Abstract
Gut-derived glucagon like peptide-1 (GLP-1) acts in the postprandial period to stimulate insulin secretion and inhibit gastrointestinal motor and secretory function; whether endogenous peripheral GLP-1 inhibits food intake is less clear. We hypothesized that GLP-1 inhibits food intake in the fed, but not fasted, state. There is evidence that GLP-1 acts via stimulation of vagal afferent neurons (VAN); we further hypothesized that the satiating effects of endogenous GLP-1 in the postprandial period is determined either by a change in GLP-1 receptor (GLP-1R) expression or localization to different cellular compartments in VAN.
METHODS
Food intake was recorded following administration of GLP-1 (50 μg/kg or 100μg/kg) or saline (IP) in Wistar rats fasted for 18h or fasted then re-fed with 3g chow. GLP-1R protein expression and localization on VAN was determined by immunocytochemistry and immunoblots in animals fasted for 18h or fasted then re-fed for 40mins. GLP-1R mRNA level was detected in animals fasted for 18h or fasted and re-fed ad libitum for 2h.
RESULTS
GLP-1 (100μg/kg) significantly reduced 40 min food intake by 38% in re-fed but not fasted rats (p<0.05). GLP-1R mRNA or protein levels in VAN were unchanged in re-fed compared to fasted rats. However, GLP-1R localization to the plasma membrane was significantly increased in VAN by feeding.
CONCLUSION
Feeding changes the ability of peripheral GLP-1 to inhibit food intake. GLP-1Rs are trafficked to the plasma membrane in response to a meal. GLP-1 may play a role in regulating food intake in the postprandial period.
Keywords: Glucagon-like-peptide-1, vagal afferent neurons, food intake, receptor trafficking
Introduction
Considerable interest is focused on the mechanisms by which signals from the gastrointestinal (GI) tract influence food intake and play a role in the maintenance of body weight. The GI tract is the primary epithelial surface involved in nutrient sensing, resulting in the release of multiple peptide hormones that can influence food intake [1]. The vagus nerve provides the major neuronal link between the GI tract and the central nervous system (CNS). Vagal afferent neurons (VAN) transmit information to terminals in the brainstem, resulting in activation of parasympathetic reflexes that regulate GI and pancreatic function, and activation of higher order neurons to change feeding behavior.
Glucagon-like-peptide-1 (GLP-1) is a gut hormone derived from the pre-proglucagon gene synthesized and released by intestinal L-cells found along the length of the small intestine and proximal colon [2]. The insulinotropic action of GLP-1 is well established; GLP-1 directly stimulates insulin release from pancreatic β cells. GLP-1 receptor (GLP-1R) knockout mice exhibit perturbations in fasting glucose levels, glucose-stimulated insulin secretion and β-cell signal transduction [3, 4]. The role of GLP-1 in regulating glucose homeostasis appears to be physiological; however, its role in feeding is less clear.
GLP-1 secretion is low in a fasting state and increases rapidly postprandially, especially with meals containing fats and carbohydrates [5]. Exogenous administration of GLP-1 has been shown to inhibit food intake in humans; diabetic patients treated with GLP-1, or its stable receptor agonist, progressively lose weight [6]. Intraperitoneal (IP) administration of GLP-1 decreases food intake dose-dependently in rodents [7], although this is not found consistently across studies. GLP-1 is rapidly degraded in the extracellular space; this may account for the variability in the biological activity of the native peptide to influence feeding behavior. Continuous infusion of GLP-1 either into the hepatic portal vein or intraperitoneally in rats is effective at decreasing overall food intake through reductions in meal size and duration [8, 9]. Other studies have used the stable synthetic GLP-1 analog, exendin 4 (Ex-4), to study the effects of GLP-1. However, there is good evidence that Ex-4 can access the central nervous system and activate discrete central pathways to inhibit food intake, thus these studies do not assist in understanding the role of peripheral GLP-1. There is some evidence that the ability of native GLP-1 to inhibit food intake only occurs in the fed and not the fasted state [7, 10]. GLP-1 inconsistently decreases food intake when administered intraperitoneally; for example, Neary et al. found no difference in food intake in 20hr fasted mice following a low doses of GLP-1 compared to vehicle [11].
