture of fiber types. Mouse gastrocnemius muscle is composed primarily of fast glycolytic type IIb fibers, with a small percentage of fast oxidative type IIa, slow oxidative type I fiber, and type IIx fibers, an intermediate fiber type in between IIb and IIa. In contrast, the soleus contains mostly type I and type IIa fibers (14). However, the fiber type for P. leucopus gastrocnemius and soleus has not been reported. Therefore, we first asked whether hind limb muscles of P. leucopus and M. musculus are similar in fiber types as measured by histological and biochemical analyses.
As shown in Fig. 1, P. leucopus and M. musculus have similar overall staining patterns for activities of succinate dehydrogenase (SDH; Fig. 1, A and B) and COX (Fig. 1, C and D). Indirect immunofluorescence staining of myosin heavy chain reveals that the gastrocnemius muscle of both P. leucopus and M. musculus is composed of a mixture of different fiber types with predominantly type IIb fibers and small percentage of type IIa, IIx, and very few type I/IIa fibers (Fig. 1E). In contrast, the soleus muscle in both species is primarily type I and type IIa fibers (Fig. 1E). High mitochondrial content/activity is found in more oxidative fiber types and higher glycolytic enzyme content/activity is associated with glycolytic fibers. Similarly, using electron microscopy, we found that gastrocnemius muscle from P. leucopus compared with M. musculus shows similar morphology in respect to mitochondrial density (Fig. 1F). However, despite the similarity between the two species in regard to fiber types, there are important differences in metabolism, mitochondrial composition, and function, as shown in the data described below.
Fig. 1.
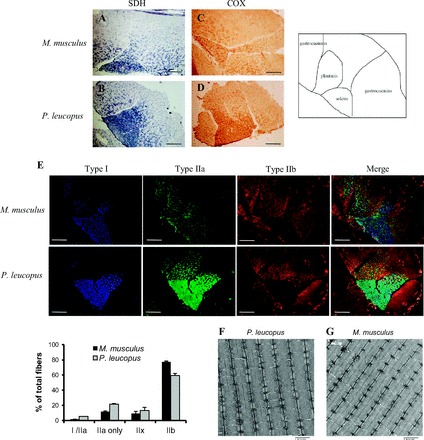
Hind limb muscle fiber types. Representative images of hind limb muscle cross sections of succinate dehydrogenase (SDH) activity staining (A, B), cytochrome-c oxidase (COX) staining (C, D) and immunofluorescence with antibodies against myosin heavy chain I (MHC I; blue), MHC IIa (green), and MHC IIb (red) (E). An illustration of different muscles in the cross-section view is shown on the right. A, C, E: top four panels are images of M. musculus. B, D, E: bottom four panels are images of P. leucopus. Scale bar = 100 μm. E, bottom: quantitative result of the percentage of different fiber types in the gastrocnemius muscle from M. musculus (black bars) and P. leucopus (gray bars). Quantification was performed from n = 4 animals per species and by counting at least 1,000 fibers per animal. Data are expressed as means ± SE. Representative transmission electron micrograph (TEM) images of gastrocnemius muscles from P. leucopus (F) and M. musculus (G). Scale bar equals 2 μm. All images were captured with same exposure settings and scaled equivalently postacquisition without gamma adjustment.
Glycolytic and oxidative enzymatic activities.
Analyses of glycolytic and oxidative enzymatic activities in gastrocnemius muscle homogenates from P. leucopus and M. musculus confirmed that muscles from the two species have similar metabolic profiles with some slight variation (Fig. 2A). HK and PK activity is not different in muscles from the two species. Activity of GAPDH is significantly elevated in muscles from P. leucopus compared with M. musculus. COX, a component of the mitochondrial electron transport chain, shows similar activity between the muscles from two species, whereas CS activity is slightly but significantly decreased in muscle of P. leucopus. To determine whether the activity of individual electron transport chain complexes is different between the two species, we measured the activity of complex I, II, III, and IV in vitro and normalized it to total mitochondrial protein content. In addition, we measured ATPase activity by blue native gel electrophoresis. Interestingly, we found significant differences in mitochondria ETC activities between the two species. Activities of complexes I and III are significantly lower, and activities of complex IV and ATPase are higher (Fig. 2B) in P. leucopus compared with M. musculus. There is no difference in complex II activity between the two species (Fig. 2B). These differences in complexes I and III can be explained, in part, by the differences in the amount of complex protein (Fig. 2, C and D). Moreover, the lower activities of complexes I and III in P. leucopus are associated with a reduction in both ATP production (Fig. 2E) and membrane potential (Fig. 2F) in isolated intact mitochondria compared with mitochondria from M. musculus.
Fig. 2.
