Abstract
Objective
Higher body mass index (BMI) increases the risk of meniscus injury and knee osteoarthritis (OA). However, it is unknown if and how obesity affects meniscus biology. We analyzed transcriptome-wide gene expression profiles of injured human menisci to test the hypothesis that meniscal gene expression signatures relate to patient BMI.
Methods
Meniscus samples were collected from patients undergoing arthroscopic partial meniscectomy. Transcriptome-wide analysis of gene expression followed by validation of selected transcripts by QuantiGene Plex assay was performed. Correlations of gene expression with BMI and relative fold-changes in three BMI categories [lean (LN; BMI=18.5–24.9 kg/m2), overweight (OW; BMI=25.0−29.9 kg/m2), obese (OB; BMI>30.0 kg/m2)] were analyzed and integrated functional classifications were probed computationally.
Results
OB/OW comparison resulted in the largest set of differences (565-transcripts) followed by OB/LN (280-transcripts) and OW/LN (125-transcripts) comparisons. Biologic reproducibility was confirmed by cluster analysis of expressed transcripts. Differentially regulated transcripts represented important functional classifications. Transcripts associated with oxygen-transport, calcium-ion-binding, and cell-homeostasis were elevated with BMI while those related to extracellular-matrix-deposition, cell-migration, and glucosamine-metabolic-processes were repressed. While these functional classifications may play key roles in cartilage/meniscus homeostasis, failure of extracellular-matrix-deposition and increase in calcium-ion-binding likely contribute to OA development following meniscal injury.
Conclusion
Our results indicate greater differences in gene expression between OB/OW category rather than OW/LN category. This may indicate that there is a weight-threshold at which injured meniscus responds severely to increased BMI. BMI-related changes in gene expression present a plausible explanation for the role of meniscal injury in OA development among obese patients.
Keywords: Body mass index, Obesity, Meniscus, Transcriptome analysis, Osteoarthritis
INTRODUCTION
Obesity, a pandemic health problem characterized by high body mass index (BMI), increases the mechanical burden on weight-bearing joints like the knee and hip (1, 2). In the knee, increased BMI is associated with a greater risk for meniscal tears, meniscectomy (3–5), and development of osteoarthritis (OA) (6–8). Meniscectomy, in which the torn fragment of meniscus is resected, is extremely prevalent such that in the US alone 465,000−690,000 partial meniscectomies are performed annually (9, 10).
There is overwhelming evidence that obesity affects both weight-bearing joints and non-weight bearing joints through increased loading as well as over expression of proinflammatory metabolic factors (9, 11, 12). Additionally, a general consensus is that both mechanical and biochemical links exist between obesity and OA (12, 13). Elevated BMI is a strong, but potentially preventable and modifiable, risk factor for OA of the knee (8, 14–16). While biomechanical and biochemical theories abound, there is surprisingly little scientific evidence that links molecular changes in the biology of the knee to obesity.
A recent study in our laboratory demonstrated a relatively benign effect of BMI on the expression levels of selected obesity- and OA-related genes in injured meniscus (17). However, it is not clear how obesity modulates overall gene expression in the meniscus, which might in turn affect knee joint homeostasis. In the present study, we have investigated the effects of BMI on the gene expression profile of human injured meniscus through transcriptome-wide gene expression analysis to test the hypothesis that meniscal gene expression signatures relate to patient BMI. If BMI is an important determinant of gene expression changes in the meniscus, these findings could provide molecular insights into understanding of how and why meniscus tears, and the subsequent development of OA, are associated with obesity.
MATERIALS AND METHODS
Patients and tissue samples
The study was approved by Institutional Review Board and a written informed consent was obtained by study subjects. The torn segment of the injured meniscus was resected from 12 patients during their meniscal surgeries. This is the same set of tissues previously used to see gene expression differences by age (18). For the current study, these tissues were divided into three categories based on patients’ BMI: LN (BMI 18.5–24.9 kg/m2), OW (BMI 25.0−29.9 kg/m2) and OB (BMI >30.0 kg/m2) (19). The mean BMI of LN category was 22.20±1.68 kg/m2, of OW category was 26.32±0.32 kg/m2 and that of OB category was 34.49±3.10 kg/m2 (Table-1).
Table 1.
Clinical description of meniscus tears, chondrosis, RNA quality and patient demographics
| Meniscus | Type of tear | Location of tear | Time from injury |
Related injury |
Age (years) |
Sex | BMI (kg/m2) |
BMI category |
Chondrosis | RIN |
|---|---|---|---|---|---|---|---|---|---|---|
| Medial | Complex | Posterior horn | 2 months | ACL | 28 | Male | 22.24 | LN | No | 6.3 |
| Medial | Parrot beak | Posterior horn | 2.5 months | None | 40 | Female | 30.89 | OB | Yes | 6.7 |
| Medial | Bucket handle | Posterior horn | 1 month | None | 16 | Female | 20.36 | LN | No | 7.7 |
| Medial | Complex | Posterior horn | 3.5 months | None | 55 | Male | 26.5 | OW | Yes | 6.9 |
| Lateral | Complex | Posterior horn | 10 months | None | 28 | Male | 36.61 | OB | No | 6.8 |
| Lateral | Complex | Posterior horn/body | 4 months | None | 53 | Male | 32.95 | OB | Yes | 7.3 |
| Medial | Complex | Posterior horn | 3 months | None | 36 | Male | 25.85 | OW | No | 7.2 |
| Medial | Parrot beak | Posterior horn | 1 year | None | 40 | Male | 26.44 | OW | Yes | 7.5 |
| Medial | Complex | Posterior horn | 9 months | None | 48 | Male | 21.81 | LN | No | 6.9 |
| Medial | Complex | Posterior horn | 4 months | None | 43 | Male | 24.41 | LN | No | 7.1 |
| Medial | Complex | Posterior horn | 1.5 months | None | 55 | Female | 37.49 | OB | Yes | 6.9 |
| Lateral | Complex | Posterior horn | 4 months | None | 47 | Male | 26.5 | OW | No | 6.9 |
ACL=Anterior cruciate ligament; BMI=Body mass index; RIN=RNA integrity number; LN=lean; OB=obese; OW=overweight
Microarray hybridization
RNA was extracted using a combination of TRIzol reagent (Invitrogen, Carlsbad, CA) and Minispin columns (Qiagen, Valencia, CA) (17). RNA quality control was performed by 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Microarray hybridization was done at Washington University Genome Technology Access Center according to standard protocols (18). Briefly, aliquots of total RNA were amplified according to the specifications of the ABI-Ambion MessageAmp Totalprep RNA Amplification Kit (Life Technologies, Grand Island, NY). The labeled samples were hybridized to HumanHT-12-v4 Expression Beadchip (Illumina Inc., San Diego, CA), incubated with streptavidin-Cy3 and scanned on the Illumina BeadArray Reader. The raw microarray data (GSE 45233) has been deposited in the Gene Expression Omnibus (GEO, http:www.ncbi.nlm.nih.gov/projects/geo) (20).
Data mining and statistical analysis
To ensure that the data was normally distributed, all the data were converted to log-2 space. Quantile normalization was applied to adjust gene expression values. To identify differentially expressed genes in the injured meniscus in response to BMI two-way Analysis of Variance (ANOVA) was performed using Partek Genomics Suite v6.6 (Partek Inc., St. Louis, MO) in which the data was adjusted for age. We did not include an interaction term for BMI and age. Age was added as a factor along with BMI and was treated as a nominal variable (≤40 years or >40 years). The analysis further used Fisher’s Least Significant Difference post hoc test to compare the gene expression differences among three BMI categories. Genes were considered differentially expressed only with an unadjusted P value of the comparison ≤0.05 since a false discovery rate (FDR) at 0.05 level was very restrictive. Pearson’s correlations were computed to see which genes were significantly positively or negatively correlated with BMI.