Under physiological conditions, because of the short plasma half-life of GLP-1 [12], it is likely that it acts locally within the intestinal wall to influence food intake. VAN are a common site of action for gut-derived satiating signals. There are reports that VAN express mRNA for GLP-1R [13] and systemic administration of GLP-1 increased electrophysiological activity in neurons of the nodose ganglion [14]. Furthermore, subdiaphragmatic deafferentation attenuated the ability of peripheral GLP-1 to decrease food intake in humans [8].
Unlike other gut hormones regulating food intake, the anorexigenic effect of peripheral GLP-1 may be dependent on nutritional status with one report suggesting that exogenous GLP-1 induced satiation in freely fed rats but had no effect in fasted animals [7]. Previous work has shown that VAN change their neurochemical phenotype accordingly to nutritional status [15]. We hypothesized that GLP-1 inhibits food intake in the fed, but not fasted, state. Additionally, we hypothesized that the expression level of GLP-1Rs change according to feeding status, and this change in receptor expression determines the ability of GLP-1 to inhibit food intake.
Methods
Animals
Animals were maintained and handled in accordance with policies of the Institutional Animal Care and Use Committee at the University of California in Davis. Male Wistar rats (initial weight 200g; Harlan, San Diego) were individually housed at 22°C under a shifted 12h light-dark cycle (light 11pm-11am) with ad libitum access to food and water. Rats were fed a regular chow diet (Lab Diet 5001). Animals were handled for 7 days prior to experiments and habituated to the new environment and to intraperitoneal (IP) injections of 400μl physiological saline prior to food intake studies.
Feeding Studies
In all experiments, rats were fasted on wire bottom cages (6pm-11am) with ad libitum access to water (n=8 in each group). Experiments were performed at the start of the dark phase when the animals have the strongest drive to eat. GLP-1 was obtained by Bachem Bioscience Inc (King of Prussia, PA) and reconstituted in 0.9% saline. GLP-1 (50μg/kg or 100μg/kg IP) or 400μl physiological saline (IP) were administered in two different protocols. The higher dose of GLP-1 was chosen based on Williams et al [7] and the lower dose was chosen as a subthreshold dose. In the first protocol, saline or GLP-1 (50μg/kg or 100μg/kg, IP) were administered at the start of dark phase (11am) after an overnight fast (6pm-11am) and food was immediately returned to the cage. Food weight and spill was recorded every 20 minutes for 2 h. In the second protocol, 40 minutes before the onset of the dark phase (11am), a small premeal (3g) was given to overnight fasted rats (6pm-10:20am). Saline or GLP-1 (50μg/kg or 100μg/kg, IP) was administered at the start of dark phase (11am) and food was immediately returned to the cage. Food weight and spill was recorded every 20 minutes for 2 h. Food intake was determined by measuring the difference between the baseline and the weight of the food and spill every 20 minutes for 2 h. A within subject study design was used for these experiments; each animal received both saline and GLP-1 and was compared for statistical analysis. Food intake following administration of saline in one rat was statistically an outlier and was removed from the study. Two way ANOVA repeated measures was used to compare groups over 2 h and unpaired Student t-test was used for 40 min food intake.
Tissue collection
Rats were euthanized by CO2 inhalation at least 1 h into the dark cycle and nodose ganglia was rapidly removed and post-fixed for 2 h in 4% paraformaldehyde in PBS, followed by 25% sucrose in PBS overnight at 4°C. The quantification of the total number of GLP-1R-expressing neurons on nodose ganglion sections were collected from ad libitum fed animals. For quantification (n=3) and localization (n=4) of GLP-1R protein, nodose ganglia were collected from rats fasted overnight or fasted overnight followed by 40 min re-fed; this time point was identified as the maximal change in food intake in response to administration of GLP-1. For mRNA analysis, nodose ganglia was collected from rats fasted overnight or fasted overnight and then re-fed for 2 h (n=5).