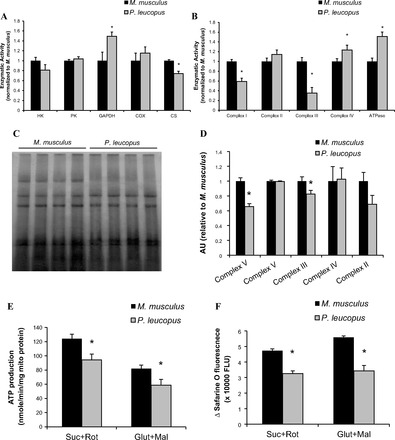
Activity of glycolytic and oxidative enzymes and electron transport chain complexes. A: skeletal muscle homogenates were used to test glycolytic enzyme activities: hexokinase (HK), pyruvate kinase (PK), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and oxidative enzymes, cytochrome-c oxidase (COX), and citrate synthase (CS) (n = 6–12). B: activities of respiratory complex chains, including NADH dehydrogenase (complex I), succinate dehydrogenase (complex II), ubiquinone:cytochrome-c oxidoreductase (complex III), cytochrome-c oxidase (complex IV), and ATPase. Mitochondrial proteins were solubilized with dodecyl maltoside (1%), and membrane-enriched soluble proteins were then used to measure electron transport chain (ETC) activity either using a spectrophotometric assay or an in-gel assay, see details in materials and methods (n = 6–10). C: representative blue-native gel image of electron transport chain complexes. D: quantification of protein contents of ETC (n = 8). E: production of ATP was measured in isolated skeletal muscle mitochondria using a luciferase/luciferin assay kit as described in materials and methods (n = 6). F: mitochondrial membrane potential (ΔΨm) was measured using fluorescent safarine O dye. The quenching of safarine O fluorescence is proportional to the electrical potential across the mitochondrial inner membrane (n = 6). Black bar represents M. musculus, and gray bar represents P. leucopus. Data are expressed as means ± SE. *P < 0.05 compared with M. musculus.
ROS production is lower in muscle mitochondria from P. leucopus compared with M. musculus. Skeletal muscle tissue is a highly active tissue for whole body metabolism. In fact, muscle contributes to the highest percentage of the total resting metabolic rate among all major oxygen-consuming organs given its large contribution to total body mass. Because of the highly metabolic nature of skeletal muscle and the enrichment of mitochondria, ROS originated from mitochondria and dysfunction of mitochondria have been implicated in age-related changes in muscle mass and functional decline. We predicted that mitochondria from long-lived P. leucopus would produce less ROS. We measured the ROS production from mitochondria isolated from hind limb muscles of young adult P. leucopus and M. musculus. In general, our data described below support a reduction in ROS production in the skeletal muscle of P. leucopus.
We measured H2O2 generation from isolated muscle mitochondria using the Amplex Red and HRP method. We found that in mitochondria isolated from P.leucopus respiring on either NADH-linked substrates (glutamate + malate) or FADH2-linked substrates (succinate + rotenone); H2O2 was reduced 36% and 39%, respectively, compared with M. musculus (Fig. 3A). In addition, when the complex I inhibitor rotenone, a known stimulator of superoxide O2·− production was added, the maximal H2O2 generation rate at the complex I site by mitochondria from M. musculus was almost three-fold higher than those from P. leucopus (593 ± 20 pmol·min−1·mg−1 vs. 207 ± 43 pmol·min−1·mg−1 for M. musculus and P. leucopus, respectively, Fig. 3B). Moreover, when antimycin A, which inhibits electron flow from the Qi-site to the Qo-site within complex III, was applied to maximize H2O2 release at complex III, P. leucopus mitochondria produced significantly lower levels of H2O2 compared with those from M. musculus (Fig. 3B).
Fig. 3.
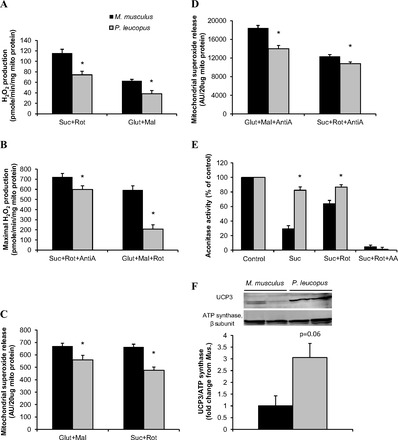
Mitochondrial reactive oxygen species (ROS) production. Mitochondrial H2O2 production was measured using Amplex-Red probe at nonphosphorylating state (A) and in the presence of inhibitors (B) (n = 6). Mitochondrial superoxide release was measured by electron paramagnetic resonance spectroscopy (EPR) with spin trap DIPPMPO with substrates (C) and with antimycin A (D) (n = 8); matrix aconitase activity was measured in the presence of succinate, succinate+rotenone and succinate+rotenone+antimycin A (E) (n = 8). The inactivation reflects the net production of superoxide in the mitochondrial matrix compartment. F: uncoupling protein 3 (UCP3) was blotted in mitochondrial fractions from muscles of M. musculus and P. leucopus. Top: representative images of Western blot analysis. Bottom: quantification results (n = 3). Black bar represents M. musculus, and gray bar represents P. leucopus. Data are expressed as means ± SE. *P < 0.05 compared with M. musculus.
Mitochondrial superoxide (O2·−) release was determined using electron paramagnetic resonance (EPR) and the spin-trap DIPPMPO, as previously described (44). Basal O2·− release was slightly, but significantly, reduced in P. leucopus than M. musculus (Fig. 3C). When antimycin A was added to mitochondria with either complex I substrates or complex II substrates, maximal O2·− release was observed due to stabilization of ubisemiquinone radicals at complex III. Mitochondria from P. leucopus displayed 24% and 12% lower levels of O2·− release compared with those from M. musculus (Fig. 3D).