To restrict the number of differentially regulated transcripts to only the most significant changes, an arbitrary cutoff of absolute fold-change of ≥1.5 was applied. Three analytical ANOVA comparisons viz., OB versus LN (OB/LN), OW versus LN (OW/LN), and OB versus OW (OB/OW) were made with each category having four biological replicates (Table-1).
From the microarray analysis the following information was extracted: 1) number of transcripts differentially expressed between any two BMI categories and among all three ANOVA comparisons, 2) fold-change differences of gene expression, 3) correlation coefficients for genes correlated with BMI and 4) functional classifications based on enrichment score and statistical significance.
To enhance the biological interpretation of large set of genes derived from microarray, grouping of genes based on functional similarity was achieved using the Database for Annotation, Visualization and Integrated Discovery (DAVID; http://david.abcc.ncifcrf.gov/home.jsp) (21). These tools generated a gene-to-gene similarity matrix based on a shared functional annotation using more than 75,000 terms from 14 functional annotation sources. The clustering algorithms classified highly related genes into functionally related groups. Summary information provided by the Functional Classification Tool is extensively linked to DAVID Functional Annotation Tools and to external databases allowing further detailed exploration of gene and term information. The Functional Classification Tool provides a rapid means to organize large lists of genes into functionally related groups to help unravel the biological content captured by high throughput technologies. The significance of the association between the differentially expressed transcripts and the specific biological processes in DAVID was analyzed using highest stringency so that only highly significant and most relevant functional classifications could be identified. In addition, selecting high stringency removed the otherwise loose, broader and larger numbers of clusters and kept only the more tightly associated genes in each cluster.
Heat maps, Venn diagrams and volcano plots were generated to get a pictorial overview of the differentially expressed genes. Heat maps used a grid linked by a dendrogram to hierarchically cluster genes. Venn diagrams are graphic techniques commonly used to illustrate overlap of genes among various groups. The volcano plots were used to visualize both P values and fold changes of genes at the same time. These plots also allowed for quick identification of differentially expressed genes with a quantifiable level of expression.
QuantiGene Plex assay validation
To validate the differences in gene expression variation by BMI by ANOVA, we used Affymetrix QuantiGene Plex assay (Panomics Inc., Fremont, CA) (18), a branched-chain DNA-based technology. The quantification of RNA was carried out in biological and technical replicates using the QuantiGene Plex 2.0 assay kit (Plex set # 312184, http://www.panomics.com).
RESULTS
Quantitative transcriptomic differences based on BMI
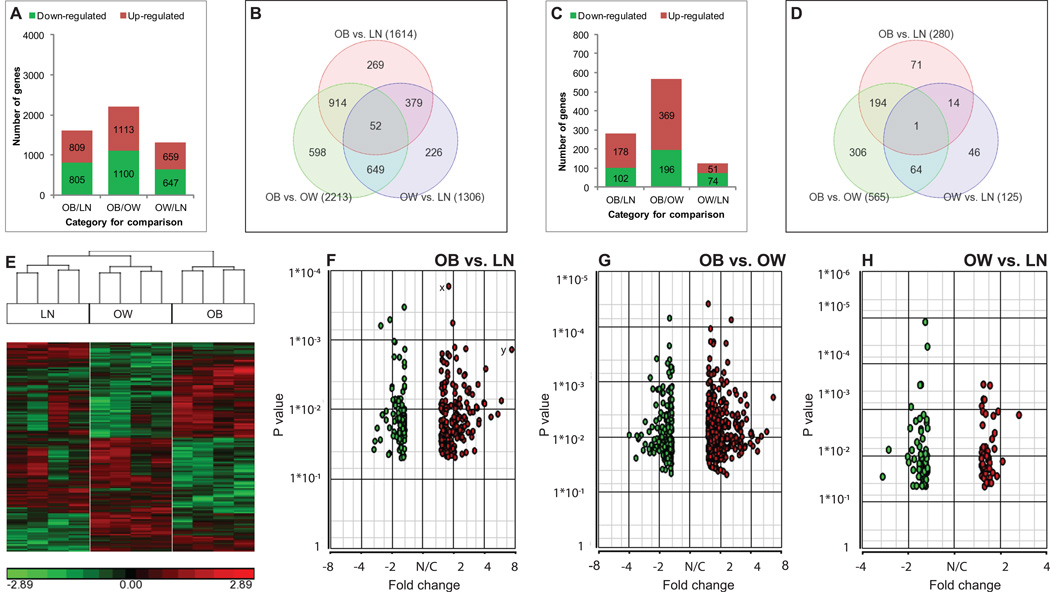
The largest significant differences in age adjusted gene expression among the three BMI categories was seen between OB and OW comparison (2213, 4.6%; 1113 up-regulated, 1100 down-regulated followed by OB/LN (1614, 3.4%; 809 up-regulated, 805 down-regulated) and OW/LN comparisons (1306, 2.7%; 659 up-regulated, 647 down-regulated). The numbers of genes up-regulated or down-regulated were relatively similar within each comparison (Fig. 1A). This accounted to a total of 5133 transcripts differentially regulated across all three BMI categories. There were 914 transcripts common to OB/LN and OB/OW comparisons, 649 transcripts common to OB/OW and OW/LN, and 379 transcripts common to OB/LN and OW/LN comparisons (Fig. 1B). To identify transcripts with the highest significant difference, the analysis was restricted to transcripts showing fold-changes ≥1.5 and P< 0.05. By these criteria, the number of differentially regulated transcripts was reduced to about 19% (970 transcripts from 5133 transcripts). The largest set of differences in number of transcripts was again seen in OB and OW comparison (565), followed by OB and LN (280) and OW and LN comparisons (125) (Fig. 1C). Table 2 lists the top 15 genes exclusively regulated for each comparison.
Fig. 1. Pictorial representation of microarray data.
Transcriptome analysis of human injured meniscus from lean (LN, BMI 18.5–24.9 kg/m2), overweight (OW, BMI 25–29.9 kg/m2) and obese (OB, BMI ≥30 kg/m2) patients was performed. The numbers of up-regulated and down-regulated genes in the three comparisons (OB/LN, OB/OW, and OW/LN) without (A) or with (C) restriction of fold-change are shown. Venn diagrams representing the number of differentially expressed genes in each comparison and the overlaps between the three comparisons are shown for any fold-change (B) or at a fold-change set at ≥1.5 only (D), with the numbers of differentially expressed genes shown in parenthesis for all three comparisons. The numbers shown in overlapping areas depict the number of genes common to two or more comparisons. Hierarchical clustering representing the transcripts that were significantly (P<0.05) and differentially regulated by BMI is shown (E). Each vertical row represents a sample and each horizontal line represents a single gene. Down-regulated genes are shown in green while up-regulated genes are shown in red. The volcano plots of differentially expressed genes (P<0.05) for the three comparisons are also shown (F–H). The X-axis represents the fold-change and Y-axis represents the P value of the ANOVA analysis. Using a stringent criterion (P<0.05, fold-change ≥1.5-fold), there were 257, 118 and 508(after removing duplicate and non-annotated genes) for OB/LN, OW/LN and OB/OW categories respectively were selected. Gene x has the lowest P value in this category and therefore is statistically the most significant, while gene y is around 8-fold up-regulated depicting its biological significance. Green circles represent down-regulated genes whereas red circles denote up-regulated genes. N/C = no change
Table 2.