Immunohistochemistry
Cryostat sections of fixed nodose ganglia (9μm) were mounted onto Superfrost/Plus Slides (Fisher Scientific, Pittsburgh, PA) and processed for immunohistochemistry. Sections were blocked with 20% donkey serum (DS) (Vector Laboratories, Burlingame, CA), 0.2% Triton-X100 and 0.1% bovine serum albumin dissolved in PBS for 30mins at 37°C. Sections were incubated with an antibody raised against GLP-1R (Abcam 39072, Cambridge, MA) diluted at 1:1000 and plasma membrane marker, pan cadherin (Abcam 6528, Cambridge, MA) diluted at 1:100 in DS-PBS (2% donkey serum, 0.2% triton X100 and 0.1% bovine serum albumin dissolved in PBS) for 1 h at room temperature and then overnight at 4°C. The specificity of GLP-1R was verified in two different ways; immunostaining of kidney samples as a positive control and liver samples as a negative control and secondly by using GLP-1R immunogen blocking peptide (Abcam 39071, manufacturer's protocol was followed). Secondary antibody used was donkey anti-rabbit IgG conjugated with AlexaFluor 488 (Life technologies, Grand Island, NY) diluted at 1:1000 and donkey anti-mouse IgG conjugated with AlexaFluor 546 (Life technologies, Grand Island, NY) diluted at 1:500 in DS-PBS. Sections were incubated for 1 h at 37°C. Images were collected using an Olympus spinning disc confocal microscope (BX61 System, Olympus, Melville, NY). All images were collected using the same confocal parameters.
To determine the percentage of GLP-1R expression on VAN under fasted and refed conditions, images were taken at 40X magnification. The quantification of positive immunoreactivity for GLP-1R was analyzed by Scion Image software (Beta 4.0.2, Scion Corp., Frederick, MD, USA, 2000) and presented as percentage of positive neurons and pixels. The percentage of positive neurons and pixels were quantified by determining the total number of neurons and pixels and the total number of positive neurons and pixels within an area. Positive neurons and pixels were defined as the immunofluorescence with a gain above the threshold for background staining. The data were expressed as percentage of positive neurons, and positive pixels.
Photomicrographs were taken to determine the distribution of GLP-1R in refed and fasted rats at 60X magnification. We quantified the amount of receptor present on the membrane. This was quantified by determining the total number of pixels within an area and the total number of marked pixels. Positive pixels were defined as the immunofluorescence with a gain above the threshold for background staining. We used pan cadherin to mark the plasma membrane. We defined the plasma membrane and the cytoplasmic area using Photoshop (Adobe systems) and quantified pixels with Scion. The plasma membrane for each neuron was outlined just outside the plasma membrane and also on the inside edge of the plasma membrane where positive pixels for pan cadherin were present. The cytoplasmic area was defined as the remaining region of the cell from the inside edge of the plasma membrane. The data is expressed as a percentage of the number of positive pixels for GLP-1R present in the plasma membrane over the total number of positive pixels on the whole cell.
Western Blots
Tissue was prepared as described by Freeman et al., 2006 [16]. Briefly, 5μg of protein was loaded onto the gel. Samples were loaded into precast 10% BisTris-gel and ran for 50 minutes at 200V (Invitrogen Power Case 500). The proteins were transferred on a PDVF membrane for 1 h at 30V. Membrane was blocked using 10% milk in TBST for 1 h at room temperature. GLP-1R was diluted at 1:1000 and GADPH (14C10, rabbit mAB; Cell Signaling Technology) was used as a loading control. The specificity of GLP-1R was verified in two different ways; 1) immunostaining of lung and hindbrain samples as a positive control and liver, spleen and testes samples as a negative control and 2) GLP-1R immunogen blocking peptide. Primary antibodies were applied on the membrane and developed on separate but consecutive days. Antibodies were incubated for 1 h at room temperature and then overnight at 4°C. The membrane was imaged using ECL substrate (Thermo Scientific) and with ChemiDoc XRS Imager (BioRad, Hercules, CA). The membrane was analyzed by Image Lab version 5.0 software (Hercules, CA).