We also measured O2·− production indirectly by aconitase inactivation. Aconitase activity is highly sensitive to inactivation by O2·−. Differences in O2·− generation by the ETC toward the mitochondrial matrix would be predicted to be reflected in aconitase activity (30). In M. musculus, the activity of aconitase was inhibited to 29% and 64% of control level when the mitochondria were treated with succinate alone or succinate plus rotenone, respectively. Succinate, which is known to stimulate reverse electron transfer to complex I, increases O2·− production at the complex I site. Rotenone, which blocks this reverse electron transfer, reduces succinate-supported matrix O2·− production. The succinate inhibition of aconitase was markedly attenuated in P. leucopus mitochondria. In addition, the reverse electron transfer by succinate was almost absent (18% reduction from control by succinate vs. 13% reduction from control by succinate+rotenone; Fig. 3E). On the other hand, when antimycin A was added to stimulate maximal O2·− production both toward the matrix and intermembrane space, aconitase from both species was almost completely inactivated.
Mitochondrial uncoupling has been proposed as an important mechanism to minimize ROS production. Therefore, we measured the level of UCP3 in isolated mitochondria. When normalized to β subunit of ATP synthase, we observed that mitochondria from muscles of P. leucopus have elevated UCP3 expression compared with those from M. musculus (Fig. 3F). Therefore, it is plausible that uncoupling could contribute to the reduced ROS production seen in longer-lived P. leucopus.
Some, but not all, antioxidant enzyme exhibit elevated activities in skeletal muscles of P. leucopus. The oxidative stress hypothesis of aging predicts that long-lived animals would have greater capacity for ROS detoxification. We tested whether long-lived P. leucopus has superior skeletal muscle antioxidant enzyme activity, compared with short-lived M. musculus. The activity of four primary antioxidant enzymes, cytosolic copper zinc superoxide dismutase (CuZnSOD, SOD1), glutathione peroxidases (GPX), catalase, and mitochondrial manganese superoxide dismutase (MnSOD, SOD2), was measured. SODs catalyze the dismutation of O2·− to H2O2 in both the cytosol (CuZnSOD) and mitochondria (MnSOD). Glutathione peroxidase catalyzes reaction of lipid hydroperoxides to less toxic alcohol, as well as converting H2O2 to water. Catalase is responsible for catalyzing H2O2 to oxygen and water. Consistent with our prediction, P. leucopus demonstrate greater activities of CuZnSOD and catalase than M. musculus (Fig. 4, A and C), although the activity of MnSOD is not different between the two species (Fig. 4B). Most strikingly, the activity of GPX is over 10-fold higher in P. leucopus than in M. musculus (Fig. 4D). Accordingly, the abundance of CuZnSOD and GPX1 is higher in skeletal muscle homogenate from P. leucopus than that from M. musculus. However, the protein level of mitochondrial localized antioxidant enzymes, MnSOD, glutathione peroxidases 4 (GPX4), and peroxiredoxin 3 (PRDX3) were found to be lower in P. leucopus (data not shown).
Fig. 4.
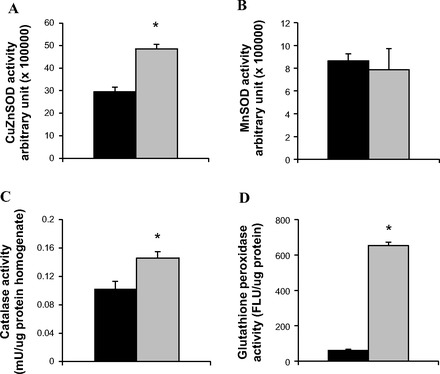
Antioxidant enzyme activities. Activities of CuZnSOD (A), MnSOD (B), catalase (C) and glutathione peroxidase (D) were measured in skeletal muscle homogenates from M. musculus and P. leucopus, as described in detail in materials and methods. n = 4 for (A) and (B); n = 6 for (C) and (D). Black bar represents M. musculus, and gray bar represents P. leucopus. Data are expressed as means ± SE. *P < 0.05 compared with M. musculus.
Skeletal muscles of P. leucopus have distinct patterns of age-dependent accumulation of oxidative damage to proteins and lipids compared with short-lived M. musculus. Oxidative stress, as a result of excessive ROS production and inefficient detoxification, leads to damage to macromolecules, such as protein, lipids, and nucleotides. On the basis of the attenuated ROS production and increased antioxidant activity observed in P. leucopus, we predict that muscles of P. leucopus would have reduced steady-state oxidative damage, even at a young age. Alternatively, P. leucopus would have diminished accumulation of oxidative damage with age. We examined two markers of oxidative damage-protein carbonyl and F2 isoprostanes in muscles from P. leucopus and M. musculus across different ages. F2 isoprostanes (IsoPs) are prostaglandin-like compounds that are produced in vivo primarily by free radical-induced peroxidation of arachidonic acid. It represents one of the most reliable approaches to assess oxidative stress status in vivo (47, 52). The level of skeletal muscle F2 isoprostane is positively correlated with chronological age in both P. leucopus and M. musculus (n = 23, r = 0.44, P < 0.05; n = 30, r = 0.61, P < 0.001, respectively; Fig. 5A). The positive correlation remains true when the age was normalized to percentage of maximal life span potential (Fig. 5B). Although the slopes between two linear regression lines are not significantly different (0.006785 ± 0.003181 vs. 0.005663 ± 0.001414 for M. musculus and P. leucopus, respectively, Fig. 5B), the value of isoprostanes in long-lived P. leucopus is almost always below that in M. musculus. Protein carbonyl derivatives can be formed directly by free radical oxidation on amino acids. They can also originate from secondary reactions of amino acids with reactive aldehydes formed during lipid peroxidation and glycation/glycoxidation of lysine amino groups (8, 59). Therefore, the protein carbonyl level reflects accumulated damaged protein from multiple ROS sources over time that escapes proteolytic degradation. In contrast to specific lipid peroxidation production-isoprostanes, carbonyl content is 30% higher in 8-mo-old P. leucopus than in 4-mo-old M. musculus. However, protein carbonyls in the muscle extract of M. musculus increase significantly with chronological age (n = 23, r = 0.72, P < 0.001; Fig. 5C) and physiological age (n = 23, r = 0.72, P < 0.001, Fig. 5D). The levels of protein oxidation in P. leucopus remains unchanged with age up to 48 mo of age (∼50% of MLSP; n = 24, r = 0.02, P = 0.91, Fig. 5, C and D).