Top 15 genes differentially regulated for each of the three comparisons
| Genes up-regulated in OB/LN category | Genes down-regulated in OB/LN category | ||||||
|---|---|---|---|---|---|---|---|
| Symbol | Gene name | FC | P | Symbol | Gene name | FC | P |
| HBM | hemoglobin, mu | 7.41 | 0.001 | CXCL12 | chemokine (C-X-C motif) ligand 12 | −3.01 | 0.038 |
| ALAS2 | aminolevulinate, delta-, synthase 2 | 5.87 | 0.007 | SERPINF1 | serpin peptidase inhibitor, clade F, member 1 | −2.95 | 0.029 |
| S100A8 | S100 calcium binding protein A8 | 5.41 | 0.012 | ROR2 | receptor tyrosine kinase-like orphan receptor 2 | −2.80 | 0.019 |
| HBG1 | hemoglobin, gamma A | 4.64 | 0.013 | IL13RA2 | interleukin 13 receptor, alpha 2 | −2.61 | 0.027 |
| CA1 | carbonic anhydrase I | 4.12 | 0.003 | NID2 | nidogen 2 (osteonidogen) | −2.59 | 0.001 |
| HBG2 | hemoglobin, gamma G | 4.03 | 0.008 | MFAP2 | microfibrillar-associated protein 2 | −2.49 | 0.012 |
| S100A9 | S100 calcium binding protein A9 | 3.95 | 0.008 | C1QTNF6 | C1q and tumor necrosis factor related protein 6 | −2.49 | 0.012 |
| KAL1 | Kallmann syndrome 1 sequence | 3.55 | 0.014 | RCAN2 | regulator of calcineurin 2 | −2.29 | 0.014 |
| NFE2 | nuclear factor (erythroid-derived 2), 45kDa | 3.52 | 0.016 | LHX2 | LIM homeobox 2 | −2.29 | 0.011 |
| LTB | lymphotoxin beta | 3.46 | 0.004 | CPXM1 | carboxypeptidase X (M14 family), member 1 | −2.17 | 0.045 |
| CD3D | CD3d molecule, delta (CD3-TCR complex) | 3.41 | 0.010 | CTHRC1 | collagen triple helix repeat containing 1 | −2.13 | 0.043 |
| AQP9 | aquaporin 9 | 3.33 | 0.009 | LOC645166 | lymphocyte-specific protein 1 pseudogene | −2.12 | 0.018 |
| MT1H | metallothionein 1H | 3.20 | 0.039 | C21orf100 | C21 open reading frame 100 | −2.10 | 0.001 |
| CCND2 | cyclin D2 | 3.10 | 0.011 | MAGED4B | melanoma antigen family D, 4B; melanoma antigen family D, 4 | −2.01 | 0.005 |
| HBD | hemoglobin, delta | 3.09 | 0.021 | ADAMTSL1 | ADAMTS-like 1 | −2.00 | 0.005 |
| Genes up-regulated in OB/OW category | Genes down-regulated in OB/OW category | ||||||
| HBM | hemoglobin, mu | 6.65 | 0.008 | SERPINF1 | serpin peptidase inhibitor, clade F, member 1 | −3.99 | 0.009 |
| ALAS2 | aminolevulinate, delta-, synthase 2 | 5.73 | 0.010 | CXCL12 | chemokine (C-X-C motif) ligand 12 | −3.42 | 0.024 |
| HBG1 | hemoglobin, gamma A | 5.07 | 0.006 | IL13RA2 | interleukin 13 receptor, alpha 2 | −3.35 | 0.009 |
| MT1H | metallothionein 1H | 4.66 | 0.009 | FABP5L2 | fatty acid binding protein 5-like 2 | −3.25 | 0.011 |
| S100A8 | S100 calcium binding protein A8 | 4.65 | 0.013 | ROR2 | receptor tyrosine kinase-like orphan receptor 2 | −3.21 | 0.010 |
| PAMR1 | peptidase domain containing associated with muscle regeneration 1 | 4.11 | 0.002 | CPXM1 | carboxypeptidase X (M14 family), member 1 | −3.04 | 0.009 |
| NFE2 | nuclear factor (erythroid-derived 2) | 4.07 | 0.003 | AGTR1 | angiotensin II receptor, type 1 | −2.90 | 0.017 |
| HBG2 | hemoglobin, gamma G | 4.03 | 0.010 | NCAM2 | neural cell adhesion molecule 2 | −2.77 | 0.005 |
| CD247 | CD247 molecule | 3.97 | 0.008 | LOC401233 | similar to HIV TAT specific factor 1 | −2.55 | 0.017 |
| CILP | cartilage intermediate layer protein | 3.93 | 0.002 | RCAN2 | regulator of calcineurin 2 | −2.54 | 0.008 |
| F5 | coagulation factor V | 3.66 | 0.016 | CTHRC1 | collagen triple helix repeat containing 1 | −2.50 | 0.019 |
| CSF3R | colony stimulating factor 3 receptor | 3.61 | 0.015 | NID2 | nidogen 2 (osteonidogen) | −2.47 | 0.001 |
| MMP21 | matrix metallopeptidase 21 | 3.52 | 0.006 | C1QTNF6 | C1q and tumor necrosis factor related protein 6 | −2.42 | 0.014 |
| EPB42 | erythrocyte membrane protein band 4.2 | 3.50 | 0.015 | C21orf34 | chromosome 21 open reading frame 34 | −2.37 | 0.007 |
| KAL1 | Kallmann syndrome 1 sequence | 3.47 | 0.012 | PHLDB2 | pleckstrin homology-like domain, family B, member 2 | −2.35 | 0.018 |
| Genes up-regulated in OW/LN category | Genes down-regulated in OW/LN category | ||||||
| IKZF3 | IKAROS family zinc finger 3 (Aiolos) | 2.64 | 0.001 | MMP21 | matrix metallopeptidase 21 | −2.95 | 0.028 |
| NTNG1 | netrin G1 | 2.06 | 0.013 | SLN | sarcolipin | −2.69 | 0.007 |
| C21orf34 | chromosome 21 open reading frame 34 | 1.89 | 0.027 | ZMYND11 | zinc finger, MYND domain containing 11 | −1.99 | 0.014 |
| RXRG | retinoid X receptor, gamma | 1.83 | 0.003 | HMGCLL1 | 3-hydroxymethyl-3-methylglutaryl-Coenzyme A lyase-like 1 | −1.95 | 0.003 |
| PRAMEF18 | PRAME family member 18; PRAME family member 3 | 1.82 | 0.007 | MYADM | myeloid-associated differentiation marker | −1.90 | 0.007 |
| ENPP2 | ectonucleotide pyrophosphatase/phosphodiesterase 2 | 1.78 | 0.023 | TNNT3 | troponin T type 3 (skeletal, fast) | −1.87 | 0.045 |
| PCDHA12 | protocadherin alpha 12 | 1.74 | 0.039 | GALNTL2 | N-acetylgalactosaminyltransferase-like 2 | −1.86 | 0.020 |
| RIOK3 | RIO kinase 3 (yeast) | 1.70 | 0.014 | BDKRB1 | bradykinin receptor B1 | −1.84 | 0.012 |
| PIBF1 | progesterone immunomodulatory binding factor 1 | 1.69 | 0.019 | PTHLH | parathyroid hormone-like hormone | −1.79 | 0.002 |
| ANGPT1 | angiopoietin 1 | 1.68 | 0.011 | SALL1 | sal-like 1 (Drosophila) | −1.77 | 0.045 |
| PRH1 | proline-rich protein HaeIII subfamily 1 | 1.68 | 0.019 | PIPOX | pipecolic acid oxidase | −1.76 | 0.005 |
| SULT2B1 | sulfotransferase family, cytosolic, 2B, member 1 | 1.68 | 0.039 | BRDT | bromodomain, testis-specific; hCG1811337 | −1.71 | 0.028 |
| NR3C2 | nuclear receptor subfamily 3, group C, member 2 | 1.66 | 0.011 | MICALCL | MICAL C-terminal like | −1.70 | 0.020 |
| HERC5 | hect domain and RLD 5 | 1.66 | 0.019 | FOXR2 | forkhead box R2 | −1.69 | 0.002 |
| CT45A5 | cancer/testis antigen family 45, member A5 | 1.65 | 0.012 | GABRG1 | gamma-aminobutyric acid (GABA) A receptor, gamma 1 | −1.67 | 0.016 |
LN = lean; OW = overweight; OB = obese; FC = fold change
After fold-change adjustment at 1.5, the number of down-regulated transcripts for OB/LN and OB/OW comparisons was lower than the number of up-regulated transcripts. However, in OW/LN comparison less transcripts (51) were up-regulated than down-regulated (74) (Fig. 1C). The number and overlaps of differentially expressed transcripts for each comparison are shown in Fig. 1D. The number in the overlapping parts of the circles represents transcripts common to both comparisons. Interestingly, only one gene, CHST15 [carbohydrate (N-acetylgalactosamine 4-sulfate 6-O) sulfotransferase 15] was found to be significantly differentially regulated in all three BMI categories.