Real Time PCR
Vagus nerve samples were homogenized in a GenoGrinder2000 (SpexCertiprep). Total RNA was extracted from the tissue lysates using a 6100 automated nucleic acid (ANA) workstation (Applied Biosystems) according to the manufacturer's instructions. RNA concentrations were determined via absorbance at 260/280 nm using a NanoDrop (ThermoFisher Sciencific, Wilmington, D). The QuantiTect Reverse transcription kit (Qiagen) was used for cDNA synthesis following the manufactures directions. Taqman gene expression assay was used consisting of fluorescent labeled TaqMan probe (5’ end, reporter dye FAM (6-carboxyflourescein), 3’ end, quencher dye MGB (Minor Grove Binding Protein)) and primers for rGCG (Rn00562293_m1) and rGAPDH (Rn99999916_s1) from Applied Biosystems (Foster City, CA). Primers were validated using defined protocols (Leutenegger et al., 1999). Each qPCR reaction contained 20× primer and probes for the respective qPCR system with a final concentration of 400 nM for each primer and 80 nM for the TaqMan probe and commercially available qPCR mastermix (TaqMan Universal PCR Mastermix, Applied Biosystems) and 5 μl of the diluted cDNA sample in a final volume of 12 μl. The samples were placed in 384 well plates and amplified in an automated fluorometer (ABI PRISM 7900 HTA FAST, ABI). ABI's standard amplification conditions were used: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C and 60 s at 60°C. Final quantitation was done using the comparative CT method (User Bulletin #2, Applied Biosystems). GAPDH was used to normalize expression of the target genes. Data is reported as the n-fold difference (2-ΔΔCt) relative to a calibrator cDNA (the average of the control group). The sample expression levels show relative amounts found in the average fasted nodose ganglia expression level.