Fig. 5.
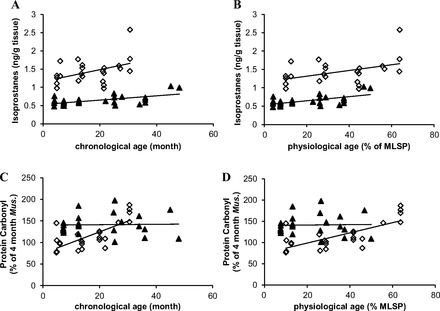
Age-associated oxidative damage to lipids and proteins. The scatterplots of skeletal muscle isoprostanes in M. musculus and P. leucopus over chronological age (A) and physiological age (B). The association between protein carbonyl levels in muscle with chronological age (C) and physiological age (D) in M. musculus and P. leucopus. Each point in the graphs represents one individual animal. Black triangles (▲) represent P. leucopus and open diamonds (◇) represents M. musculus. Linear regression line is shown on top of each data set. The correlation was tested using Pearson two-tailed test with P < 0.05 considered to be significant.
DISCUSSION
Skeletal muscle is a major component to body mass and accounts for 20–30% of standard metabolic rate (53). Because of the highly metabolic nature of skeletal muscle, it has a high potential for mitochondrially generated oxidative stress. Indeed, there is a significant amount of evidence demonstrating a strong association between oxidative stress and mitochondrial dysfunction with age within a single organism (15, 28, 43, 46). Therefore, it is reasonable to speculate that longer-living species would have developed mechanisms to maintain lower levels of oxidative stress in this tissue compared with shorter-lived species, even at a young age.
On the basis of the predictions of the oxidative stress theory of aging, we hypothesized that the long-lived P. leucopus, compared with short-lived M. musculus, would produce less ROS by the skeletal muscle mitochondria, have enhanced antioxidant capacity, and would show reduced oxidative damage to macromolecules. Consistent with our predictions, we found that skeletal muscles from P. leucopus exhibit 1) lower mitochondrial ROS production (both H2O2 and O2·−); 2) elevated antioxidant activity (CuZnSOD, catalase, and glutathione peroxidase); 3) lower levels of oxidative damage to lipids with age, and 4) an attenuated increase of protein carbonyl with age than observed in muscles from M. musculus, although higher levels at young age. Taken together, these data suggest that the longer life span in P. leucopus is associated with reduced free radical production and diminished accrual of oxidative damage in skeletal muscle and are consistent with the oxidative stress theory of aging.
The electron transport chain of mitochondria generate O2·− when single electron leak to O2 as electron pairs flow down the chain (16). There are seven main sites known for mitochondrial ROS production, and among them, complex I and complex III are the major sources of mitochondrial O2·− (10). Measurement of H2O2 in isolated mitochondria in the presence of NADH-substrates (e.g., glutamate+malate) and a Q binding site inhibitor, such as rotenone, indirectly reflects the capacity of O2·− production in complex I at sites upstream of Q binding site, mostly likely flavin mononucleotide and iron-sulfur clusters. Mitochondria from muscle of P. leucopus showed a significantly reduced rate of H2O2 production in response to glutamate+malate and rotenone (207 ± 47 pmol·min−1·mg−1 vs. 593 ± 20 pmol·min−1·mg−1; Fig. 3B), suggesting that the complex I of P. leucopus has reduced maximal propensity to leak electron to oxygen to form O2·−. Furthermore, antimycin A, a Qi site inhibitor of complex III, which stimulates O2·− production at Qo site toward both the matrix and intermembrane space, resulted in a marked increase in H2O2 and O2·− release in both P. leucopus and M. musculus mitochondria (Fig. 3, B and D). In addition to the reduction in H2O2 production, the generation of O2·− is also significantly diminished in skeletal muscle mitochondria from P. leucopus (Fig. 3, C and D). Production of O2·− from complex I and III has been shown to be partially sensitive to membrane potential (ΔΨ) (60, 64, 69). We measured the changes in safarine O fluorescence, the quenching of which is dependent on ΔΨ (2). Mitochondria from P. leucopus showed reduced ΔΨ in basal conditions without inhibitors (Fig. 2F), which could potentially contribute to the reduced production of ROS under these conditions. Moreover, UCP3 protein is elevated in mitochondria from P. leucopus (Fig. 3F). Taken together, it suggests that mitochondria of P. leucopus may utilize uncoupling mechanisms to mitigate ROS generation. UCP3, among other known uncoupling proteins (UCPs 1–3), and adenine nucleotide translocase are known to be activated by ROS and ROS by-products. It induces “mild uncoupling”, i.e., a small dissipation of membrane potential, which is estimated to account for 20–50% of total reparation of the mitochondria. Therefore, it serves as a feedback mechanism to reduce ROS production as the first line of defense against oxidative stress. Studies have shown mitochondria of UCP3 knockout mice exhibit a higher rate of ROS production (56, 63, 68) and mice, which have UCP3 overexpressed in the muscle mitochondria, produce lower levels of H2O2 (27). Hence, the elevated expression of UCP3 in P. leucopus mitochondria allows proton leak from intermembrane space back to matrix (uncoupled respiration) to decrease the proton-motive force and, thereby, reduce ROS emission. It is consistent with reduced production of H2O2 and O2·− by mitochondria from P. leucopus reported here.