There were 280 transcripts (Supplementary Table-1) differentially regulated in OB/LN comparison. Since this comparison represents two extremes of BMI, we assumed that an OB/LN comparison would allow for the largest change in the number of transcripts differentially expressed than in other comparisons, and that the changes between OW and LN or between OB and OW categories would be subsets of the changes between OB and LN categories. However, this was only partially true for differentially expressed genes for OB/OW comparison since 30% of the transcripts (194) were common between OB/LN and OB/OW comparisons while only a small (< 4%) number of transcripts (14) were common between OB/LN and OW/LN comparisons (Fig. 1D). This indicates that more changes in gene expression exist between LN and OB categories and between OB and OW categories with fewer differences between OW and LN subjects. In addition, the highest number of differentially regulated transcripts was observed for OB/OW comparison (565, Supplementary Table-2) with a lower number of transcripts (118) in OW/LN comparison (Supplementary Table-3). There were 64 transcripts common to both OB/OW and OW/LN comparisons (Fig. 1D).
We found 3086 transcripts differentially regulated by BMI indicating that gene signatures in meniscus diverge based on BMI. To visualize the trend of differentially regulated transcripts and to appreciate the differences among the three BMI categories, we generated a heat-map. The hierarchical clustering showed that based on gene expression signatures, the three BMI categories indeed clustered uniquely (Fig. 1E).
To pictorially depict P values and the fold-changes simultaneously for the differentially regulated transcripts, volcano plots were generated (Fig. 1F–H). These plots indicate the trend of transcript expression in both direction and significance. The transcripts landing on upper right or left regions have the smallest P value with larger absolute fold-change. In contrast, the transcripts landing on the upper middle region have a smaller fold-change though with significant P values (P < 0.05). The transcripts appearing on the lower left or right regions have larger fold-changes (biologically significant) though with higher yet statistically significant P values.
Genes commonly regulated among the three BMI categories
Several genes were commonly regulated in any comparison between any two BMI categories. There were 52 genes common to all three BMI categories without restriction of fold change (Fig. 1B). However, after the fold-change adjustment only a single gene (CHST15) was found to be significantly differentially regulated (P=0.003) by BMI in all three categories. CHST15 was found to be down-regulated (1.53-fold) in OW category compared to LN (P=0.033) and up-regulated in OB category compared to both OW (2.31-fold, P=0.0009) and LN (1.51-fold, P=0.038) categories.
There were 194 transcripts (Supplementary Table-4) common to OB/LN and OB/OW comparisons. Among these, 130 transcripts were up-regulated while 64 were down-regulated in OB compared to both LN and OW categories. The up-regulated transcripts were mainly associated with oxygen transport (enrichment score = 4.93; P < 0.001), oxygen carrier (enrichment score = 4.11; P < 0.001) and calcium binding region (enrichment score = 3.28; P <0.001). The down-regulated transcripts in OB mainly represented extracellular matrix (enrichment score = 2.09; P=0.0035) and cell morphogenesis involved in cell differentiation (enrichment score = 1.36; P = 0.02). Between OB/OW and OW/LN comparisons, 64 transcripts were commonly regulated (Supplementary Table-5) with 14 transcripts being up-regulated and 50 transcripts being down-regulated in OW compared to both OB and LN categories. No significant differences by biological process were identified when analyzed by DAVID. Finally, only 14 transcripts (supplementary Table-6) were common to both OB/LN and OW/LN comparisons of which 4 transcripts were up-regulated in LN and 10 transcripts were down-regulated in LN category compared to both OB and OW categories. Again, none of these transcripts represented a common biological process.
Validation of microarray
To confirm the accuracy of the microarray data, 26 of the differentially expressed transcripts were validated by QuantiGene Plex assay. The criterion for their selection was significant differential expression in any two comparisons. A microarray transcript was validated if the fold-change obtained for the analysis of the QuantiGene Plex assay was in the same direction as the fold-change obtained from microarray analysis for the 26 selected genes. Overall the QuantiGene Plex assay results were highly concordant with microarray data. The differential expression of 25 out of 26 transcripts demonstrated by QuantiGene Plex assay showed their expression to be in the same direction as that indicated by microarray suggesting that the expression of these genes was truly different between the different BMI categories (Table-3).
Table 3.