Statistical Analysis
Results are expressed as mean ± SEM. All studies were analyzed by using unpaired Student t-test or two way ANOVA repeated measures to validate statistical significance unless otherwise stated.
Results
Administration of GLP-1 inhibits food intake in re-fed, but not fasted, rats
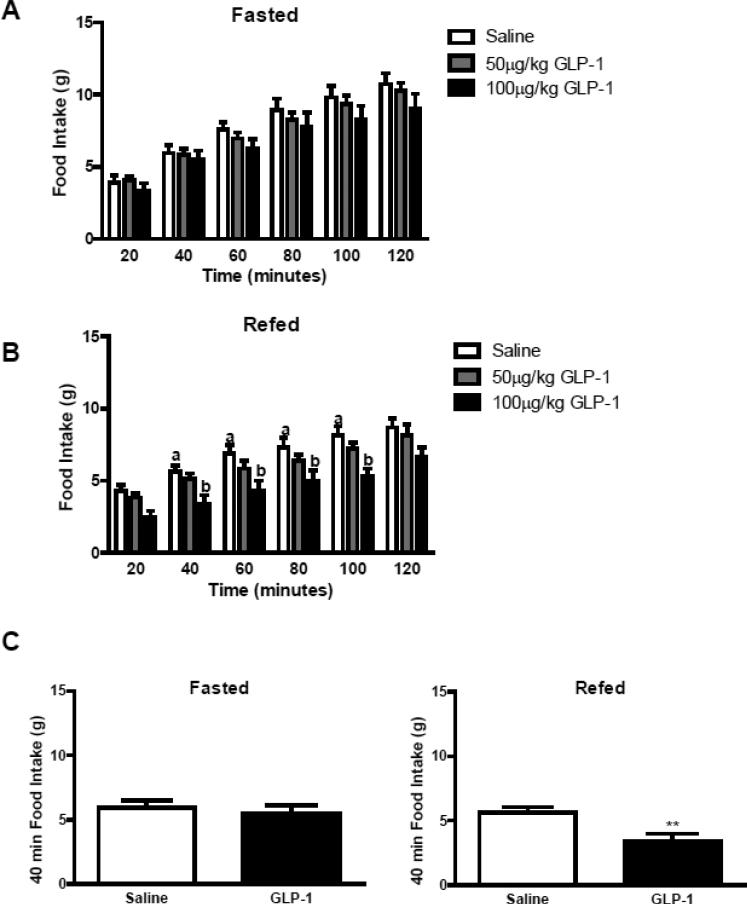
In overnight fasted rats, peripheral administration of GLP-1 (50μg/kg or 100μg/kg IP) failed to reduce food intake over 2 h (Figure 1A, p>0.05). However, in re-fed rats, administration of GLP-1 (100μg/kg, IP) significantly decreased food intake compared to saline (p<0.05; Fig 1B). There was no difference in food intake following administration of GLP-1 (50μg/kg) in either fasted or re-fed rats (Figure 1A and B). Food intake was significantly reduced (39%) 40 min after administration of GLP-1 (100μg/kg) compared to saline treatment in re-fed rats (p<0.01; Fig. 1C). GLP-1 significantly decreased food intake for 100 mins after administration (p<0.01; Fig. 1B).
Figure 1.
Food intake was measured in animals either fasted overnight or fasted then refed prior to administration of GLP-1 (50μg/kg or 100μg/kg, IP) (A) GLP-1 had no effect on food intake in fasted rats; (B) 100μg/kg of GLP-1 significantly decreased food intake in rats fasted then refed for 40mins prior to administration of GLP-1 (saline vs. 100μg/kg, P<0.05; n=7-8 in each group). (C) The anorexigenic effect of GLP-1 (100μg/kg) was significant at 40-min in refed rats (saline vs. 100μg/kg GLP-1, P<0.05, n=7 in each group). Data are represented as mean ± SEM. Statistical differences were measured using two-way ANOVA and unpaired Student t-test.
Vagal afferent neurons express GLP-1R
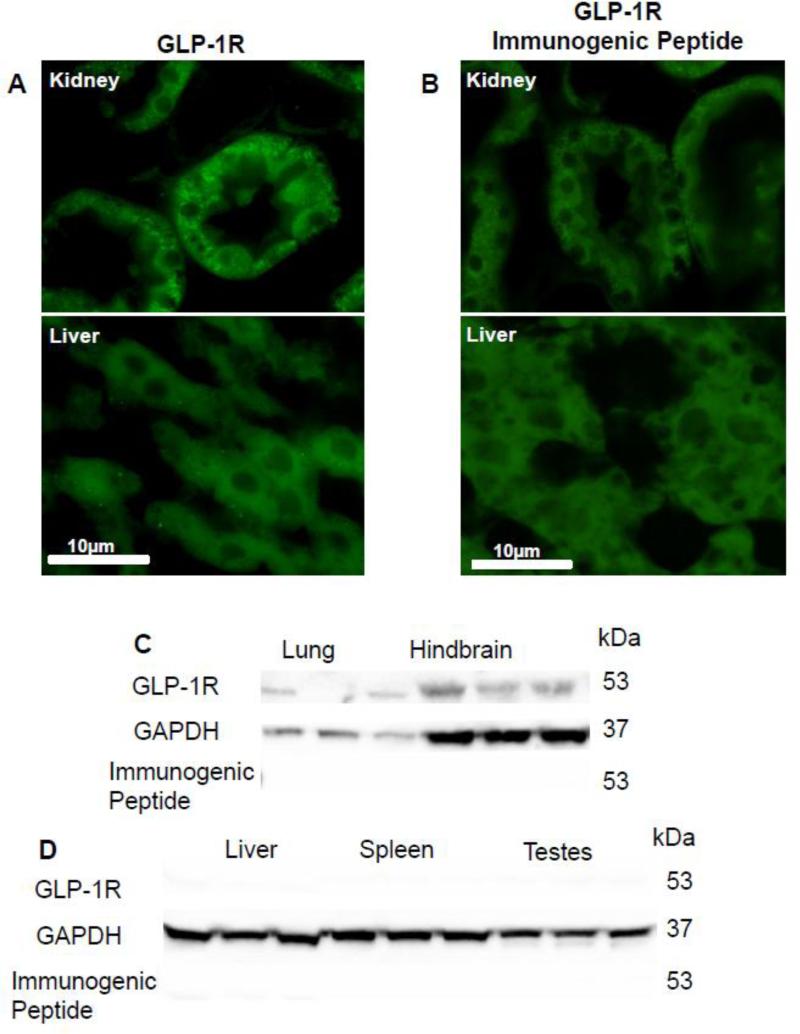