Our data showing reduced production of H2O2 and O2·− in skeletal muscle mitochondria are consistent with previous studies from other tissues in P. leucopus (12, 21, 39, 66). In the brain, heart, and endothelial cells of P. leucopus, investigators demonstrate a low rate of mitochondrial O2·− and H2O2 generation (39, 58), high activities of catalase and glutathione peroxidase (21, 58), low levels of oxidative damage to proteins (32, 58), delayed accumulation of DNA damage (62), and enhanced resistance to oxidative stress in vitro (21, 66) compared with M. musculus. Interestingly, we found that the levels of H2O2 and O2·− are lower, even when inhibitors (rotenone or antimycin A) were added to stimulate their maximal production. Another indicator showing differences in ROS production/damage is the aconitase inactivation assay. Aconitase, which catalyzes the isomerization of citrate to isocitrate, is sensitive to matrix O2·− and becomes inactivated following an increase in ROS production. In P. leucopus, this inactivation is significantly attenuated compared with M. musculus (Fig. 3E). The lessened inactivation of aconitase from P. leucopus could be attributed to reduced production of O2·− and/or efficient removal of O2·− by antioxidants, such as MnSOD. However, because the activity of MnSOD is not different between P. leucopus and M. musculus (Fig. 4B), the difference is most likely due to lower production of O2·− toward the matrix by mitochondria from P. leucopus. Another potential mechanism to decrease ROS production is termed “spare oxidative capacity” (41). Previously, Lane had suggested that an increase in activity of ETC components at or downstream of the known ROS-producing sites should lower the reduction state of these sites. Therefore, the possibility to move electrons past these ROS generators would be higher than the capacity to leak electrons to the sites, thereby producing ROS. This concept has previously been suggested as one of the explanations for the relatively low ROS production seen in long-lived house sparrow (MLSP = 13.5 yr) and big brown bat (MLSP = 19 yr), compared with mouse (MLSP = 3.5 yr) (11). In our study, we observed that the maximal in vitro activity of complex IV, the final electron transport complex was significantly elevated in P. leucopus compared with that of M. musculus (Fig. 2B). This is consistent with the concept that long-lived P. leucopus utilizes the spare oxidative capacity strategy to minimize ROS generation. Finally, the difference in mitochondrial composition (proteins and membrane lipids) and assembly are also likely to affect the probability of ROS production. For example, the reduced production of heart mitochondrial ROS from pigeon compared with rat was attributed to the low complex I content in pigeon mitochondria (40). We also observed reduced complex I content and activity in mitochondria from P. leucopus compared with those of M. musculus (Fig. 2, B–D). Another interesting finding is that we observed reduced citrate synthase activity in isolated muscle mitochondria from P. leucopus. When mitochondrial ROS production was normalized to citrate synthase activity, the only difference between two species remains statistically significant is the H2O2 production in the presence of glutamate, malate, and rotenone (data not shown). These two pieces of evidence indicate that the site of ROS production at complex I is probably the greatest difference between these two rodent species. This hypothesis needs to be further tested and validated.