Validation of microarray data by QuantiGene Plex assay
| Symbol | Gene name | OB/LN comparison | OB/OW comparison | ||||
|---|---|---|---|---|---|---|---|
| Microarray assay |
QGP assay |
Microarray assay |
QGP assay |
||||
| FC | P | FC | FC | P | FC | ||
| HBM | hemoglobin, mu | 7.41 | 0.001 | 8.96 | 6.65 | 0.008 | 7.63 |
| ALAS2 | aminolevulinate, delta-, synthase 2 | 5.87 | 0.007 | 21.44 | 5.73 | 0.010 | 20.8 |
| HBG1 | hemoglobin, gamma A | 4.64 | 0.013 | 13.62 | 5.07 | 0.006 | 14.55 |
| MT1H | metallothionein 1H | 3.20 | 0.039 | 3.40 | 4.66 | 0.009 | 3.03 |
| S100A8 | S100 calcium binding protein A8 | 5.41 | 0.012 | 11.88 | 4.65 | 0.013 | 6.48 |
| NFE2 | nuclear factor (erythroid-derived 2) | 3.52 | 0.016 | 3.25 | 4.07 | 0.003 | 2.7 |
| HBG2 | hemoglobin, gamma G | 4.03 | 0.008 | 4.42 | 4.03 | 0.010 | 4.37 |
| CD247 | CD247 molecule | 3.01 | 0.025 | 2.89 | 3.97 | 0.008 | 2.51 |
| F5 | coagulation factor V (proaccelerin, labile factor) | 3.05 | 0.006 | 3.08 | 3.66 | 0.016 | 2.69 |
| KAL1 | Kallmann syndrome 1 sequence | 3.55 | 0.014 | 4.25 | 3.47 | 0.012 | 2.38 |
| AQP9 | aquaporin 9 | 3.33 | 0.009 | 7.00 | 3.27 | 0.009 | 5.22 |
| LTB | lymphotoxin beta (TNF superfamily, member 3) | 3.46 | 0.004 | 1.81 | 3.23 | 0.018 | 1.73 |
| S100A9 | S100 calcium binding protein A9 | 3.95 | 0.008 | 10.71 | 3.07 | 0.021 | 5.39 |
| CA1 | carbonic anhydrase I | 4.12 | 0.003 | 11.82 | 2.94 | 0.011 | 12.02 |
| MT1E | metallothionein 1E | 1.53 | 0.006 | 1.58 | 1.53 | 0.006 | 1.53 |
| CDC25C | cell division cycle 25C | −1.48 | 0.026 | −1.23 | −1.54 | 0.017 | −1.05 |
| CCL17 | chemokine (C-C motif) ligand 17 | −1.90 | 0.015 | 1.81 | −2.11 | 0.007 | 1.53 |
| MFAP2 | microfibrillar-associated protein 2 | −2.49 | 0.012 | −2.43 | −2.28 | 0.019 | −2.43 |
| C1QTNF6 | C1q and tumor necrosis factor related protein 6 | −2.49 | 0.012 | −2.57 | −2.42 | 0.014 | −2.66 |
| NID2 | nidogen 2 (osteonidogen) | −2.59 | 0.001 | −2.15 | −2.47 | 0.001 | −1.82 |
| RCAN2 | regulator of calcineurin 2 | −2.29 | 0.014 | −3.00 | −2.54 | 0.008 | −2.74 |
| CPXM1 | carboxypeptidase X (M14 family), member 1 | −2.17 | 0.045 | −3.06 | −3.04 | 0.009 | −4.52 |
| ROR2 | receptor tyrosine kinase-like orphan receptor 2 | −2.80 | 0.019 | −2.87 | −3.21 | 0.010 | −2.78 |
| IL13RA2 | interleukin 13 receptor, alpha 2 | −2.61 | 0.027 | −4.06 | −3.35 | 0.009 | −3.5 |
| CXCL12 | chemokine (C-X-C motif) ligand 12 | −3.01 | 0.038 | −4.44 | −3.42 | 0.024 | −3.81 |
| SERPINF1 | serpin peptidase inhibitor, clade F, member 1 | −2.95 | 0.029 | −5.19 | −3.99 | 0.009 | −6.48 |
LN = lean; OW = overweight; OB = obese; QGP = QuantiGene Plex assay; FC = fold change
Transcript correlations with BMI
To further investigate genes highly correlated with BMI, we calculated Pearson’s correlation coefficients. We found that a total of 2841 transcripts were correlated with BMI. Among these, 1360 transcripts were positively correlated with BMI i.e. their expression increased with rise in BMI and 1481 transcripts were negatively correlated with BMI i.e. their expression decreased with rise in BMI. The top 20 transcripts in each correlation are shown in Table 4.
Table 4*.
Pearson correlation coefficients for genes correlated with BMI
| Genes positively correlated with BMI | |||
|---|---|---|---|
| Symbol | Gene name | r | P |
| NAIP | NLR family, apoptosis inhibitory protein | 0.89 | <0.001 |
| GPR98 | G protein-coupled receptor 98 | 0.87 | <0.001 |
| IGLL1 | immunoglobulin lambda-like polypeptide 1 | 0.86 | <0.001 |
| ADAM10 | ADAM metallopeptidase domain 10 | 0.86 | <0.001 |
| BMF | Bcl2 modifying factor | 0.85 | <0.001 |
| KRTAP9–8 | keratin associated protein 9-8 | 0.85 | <0.001 |
| STEAP1 | six transmembrane epithelial antigen of the prostate 1 | 0.85 | 0.001 |
| PASK | PAS domain containing serine/threonine kinase | 0.84 | 0.001 |
| S100A8 | S100 calcium binding protein A8 | 0.83 | 0.001 |
| HBG2 | hemoglobin, gamma G | 0.83 | 0.001 |
| S100A9 | S100 calcium binding protein A9 | 0.83 | 0.001 |
| CA1 | carbonic anhydrase I | 0.82 | 0.001 |
| SELL | selectin L | 0.82 | 0.001 |
| RAD51AP2 | RAD51 associated protein 2 | 0.82 | 0.001 |
| SNORA2A | small nucleolar RNA, H/ACA box 2A | 0.82 | 0.001 |
| ANKRD42 | ankyrin repeat domain 42 | 0.82 | 0.001 |
| HBM | hemoglobin, mu | 0.82 | 0.001 |
| FSCN2 | fascin homolog 2, actin-bundling protein, retinal | 0.82 | 0.001 |
| LTB | lymphotoxin beta | 0.82 | 0.001 |
| FFAR2 | free fatty acid receptor 2 | 0.82 | 0.001 |
| Genes negatively correlated with BMI | |||
| PBOV1 | prostate and breast cancer overexpressed 1 | −0.89 | <0.001 |
| ISG20L2 | interferon stimulated exonuclease gene 20kDa-like 2 | −0.89 | <0.001 |
| G6PC | glucose-6-phosphatase, catalytic subunit | −0.88 | <0.001 |
| EPS15L1 | epidermal growth factor receptor pathway substrate 15-like 1 | −0.88 | <0.001 |
| ENHO | energy homeostasis associated | −0.86 | <0.001 |
| YY1 | YY1 transcription factor | −0.85 | <0.001 |
| PCDHGB7 | protocadherin gamma subfamily B, 7 | −0.85 | <0.001 |
| PLEKHG2 | pleckstrin homology domain containing, family G (with RhoGef domain) member 2 | −0.84 | 0.001 |
| TNFSF12-TNFSF13 | tumor necrosis factor (ligand) superfamily, member 12 , 13 | −0.84 | 0.001 |
| NID2 | nidogen 2 | −0.84 | 0.001 |
| ZFP64 | zinc finger protein 64 | −0.84 | 0.001 |
| ZNF212 | zinc finger protein 212 | −0.84 | 0.001 |
| HIATL1 | hippocampus abundant transcript-like 1 | −0.83 | 0.001 |
| U2AF1 | U2 small nuclear RNA auxiliary factor 1 | −0.83 | 0.001 |
| TNFSF15 | tumor necrosis factor (ligand) superfamily, member 15 | −0.82 | 0.001 |
| PI15 | peptidase inhibitor 15 | −0.82 | 0.001 |
| RPUSD2 | RNA pseudouridylate synthase domain containing 2 | −0.82 | 0.001 |
| ARHGAP8 | Rho GTPase activating protein 8 | −0.82 | 0.001 |
| WHAMM | WAS protein homolog associated with actin, golgi membranes and microtubules | −0.82 | 0.001 |
| SNAI2 | snail family zinc finger 2 | −0.82 | 0.001 |
BMI = body mass index; r = correlation coefficient;
only top 20 genes from each category are shown here.
Functional classification
To identify the most meaningful biological processes linked to BMI-dependent changes in transcript expression, we performed functional classification of genes, which were exclusively regulated in each comparison (Fig. 1D). In OB/LN comparison, transcripts related to extracellular matrix (enrichment score = 2.90; P < 0.001) and metal ion binding (enrichment score = 1.33; P = 0.041) were found to be significantly down-regulated while those involved in oxygen transport (enrichment score = 4.51; P < 0.001), oxygen carrier (enrichment score = 3.78; P < 0.001) and calcium binding region (enrichment score = 2.97; P < 0.001) were up-regulated. In OW/LN comparison, transcripts related to axon guidance (enrichment score = 0.84; P = 0.031) were down-regulated in OW category while no significant processes up-regulated in OW category were recognized. Lastly, in OB/OW comparison, several biological processes including oxygen transport (enrichment score = 4.33; P < 0.001), oxygen carrier (enrichment score = 3.762; P < 0.001) and calcium binding region (enrichment score = 2.39; P = 0.002) were significantly up-regulated. Three processes were significantly down-regulated: glucosamine metabolic process (enrichment score = 1.99; P = 0.009), cell morphogenesis involved in differentiation (enrichment score = 1.33; P = 0.031) and regulation of leukocyte migration (enrichment score = 1.24; P = 0.009). The functional classifications and relevant transcripts are summarized in Table 5.