Initial experiments were aimed at characterizing the GLP-1R antibody. Sections of a kidney (positive control) and liver (negative control) [17] were used to verify the specificity of a recently available GLP-1R antibody (Fig 2A). Kidney sections expressed positive immunoreactivity for GLP-1R, however, there was no immunoreactivity detected in liver. Pre-incubation of the GLP-1R with the immunogenic peptide (1:1 ratio to antibody, 1μg/ml) abolished immunoreactivity in tissue sections of kidney (Fig 2B). The specificity of the GLP-1R antibody was also verified using immunoblots (Figure 2C and 2D). An immunoreactive band was present at 53kda in lung and hindbrain tissue (Figure 2C); no bands were detected in liver spleen or testes tissue (Figure 2D). Further, preincubation with the immunogenic peptide abolished immunoreactivity of the 53kDa band from lung and hindbrain (Fig. 2C). Taken together these data confirm the specificity of the antibody for GLP-1R.
Figure 2.
The specificity of the GLP-1R antibody was verified using positive (kidney and lung tissue) and negative control (liver tissue). GLP-1R immunoreactivity was present in section of kidneybut not liver A). Pre-incubation of the immunogenic peptide abolished immunoreactivity of the kidney (B). Immunoblots confirmed the specificity of the antisera (C) a band was present at the correct molecular weight (53kDa) for GLP-1R in lung and hindbrain (D) but not liver, spleen and testes tissue samples. Presence of the immunogenic peptide abolished immunostaining of the lung and hindbrain. All photomicrographs pictures were taken under the same conditions and at 40X magnification.
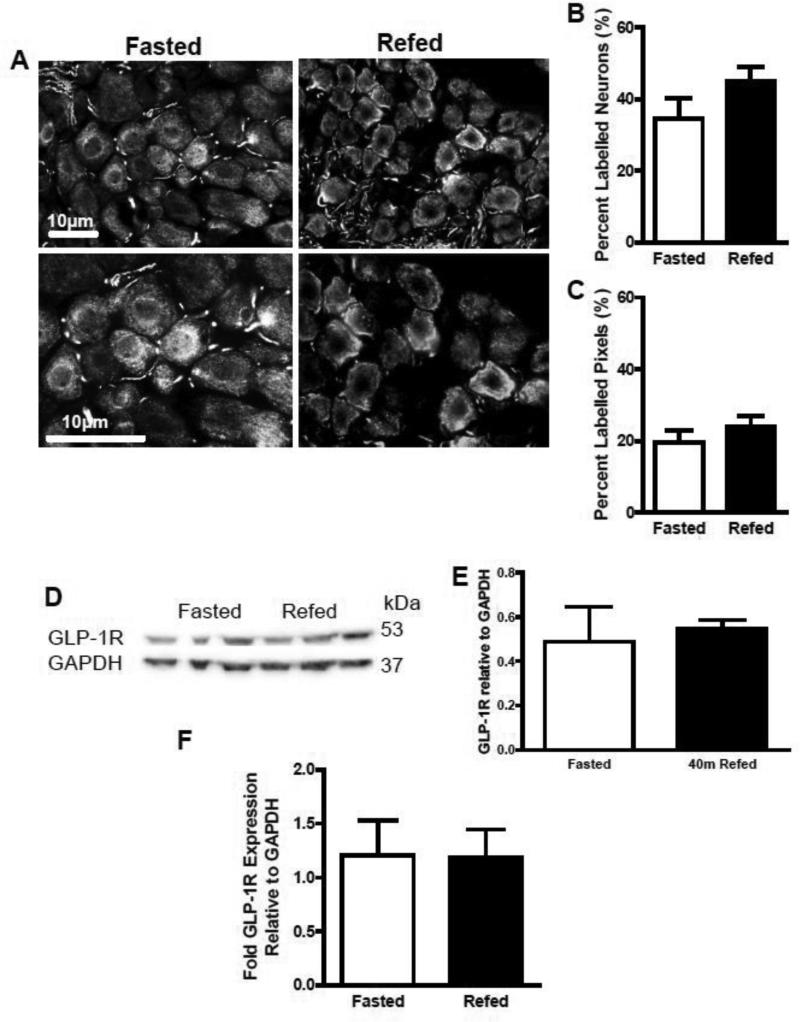