Reduced mitochondrial ROS production toward both the mitochondrial matrix and cytosol from P. leucopus could lead to decreased oxidative damage to macromolecules if the antioxidant system is maintained at an equal or a greater level than the shorter-lived M. musculus. Previous studies have shown that brain and heart tissues of P. leucopus have higher activities of catalase and glutathione peroxidase in addition to having lower protein oxidative damage when comparing with M. musculus (58). Consistent with results from other tissues, we found that oxidative damage to lipids (Fig. 5, A and B) was approximately half the level in long-lived P. leucopus compared with in M. musculus throughout life. Although P. leucopus display higher baseline levels of protein carbonyl at a young age, they do not accrue further protein oxidative damage with age in contrast to short-lived M. musculus. This latter feature has been shown in another long-lived rodent species, naked mole rat (MLSP >28 yr) (4). It hints that long-lived species may have evolved mechanisms to tolerate elevated levels of protein oxidation. In contrast, short-lived M. musculus is more vulnerable for accumulation of oxidized proteins, possibly due to continuous ROS production and compromised protein degradation processes, including ubiquitin-proteasome and lysosomal pathways. It suggests that unlike P. leucopus, short-lived M. musculus are unable to maintain the cellular protein homeostasis with age. Furthermore, the identification of oxidative modifications to specific proteins or within particular cellular compartments is likely to have differential effects on numerous important biological processes and functional pathways, as suggested by some recent studies (6, 9). All of these possibilities warrant further study. Our results corroborated these previous findings that skeletal muscles of P. leucopus exhibit higher activities of CuZnSOD, catalase, and GPX, but no change in MnSOD (Fig. 4). Enhanced GPX activity may contribute to the reduced lipid oxidation marker-isoprostanes in long-lived P. leucopus. The protein expression levels of these antioxidant enzymes were somewhat different than an earlier study, which examined the abundance of these enzymes in blood vessels (21). There were twofold and fourfold increases in the protein levels of GPX1 and catalase, respectively, in aortas of P. leucopus compared with those of M. musculus; whereas expression of CuZnSOD and MnSOD was not different (21). We found elevated protein levels of CuZnSOD and GPX1 but reduced expression of MnSOD, GPX4, and PRDX3 (data not shown). This could be related to tissue-specific regulation of antioxidant enzyme expression.
Unavoidably, there are some limitations to the current study. The premise of using P. leucopus as a “long-lived” model to study tissue oxidative stress is based on the assumption that long-lived animals apply longevity assurance mechanisms to maintain their physiological functions throughout the entirety of the life span. However, there is some evidence suggesting that P. leucopus, indeed, demonstrates an attenuated decline in multiple physiological functions with age compared with the short-lived M. musculus. It is known that both male and female P. leucopus are capable of reproduction at 5.5 yr of age and the hypothalamic-pituitary-ovarian axis remains largely intact (13, 61). In addition, P. leucopus has a much slower rate of weight loss of 0.23% per month for both sexes than shorter-lived M. musculus, which shows a steady 2% decline per month in body weight following cessation of skeletal growth (26). On the other hand, the widely used various inbred strains of M. musculus, are much shorter-lived than would be expected based upon body size. It is possible that many age-related changes regarding the oxidative stress parameters are amplified due to the short life span of M. musculus. In that sense, wild-caught animals would be ideal to study without the influence of laboratory inbreeding, which favors artificial selection. We have found that the wild-derived mice (Idaho mice, MLSP = 4 yr), with longer life span than many lab strains (MLSP = 3.5 yr), have similar levels of mitochondrial ROS and ATP production to C57BL/6 mice at young age (Van Remmen et al., unpublished data). Moreover, the age-dependent changes in oxidative stress parameters seen in C57BL/6 mice in 25-mo-old animals (∼60% of MLSP) are similar to those in 43-mo-old Idaho mice (∼90% of MLSP).
Nonetheless, by comparing a longer-lived rodent species with M. musculus, significant insights could be gained into the mechanisms of aging in a broader context. It remains to be fully investigated 1) whether P. leucopus, indeed, maintains its skeletal muscle mass and function with age or the rate of decline is attenuated; and 2) whether all parameters of oxidative stress measurements that are associated with longevity correlate with the skeletal muscle function and structure integrity. Testing these hypotheses involves functional studies using aged animals. Although we observed a consistent lower level of F2-isoprostanes and attenuated age-related increase in protein carbonyls in P. leucopus from 8 mo up to 48 mo (Fig. 5), the reduction of gastrocnemius mass at 24 mo (∼25% of its MLSP) in P. leucopus was found to be similar in physiologically age-matched M. musculus (data not shown). It is, therefore, important to chronologically document the changes in muscle mass and function. It remains to be tested whether muscular fitness is selected against throughout evolution in aged animals of P. leucopus. It is unlikely since the majority of field population rarely survive more than 1.5 yr. Another closely related species, P. maniculatus has been reported to exhibit age-dependent decline in mass-adjusted maximal aerobic performance (V̇o2 max) after around 485 days old (18). This change is not explained by the alterations in body composition (lean and fat mass), suggesting that mass-specific oxidative capacity of lean tissues, including heart, lung, and skeletal muscle decrease with age (18). The change in tissue-specific mitochondrial content and function are of crucial importance in determining the reserve capacity for oxidative metabolism.
It has been shown in numerous reports that mitochondria from muscles of different fiber types have distinct bioenergetic profiles (3, 50). In addition, muscles of different fiber types exhibit different vulnerability to age-related changes, with gastrocnemius (primarily fast-type II fibers) affected the most, while the highly recruited soleus (mostly type I and IIa fibers) minimally affected in mice (29). Therefore, it is important to identify and characterize the fiber types in the hind limb muscles of P. leucopus. What we have found is a great deal of similarity in terms of fiber composition and enzymatic activity between the two species among the hind limb muscles (gastrocnemius, plantaris, and soleus; Fig. 1). However, the mitochondria from these muscles display different properties in respect to ROS production and ATP generation. To fully understand the specific mechanisms of regulation and modulation in P. leucopus muscle mitochondria requires further investigation.