Table 5.
Functional classifications of genes differentially expressed by BMI
| Biological processes | BMI category | E score | P value | Genes in the process | ||
|---|---|---|---|---|---|---|
| LN | OW | OB | ||||
| Oxygen transport | ↓ | - | ↑ | 4.51 | <0.001 | HBG1, HBG2, HBM, HBQ1, HBD |
| Oxygen carrier | ↓ | - | ↑ | 3.78 | <0.001 | HBG1, HBG2, HBQ1, HBD |
| Calcium binding region | ↓ | - | ↑ | ↓2.97 | <0.001 | S100P, S100A12, S100A8, S100A9 |
| Chelation | ↓ | - | ↑ | 2.52 | 0.001 | MT1F, MT1X, MT1H |
| T cytotoxic cell pathway | ↓ | - | ↑ | 1.81 | 0.013 | CD247, CD3D, CD2 |
| Platelet alpha granule lumen | ↓ | - | ↑ | 1.80 | 0.006 | F5, PPBP, CLU, THBS1 |
| Negative regulation of apoptosis | ↓ | - | ↑ | 1.45 | 0.034 | IL1B, FOXO1, SNCA, STRADB, CLU, BTC, THBS1, PROK2 |
| Mitogen | ↓ | - | ↑ | 1.35 | 0.033 | IL1B, PPBP, BTC |
| Leukocyte chemotaxis | ↓ | - | ↑ | 1.27 | 0.041 | IL1B, CCL5, S100A9 |
| Cellular ion homeostasis | ↓ | - | ↑ | 1.22 | 0.044 | TMPRSS3, CCL5, IL1B, MT1H, ALAS2, SNCA, MAL, PROK2 |
| Extracellular matrix | ↑ | - | ↓ | 2.90 | <0.001 | MMP28, CTHRC1, ZP4, LAMB1, NID2, MFAP2, ADAMTSL1 |
| Glioma | ↑ | - | ↓ | 1.43 | 0.020 | CALML6, HRAS, CAMK2D |
| Metal ion binding* | ↑ | - | ↓ | 1.33 | 0.041 | ZFP64, ADA, CPXM1, CA5A, EHD1, NID2, UBR7, RCN1, ZBTB8A, PDZRN3 |
| Immunoglobulin I-set | ↑ | - | ↓ | 0.89 | 0.021 | CADM4, ROR2, ADAMTSL1, IGDCC4 |
| Region of interest:Alpha | - | ↓ | ↑ | 6.57 | <0.001 | MT1E, MT1F, MT1H, MT2A, MT1A, MT1M |
| Oxygen transport | - | ↓ | ↑ | 4.33 | <0.001 | HBG1, HBG2, HBM, HBQ1, HBD |
| Oxygen carrier | - | ↓ | ↑ | 3.62 | <0.001 | HBG1, HBG2, HBQ1, HBD |
| Calcium-binding region | - | ↓ | ↑ | 2.39 | 0.002 | S100P, S100A12, S100A8, S100A9 |
| DNA-binding region | - | ↓ | ↑ | 2.28 | 0.005 | FOXR2, FOXO1, FOXD1, FOXP4, FOXC2 |
| Heme | - | ↓ | ↑ | 2.04 | 0.007 | HBG1, HBG2, HBM, HBQ1 |
| Domain:SEC7 | - | ↓ | ↑ | 1.64 | 0.020 | IQSEC1, PSD3, KIAA1244 |
| T cytotoxic cell pathway | - | ↓ | ↑ | 1.50 | 0.028 | CD247, CD3D, CD2 |
| Domain:C-type lectin | - | ↓ | ↑ | 1.47 | 0.024 | FCER2, KLRB1, KLRC3, CD69, SELL |
| Regulation of apoptosis* | - | ↓ | ↑ | 1.38 | 0.039 | SNCA, RARB, PLAGL1, GLI3, PROK2, FOXC2, SORT1, SEMA4D, SOS1, THBS1 |
| Hemopexin-like 2 | - | ↓ | ↑ | 1.36 | 0.040 | PRG4, MMP21, MMP25 |
| Cellular ion homeostasis | - | ↓ | ↑ | 1.34 | 0.029 | SNCA, ATP2A2, PROK2, MT1H, ITPR3, MT2A, IL1B, CCL5, ALAS2, EGR2, PRKCZ, MAL |
| Negative regulation of apoptosis | - | ↓ | ↑ | 1.31 | 0.046 | SORT1, IL1B, FOXO1, SEMA4D, SNCA, STRADB, CLU, PRKCZ, THBS1, FOXC2, PROK2 |
| Metal ion binding* | - | ↓ | ↑ | 1.29 | 0.041 | S100A8, S100A9, SNCA, MAP3K8, RARB, RREB1, F5, ADAMTS1, ADAMTS5, ENPP3 |
| Platelet alpha granule lumen | - | ↓ | ↑ | 1.23 | 0.024 | F5, PPBP, CLU, THBS1 |
| Region of interest:Spacer | - | ↓ | ↑ | 1.09 | 0.040 | ADAMTS13, ADAMTS1, ADAMTS5 |
| Glucosamine metabolic process | - | ↑ | ↓ | 1.99 | 0.009 | LARGE, AMDHD2, GNPDA1 |
| Cell morphogenesis involved in differentiation | - | ↑ | ↓ | 1.32 | 0.031 | NCAM2, FEZ1, NTNG1, LAMB1, LHX2, CXCL12 |
| Regulation of leukocyte migration | - | ↑ | ↓ | 1.24 | 0.009 | ADA, F7, CXCL12 |
| Axon guidance | ↑ | ↓ | - | 0.84 | 0.031 | SEMA3B, PVRL1, NTN1 |
BMI = body mass index, LN = lean, OW = overweight, OB = obese; E = enrichment;
only 10 genes listed
DISCUSSION
This study is the first that compares comprehensive gene expression profiles of injured human meniscus in relation to patients’ BMI. The most important finding is that both individual genes and sets of coordinately expressed genes diverge based on BMI. Interestingly, the greatest difference in gene expression was between OB and OW individuals. This finding suggests that weight gain sufficient to convert overweight patients to obese could have a very detrimental effect on the biology of the meniscus. Several studies have suggested that loss in weight results in reduced incidence of OA in the knee (14–16, 22). Our study suggests that losing weight when obese could have a beneficial molecular and biological effect on the meniscus, perhaps reducing the risk for meniscal injury and subsequent progression to OA. Clinically, it has been shown that weight loss due to bariatric surgery or by other means leads to decreased knee pain and improved knee function in obese patients with OA (22, 23).