Although gene expression studies have shown that VAN express GLP-1R mRNA [18], there are no published reports of protein expression. We used immunofluorescence and immunoblots to show protein expression. GLP-1R immunoreactivity was seen in tissue sections of nodose ganglia (Fig 3A). GLP-1R immunoreactivity was present in tissue sections of nodose ganglia taken from both fasted and re-fed rats. Quantification revealed that 42% of VAN were immunopositive for GLP-1; the percentage of labeled pixels and labeled neurons in nodose ganglia did not differ with feeding status (p>0.05; Fig 3B and C). Immunoblots confirmed these findings; GLP-1R protein levels were similar for both fasted and re-fed groups (p>0.05, Fig 3D and E).
Figure 3.
The protein and mRNA levels were measured in fasted and 40-min refed NG samples. (A) photomicrographs of GLP-1R immunoreactivity in section of nodose ganglia, (B) the percent labeled neurons and (C) percent labeled pixels on NG sections did not differ between fasted and 40-min-refed animals (fasted vs. 40min refed, P>0.05, fasted and 40min refed n=4, 8 images were analyzed per rat). This was further confirmed with immunoblots; (D) and (E) protein levels relative to GAPDH did not change in a refed state compared to the fasted state (fasted vs. 40min refed, P>0.05, fasted and 40min refed n=3). mRNA levels were verified in animals that were fasted overnight or fasted overnight and then refed for 2 h. (F) There was no difference between the conditions in GLP-R mRNA levels (fasted vs. 40min refed, P>0.05, fasted and refed n=5). Data are represented as mean ± SEM. Statistical differences were measured using unpaired Student t-test.
We next tested GLP-1R mRNA levels in fasted and re-fed conditions. Given that it is unlikely that gene expression would change in a short amount of time, rats were fasted and refed for 2 h and gene expression compared to that in fasted rats. There was no difference in GLP-1R mRNA levels between fasted and 2 h re-fed nodose ganglia (p>0.05; Fig 3F).