Perspectives and Significance
Our study in skeletal muscles of long-lived P. leucopus compared with shorter-lived M. musculus largely agrees with the predictions that longevity is associated with reduced oxidative stress with the exception of protein carbonyls in young animals. We predict that long-lived P. leucopus would maintain skeletal muscle integrity and function with age. Further investigations of how P. leucopus muscle adapt with age at the molecular, cellular, and functional level will offer more insights into the etiology of sarcopenia. This study also provides further rationale for the use of P. leucopus as a valuable model to study mechanisms of aging and age-related alterations in the skeletal muscle.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.S. and H.V.R. conception and design of research; Y.S., D.A.P., Y.L., R.T.H., A.L.J., A.B., L.B.S., and W.Q. performed experiments; Y.S., Y.L., R.T.H., A.L.J., A.B., L.B.S., and W.Q. analyzed data; Y.S., D.A.P., R.T.H., A.B., W.Q., A.C., R.B., Z.I.U., S.N.A., and H.V.R. interpreted results of experiments; Y.S. and R.T.H. prepared figures; Y.S. drafted manuscript; Y.S., D.A.P., R.T.H., A.B., A.C., R.B., Z.I.U., S.N.A., and H.V.R. edited and revised manuscript; Y.S., D.A.P., Y.L., R.T.H., A.L.J., A.B., L.B.S., W.Q., A.C., R.B., Z.I.U., S.N.A., and H.V.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate Michael Lustgarten's assistance with muscle freezing and cryosections and Adam Salmon for helpful discussions in the preparation of the manuscript. We also thank Barbara Hunter in the electromicroscopy core for her excellent service.
REFERENCES
- 1. Abnous K, Storey KB. Skeletal muscle hexokinase: regulation in mammalian hibernation. Mol Cell Biochem 319: 41–50, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Akerman KE, Wikström MK. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68: 191–197, 1976 [DOI] [PubMed] [Google Scholar]
- 3. Anderson EJ, Neufer DP. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell 5: 463–471, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Augustin H, Partridge L. Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta 1790: 1084–1094, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Baraibar MA, Friguet B. Oxidative proteome modifications target specific cellular pathways during oxidative stress, cellular senescence and aging. Exp Gerontol In press [DOI] [PubMed] [Google Scholar]
- 7. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287, 1971 [DOI] [PubMed] [Google Scholar]
- 8. Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272: 20313–20316, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharya A, Leonard S, Tardif S, Buffenstein R, Fischer KE, Richardson A, Austad SN, Chaudhuri AR. Attenuation of liver insoluble protein carbonyls: indicator of a longevity determinant? Aging Cell 10: 720–723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown J, Faure P, Klaiman J, Staples J. Examining the mechanisms responsible for lower ROS release rates in liver mitochondria from the long-lived house sparrow (Passer domesticus) and big brown bat (Eptesicus fuscus) compared to the short-lived mouse (Mus musculus). Mech Ageing Dev 130: 467–476, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Brunet-Rossinni AK. Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech Ageing Dev 125: 11–20, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Burger J, Gochfeld M. Survival and reproduction in Peromyscus leucopus in the laboratory: viable model for aging studies. Growth Dev Aging 56: 17–22, 1992 [PubMed] [Google Scholar]
- 14. Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221: 177–190, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Chabi B, Ljubicic V, Keir JM, Julianna HH, Saleem A, David AH. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 7: 2–12, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Chappell JB, Perry SV. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature 173: 1094–1095, 1954 [DOI] [PubMed] [Google Scholar]
- 18. Chappell MA, Rezende EL, Hammond KA. Age and aerobic performance in deer mice. J Exp Biol 206: 1221–1231, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Chaudhuri AR, de Waal EM, Pierce A, Van Remmen H, Ward WF, Richardson A. Detection of protein carbonyls in aging liver tissue: A fluorescence-based proteomic approach. Mech Ageing Dev 127: 849–861, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Choksi KB, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free Radic Biol Med 44: 1795–1805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007 [DOI] [PubMed] [Google Scholar]
- 22. de Magalhaes JP, Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol 22: 1770–1774, 2009 [DOI] [PubMed] [Google Scholar]
- 23. de Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci 62: 149–160, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deavers DR, Hudson JW. Temperature regulation in two rodents (Clethrionomys gapperi and Peromyscus leucopus) and a shrew (Blarina brevicauda) inhabiting the same environment. Physiol Zool 54: 94–108, 1981 [Google Scholar]
- 25. Derrickson EM. Patterns of postnatal growth in a laboratory colony of Peromyscus leucopus. J Mammal 69: 57–66, 1988 [Google Scholar]
- 26. Duffy PH, Sacher GA. Age-dependence of body weight and linear dimensions in adult Mus and Peromyscus. Growth 40: 19–31, 1976 [PubMed] [Google Scholar]
- 27. Estey C, Seifert EL, Aguer C, Moffat C, Harper ME. Calorie restriction in mice overexpressing UCP3: evidence that prior mitochondrial uncoupling alters response. Exp Gerontol 47: 361–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J 390: 501–511, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol 39: 17–24, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol 349: 9–23, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Guetzow DD, Judd FW. Postnatal growth and development in a subtropical population of Peromyscus leucopus texanus. Southwestern Naturalist 26: 182–191, 1981 [Google Scholar]