The transcripts up-regulated in OB compared to both LN and OW categories represented increased oxygen transport and calcium ion binding, and suppression of extracellular matrix deposition. Interestingly, only one transcript (CHST15) was common to all three comparisons. CHST15 is a sulfotransferase, which sulfates the GalNAc residues of chondroitin polymers (24) and transfers sulfate to the carbon-6 of an already 4-O sulfated GalNAc residue producing over-sulfated chondroitin sulfate E (25). Genome-wide association studies, designed to study the genetic contribution to OA have recently shown that a related gene CHST11 is a candidate for association with OA (26). Transcripts associated with extracellular matrix synthesis were down-regulated in OB category suggesting a higher risk of developing meniscus degeneration from the excessive mechanical stress on the knee joint in the obese population. Increased degenerative changes in the meniscus are associated with aging and obesity and may be driven by the inability to maintain extracellular matrix deposition. Thus, it seems that obese people may be at a disadvantage because of the inability to synthesize extracellular matrix compounded by the up-regulation of calcium-ion binding seen in the meniscus following injury. This speculation is in line with several other experimental studies in which it has been shown that under specific conditions overload may trigger both inhibition of extracellular matrix synthesis and degradation of articular cartilage (11, 22, 27).
It is interesting that the extracellular matrix components identified in this study that change with obesity are not COL2A1 or ACAN, the classical markers for hyaline cartilage, which were shown by our analysis to be down-regulated in the aging meniscus (18). Here, other matrix components e.g. MMP28, CTHRC1, ZP4, LAMB1, NID2, MFAP2, and ADAMTSL1 were dysregulated. In addition, we have previously shown that the expression of extracellular matrix genes also varied based on age and, whether or not the anterior cruciate ligament was also injured (28). Thus, with increasing age and BMI both structural components of the meniscus deteriorate, making a combination of increased BMI and age particularly detrimental to meniscus homeostasis.
In addition to the down-regulation of extracellular matrix biological process, patients with higher BMI were found to exhibit higher calcium ion binding. Elevated calcium ion binding has previously been reported in meniscus tissues from OA knees (29). Several lines of evidence suggest that an increased deposition of calcium crystals promotes joint degradation (30–32). Furthermore, a reduction in the amount of calcium deposition has also been shown to decrease cartilage degradation in guinea pigs (33). Small calcium-binding S100 proteins have been implicated in a variety of inflammatory conditions, including OA. Therefore, it is likely that increased calcium ion binding in obese individuals due to higher BMI could foster the initiation and progression of OA. This situation is further augmented by loss of extracellular matrix deposition, commonly observed in the presence of calcium crystals (30–32).
A set of genes (HBG1, HBG2, HBD, HBM, HBQ1) associated with oxygen transport and oxygen carrier (i.e. blood gas transport) was elevated in OB category. These genes have also been reported to be up-regulated in peripheral blood mononucleated cells obtained from patients with pulmonary hypertension (34) which is associated with obesity (35). This observation is consistent with our analysis and suggests the involvement of these genes in pathophysiological changes associated with obesity.
Gene correlation analysis showed that NAIP (an apoptosis inhibitory protein) was strongly and significantly associated with BMI. Other apoptosis inhibitor genes such apoptosis inhibitor of macrophage (AIM) is involved in obesity-associated recruitment of inflammatory macrophages into adipose tissue where it induces inflammation in adipocytes (36). Since both obesity and OA are low grade inflammatory conditions [reviewed in (37)], elevated NAIP may contribute to higher inflammation in obesity and OA.
Because the gene expression signatures from samples obtained from OB category are the most different (even compared to that of OW patients), it may suggest that either there is a weight threshold at which the meniscus responds severely or that the metabolic syndrome or amount and quality of adipose tissue present in OB patients drives meniscal gene expression changes in OB patients. There is little if any understanding of the molecular response of meniscus to altered loading. Previous studies (38–40) have shown that the meniscus experiences a broad range of compressive stress both in terms of space and time, which could be an important confounding factor for meniscal gene expression. In addition, some studies have demonstrated zonal differences in the molecular profile of the meniscus, perhaps resulting from differences in weight bearing across the zones of the meniscus (41, 42). However, no previous study has established a weight threshold at which major changes in gene expression occur. Our study is the first to provide strong evidence that an increase in body weight from OW to OB has a profound effect on meniscus gene expression.
A general consensus is that genes related to inflammation and lipid metabolism are up-regulated in the adipose tissues (or fat pad) of obese individuals compared to non-obese ones (43, 44). The studies supporting this notion were conducted primarily on adipose tissues and demonstrate that genes related to inflammation and metabolic processes are up-regulated in these tissues. While it might be reasonable to expect similar changes in meniscal tissue, we could not detect any. Since gene expression related to inflammation and lipid metabolism did not differ in the meniscus based on BMI, it suggests other mechanisms mediate the effect of body weight on the meniscus.
A major limitation of the current study is a lack of data pertaining to the uninjured meniscus. However, given the association between obesity, meniscus injury and the development of OA, the injured meniscus may be the optimal model to study this relationship. While the focus of the current study is to provide a list of transcripts that are differentially regulated among various BMI categories, it would be of great value in the future to perform histological analysis of the injured and uninjured meniscus tissues to confirm these pathways and biological processes differ at the protein level by use of other techniques such as immunohistochemistry.
Another limitation is that we did not filter the data based on FDR. There is a tradeoff between increasingly stringent criteria to avoid false-positive discoveries and making criteria so strict that true effects are not identified (45). In the current investigation, similar to previous studies (46,47), we have independently validated the microarray gene expression differences using RNA from the same patient cohort through QuantiGene Plex Assay, suggesting this assay can be used to validate a subset of transcripts identified from microarray platforms with unparalleled precision. A higher-powered study could identify potential false-positive findings, if any, associated with the current analysis.
Also concerning is the possibility that transcripts may have a biologically significant increase in expression without a statistically significant change in expression. Transcripts, which are differentially expressed at very high fold change but without a significant P value (<0.05) indicate a large inter-group or inter-sample variation. Since modern methods may identify genes with arbitrarily small fold change as statistically significant, it has become increasingly common to require that differentially expressed transcripts satisfy both p value and fold-change criteria simultaneously (48–50). To circumvent the potential limitations associated with filtering criterion and to interpret the true biological significance of the fold change differences, an entirely new validation cohort could be helpful in the future.
In summary, computational cluster analysis revealed that gene expression in human injured meniscus relates to patient BMI in this hypothesis generating study. Interestingly a larger set of differentially expressed transcripts was identified between OB and OW categories than OB and LN categories. The differentially expressed transcripts identify important biological processes that could explain the biological impact of BMI on the generalized response of meniscus to injury and contribute to the elevated risk for development of OA in obese patients.
Supplementary Material
Acknowledgements
Source of Funding
This study was supported by an Orthopaedic Research and Education Foundation (OREF) grant to Dr. Brophy, National Institute of Arthritis and Musculoskeletal and Skin Diseases grants # R01-AR050847 and R01-AR045550 to Dr. Sandell and Musculoskeletal Research Center, grant # P30-AR057235. Dr. Rai is supported by Ruth L. Kirschstein National Research Service Award Fellowship from National Institutes of Health through grant # T32-AR060719. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis, Musculoskeletal and Skin Diseases or the National Institutes of Health.
We thank Washington University Genome Technology Access Center for help with transcriptome and QuantiGene Plex assays. We also acknowledge with thanks the important intellectual contribution of Dr. James M. Cheverud (Department of Biology, Loyola University, Chicago)
Footnotes
Financial conflict of interest
L.J.S. owns stock or stock options in ISTO Technologies and receives royalties from Merck/Millipore for a type IIA collagen N-propeptide enzyme-linked immunosorbent assay. M.F.R., D.P. and R.H.B. have nothing to disclose.
Competing interests
No, there are no competing interests.
Author contributions
All authors were involved in drafting and revision of the manuscript and all authors approved the final version to be published. Dr. Brophy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design: Sandell, Brophy, Rai.
Acquisition of data: Brophy, Rai, Patra.