- 32. Guo Z, Wang M, Tian G, Burger J, Gochfeld M, Yang CS. Age- and gender-related variations in the activities of drug-metabolizing and antioxidant enzymes in the white-footed mouse (Peromyscus leucopus). Growth Dev Aging 57: 85–100, 1993 [PubMed] [Google Scholar]
- 33. Hill RW. Daily torpor in Peromyscus leucopus on an adequate diet. Comp Biochem Physiol A Comp Physiol 51: 413–423, 1975 [DOI] [PubMed] [Google Scholar]
- 34. Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev 60: 199–213, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Institute of Laboratory Animal Resources Mammalian Models for Research on Aging. Washington D. C.: National Academy Press, 1981, p. 587 [Google Scholar]
- 36. Ishihara A, Naitoh H, Katsuta S. Effects of ageing on the total number of muscle fibers and motoneurons of the tibialis anterior and soleus muscles in the rat. Brain Res 435: 355, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Jakobsson F, Borg K, Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol (Berl) 80: 459–468, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Johnston J, Iser WB, Chow DK, Goldberg IG, Wolkow CA. Quantitative image analysis reveals distinct structural transitions during aging in Caenorhabditis elegans tissues. PLos One 3: e2821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946–H956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambert AJ, Buckingham JA, Boysen HM, Brand MD. Low complex I content explains the low hydrogen peroxide production rate of heart mitochondria from the long-lived pigeon, Columba livia. Aging Cell 9: 78–91, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Lane N. Power, Sex, Suicide: Mitochondria and the Meaning of Life. Oxford University Press, 2005, p. 368 [Google Scholar]
- 42. Liao CY, Rikke BA, Johnson TE, Gelfond JA, Diaz V, Nelson JF. Fat maintenance is a predictor of the murine lifespan response to dietary restriction. Aging Cell 10: 629–639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lopez ME, Van Zeeland NL, Dahl DB, Weindruch R, Aiken JM. Cellular phenotypes of age-associated skeletal muscle mitochondrial abnormalities in rhesus monkeys. Mutat Res 452: 123–138, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Lustgarten MS, Jang YC, Liu Y, Muller FL, Qi W, Steinhelper M, Brooks SV, Larkin L, Shimizu T, Shirasawa T, McManus LM, Bhattacharya A, Richardson A, Van Remmen H. Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am J Physiol Cell Physiol 297: C1520–C1532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell 10: 493–505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127: 298–306, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem 14: 703–717, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol 300: 3–12, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Picard M, Ritchie D, Melissa MT, Kathryn JW, Russell TH. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell 10: 1047–1055, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Pierce A, Mirzaei H, Muller F, De Waal E, Taylor AB, Leonard S, Van Remmen H, Regnier F, Richardson A, Chaudhuri A. GAPDH is conformationally and functionally altered in association with oxidative stress in mouse models of amyotrophic lateral sclerosis. J Mol Biol 382: 1195–1210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28: 505–513, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77: 731–758, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Sacher GA, Hart RW. Longevity, aging and comparative cellular and molecular biology of the house mouse, Mus musculus, and the white-footed mouse, Peromyscus leucopus. Birth Defects Orig Artic Ser 14: 71–96, 1978 [PubMed] [Google Scholar]
- 55. Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 77: 493–501, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Seifert EL, Bezaire V, Estey C, Harper ME. Essential role for uncoupling protein-3 in mitochondrial adaptation to fasting but not in fatty acid oxidation or fatty acid anion export. J Biol Chem 283: 25124–25131, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Smith GS, Crew MD, Walford RL. Peromyscus as a gerontologic animal: aging and the MHC. In: Genetic Effects on Aging ii, edited by Harrison DE. Caldwell, New Jersey: Telford Press, 1990, chapt. 25, p. 457–472 [Google Scholar]
- 58. Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem Biophys Res Commun 196: 7–11, 1993 [DOI] [PubMed] [Google Scholar]
- 59. Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Steger RW, Peluso JJ, Huang HH, Hodson CA, Leung FC, Meites J, Sacher G. Effects of advancing age on the hypothalamic-pituitary-ovarian axis of the female white-footed mouse (Peromyscus leucopus). Exp Aging Res 6: 329–339, 1980 [DOI] [PubMed] [Google Scholar]
- 62. Su CM, Brash DE, Turturro A, Hart RW. Longevity-dependent organ-specific accumulation of DNA damage in two closely related murine species. Mech Ageing Dev 27: 239–247, 1984 [DOI] [PubMed] [Google Scholar]
- 63. Toime LJ, Brand MD. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol Med 49: 606–611, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tretter L, Adam-Vizi V. Moderate dependence of ROS formation on DeltaPsim in isolated brain mitochondria supported by NADH-linked substrates. Neurochem Res 32: 569–575, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264: 484–509, 1996 [DOI] [PubMed] [Google Scholar]
- 66. Ungvari Z, Krasnikov BF, Csiszar A, Labinskyy N, Mukhopadhyay P, Pacher P, Cooper AJ, Podlutskaya N, Austad SN, Podlutsky A. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways, and DNA repair efficiency. Age (Dordr) 30: 121–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vander Heiden MG, Christofk HR, Schuman E, Subtelny AO, Sharfi H, Harlow EE, Xian J, Cantley LC. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol 79: 1118–1124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem 275: 16258–16266, 2000 [DOI] [PubMed] [Google Scholar]
- 69. Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79: 266–277, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5: 51–66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc 1: 418–428, 2006 [DOI] [PubMed] [Google Scholar]