Analysis and interpretation of data: Rai, Patra, Sandell, Brophy
Contributor Information
Muhammad Farooq Rai, Email: raim@wudosis.wustl.edu.
Debabrata Patra, Email: patrad@wudosis.wustl.edu.
Linda J. Sandell, Email: sandelll@wustl.edu.
Robert H. Brophy, Email: brophyr@wudosis.wustl.edu.
REFERENCES
- 1.Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22(5):533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynauld JP, Cicuttini F, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34(4):776–784. [PubMed] [Google Scholar]
- 4.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford GM, Hegmann KT, White GL, Jr., Holmes EB. Associations of body mass index with meniscal tears. American journal of preventive medicine. 2005;28(4):364–368. doi: 10.1016/j.amepre.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996;23(7):1221–1226. [PubMed] [Google Scholar]
- 7.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128(1):179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996;55(9):668–670. doi: 10.1136/ard.55.9.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 10.Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1–25. [PubMed] [Google Scholar]
- 11.Pottie P, Presle N, Terlain B, Netter P, Mainard D, Berenbaum F. Obesity and osteoarthritis: more complex than predicted! Ann Rheum Dis. 2006;65(11):1403–1405. doi: 10.1136/ard.2006.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai MF, Sandell L. Inflammatory mediators: tracing links between obesity and osteoarthritis. Critical reviews in eukaryotic gene expression. 2011;21(2):131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 13.Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol. 2013;9(4):225–235. doi: 10.1038/nrrheum.2012.224. [DOI] [PubMed] [Google Scholar]
- 14.Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130(2):278–288. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116(7):535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 16.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis care & research. 2013;65(1):15–22. doi: 10.1002/acr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai MF, Sandell LJ, Cheverud JM, Brophy RH. Relationship of age and body mass index to the expression of obesity and osteoarthritis-related genes in human meniscus. Int J Obes (Lond) 2013;37(9):1238–1246. doi: 10.1038/ijo.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai MF, Patra D, Sandell LJ, Brophy RH. Transcriptome analysis of injured human meniscus reveals a distinct phenotype of meniscus degeneration with aging. Arthritis Rheum. 2013;65(8):2090–2101. doi: 10.1002/art.37984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 20.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70(1):139–144. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 23.Gill RS, Al-Adra DP, Shi X, Sharma AM, Birch DW, Karmali S. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12(12):1083–1089. doi: 10.1111/j.1467-789X.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 24.Cooney CA, Jousheghany F, Yao-Borengasser A, Phanavanh B, Gomes T, Kieber-Emmons AM, et al. Chondroitin sulfates play a major role in breast cancer metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast cancer research : BCR. 2011;13(3):R58. doi: 10.1186/bcr2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habuchi O, Moroi R, Ohtake S. Enzymatic synthesis of chondroitin sulfate E by N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase purified from squid cartilage. Anal Biochem. 2002;310(2):129–136. doi: 10.1016/s0003-2697(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 26.Lancet. 2012;380(9844):815–823. doi: 10.1016/S0140-6736(12)60681-3. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messier SP, Loeser RF, Mitchell MN, Valle G, Morgan TP, Rejeski WJ, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48(9):1062–1072. doi: 10.1111/j.1532-5415.2000.tb04781.x. [DOI] [PubMed] [Google Scholar]
- 28.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am. 2012;94(5):385–393. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN, Jr., et al. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord. 2010;11:19. doi: 10.1186/1471-2474-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung HS, Mitchell PG, Pledger WJ. Induction of expression of c-fos and c-myc protooncogenes by basic calcium phosphate crystal: effect of beta-interferon. Cancer Res. 1989;49(1):134–138. [PubMed] [Google Scholar]
- 31.McCarthy GM, Cheung HS, Abel SM, Ryan LM. Basic calcium phosphate crystal-induced collagenase production: role of intracellular crystal dissolution. Osteoarthritis Cartilage. 1998;6(3):205–213. doi: 10.1053/joca.1998.0113. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Zeng XR, Wenger L, Cheung HS. Basic calcium phosphate crystals stimulate the endocytotic activity of cells--inhibition by anti-calcification agents. Biochem Biophys Res Commun. 2003;312(4):1053–1059. doi: 10.1016/j.bbrc.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Cheung HS, Sallis JD, Demadis KD, Wierzbicki A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54(8):2452–2461. doi: 10.1002/art.22017. [DOI] [PubMed] [Google Scholar]
- 34.Cheadle C, Berger AE, Mathai SC, Grigoryev DN, Watkins TN, Sugawara Y, et al. Erythroid-specific transcriptional changes in PBMCs from pulmonary hypertension patients. PLoS One. 2012;7(4):e34951. doi: 10.1371/journal.pone.0034951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poirier P, Eckel RH. Obesity and cardiovascular disease. Current atherosclerosis reports. 2002;4(6):448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 36.Kurokawa J, Nagano H, Ohara O, Kubota N, Kadowaki T, Arai S, et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011;108(29):12072–12077. doi: 10.1073/pnas.1101841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Critical reviews in eukaryotic gene expression. 2011;21(2):131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 38.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21(6):963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 39.Schreppers GJ, Sauren AA, Huson A. A numerical model of the load transmission in the tibio-femoral contact area. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine. 1990;204(1):53–59. doi: 10.1243/PIME_PROC_1990_204_228_02. [DOI] [PubMed] [Google Scholar]
- 40.Spilker RL, Donzelli PS, Mow VC. A transversely isotropic biphasic finite element model of the meniscus. J Biomech. 1992;25(9):1027–1045. doi: 10.1016/0021-9290(92)90038-3. [DOI] [PubMed] [Google Scholar]
- 41.Fuller ES, Smith MM, Little CB, Melrose J. Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthritis Cartilage. 2012;20(1):49–59. doi: 10.1016/j.joca.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Upton ML, Chen J, Setton LA. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24(7):1562–1570. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]
- 43.Gandhi R, Takahashi M, Virtanen C, Syed K, Davey JR, Mahomed NN. Microarray analysis of the infrapatellar fat pad in knee osteoarthritis: relationship with joint inflammation. J Rheumatol. 2011;38(9):1966–1972. doi: 10.3899/jrheum.101302. [DOI] [PubMed] [Google Scholar]
- 44.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18(14):1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 45.van den Oord EJ, Sullivan PF. False discoveries and models for gene discovery. Trends Genet. 2003;19(10):537–542. doi: 10.1016/j.tig.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Canales RD, Luo Y, Willey JC, Austermiller B, Barbacioru CC, Boysen C, et al. Evaluation of DNA microarray results with quantitative gene expression platforms. Nat Biotechnol. 2006;24(9):1115–1122. doi: 10.1038/nbt1236. [DOI] [PubMed] [Google Scholar]
- 47.Dumitriu A, Latourelle JC, Hadzi TC, Pankratz N, Garza D, Miller JP, et al. Gene expression profiles in Parkinson disease prefrontal cortex implicate FOXO1 and genes under its transcriptional regulation. PLoS Genet. 2012;8(6):e1002794. doi: 10.1371/journal.pgen.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raouf A, Zhao Y, To K, Stingl J, Delaney A, Barbara M, et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell stem cell. 2008;3(1):109–118. doi: 10.1016/j.stem.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Patterson TA, Lobenhofer EK, Fulmer-Smentek SB, Collins PJ, Chu TM, Bao W, et al. Performance comparison of one-color and two-color platforms within the MicroArray Quality Control (MAQC) project. Nat Biotechnol. 2006;24(9):1140–1150. doi: 10.1038/nbt1242. [DOI] [PubMed] [Google Scholar]
- 50.Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102(10):3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.