GLP-1R localization on VAN in response to feeding status
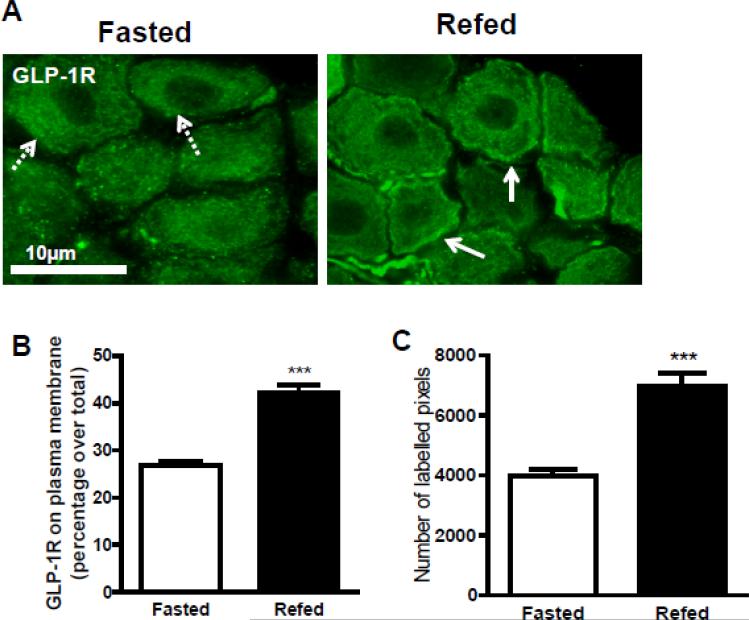
Immunohistochemistry was used to study the distribution of GLP-1R in vagal afferent neurons of re-fed and fasted rats. In tissue sections of nodose ganglia taken from fasted rats, the majority of the immunoreactivity was localized to the cytoplasm (Fig 4A). In contrast, in sections of nodose ganglia taken from re-fed rats, there was a significant increase in GLP-1R immunoreactivity at the membrane (Fig 4A). Pan cadherin was used as a plasma membrane marker [19] to quantify the immunoreactivity present on the membrane. Quantification of the immunoreactivity revealed that there was a significant increase of the number of labelled pixels at the plasma membrane of vagal afferent neurons from the re-fed compared to the fasted rats (Figure 4C). In the fasted state, 26.7 ± 1.0% of immunoreactivity for GLP-1R was localized to the plasma membrane compared to 42.3 ± 1.6% in the re-fed state; this represents a 1.8 fold increase in GLP-1R at the plasma membrane (Fig 4B).
Figure 4.
The immunohistochemical colocalization of GLP-1R at the plasma membranewas analyzed on fasted or 40min refed NG sections. (A) Photomicorgraphs of GLP-1R staining in green at 60X magnification. The merged images reveal that there was increased colocalization with GLP-1R at the membrane indicated with solid arrows in refed compared to fasted NG sections. The dotted arrows represent non-colocalization and the solid arrow represents colocalization. Pan cadherin was used to identify the plasma membrane and quantify the colocalization of GLP-1R at the membrane. The percent (B) and number of pixels (C) of GLP-1R present on the plasma membrane was a significantly increased in the refed condition compared to the fasted condition (fasted vs. 40-min refed, P<0.001, fasted vs. 40min refed n=4, 8 images per rat). Data are represented as mean ± SEM. Statistical differences were measured using unpaired Student t-test.
Discussion
In the present study, we clearly demonstrate that peripheral administration of native GLP-1 requires a postprandial state to express biological activity to inhibit food intake. In addition, we describe a novel mechanism by which GLP-1R on VAN is modulated by feeding. GLP-1R mRNA or protein expression by VAN is not altered by feeding; however, localization of the GLP-1R on the cell bodies of VAN is changed by feeding. GLP-1R co-localization at the plasma membrane of nodose ganglia neurons significantly increases in response to a short bout of food consumption compared to the fasting state. These findings provide the first line of evidence that GLP-1Rs expressed on vagal afferent neurons are trafficked to the membrane in the postprandial period. Trafficking of the receptor to the membrane provides a possible explanation to the observation that exogenously administered GLP-1 only inhibits food intake after feeding. The mechanism that is regulating trafficking of the receptor to the membrane is not known, but could depend either on the release of a signal from the gut upon intake of food that brings the receptor to the surface or the diminution of a signal that keeps the receptor localized to the cytoplasm. Our observations contribute to the understanding of the satiating mechanism of gut-derived GLP-1.
In this study, we demonstrate that peripheral GLP-1 inhibits food intake in the postprandial but not the food-deprived condition. A previous study showed that native GLP-1 failed to inhibit food intake following acute peripheral administration in fasted mice. However, in the same study, the identical dose of peripherally-administered GLP-1 (30nmol/kg) decreased energy consumption in ad libitum fed rats [11]. Taken together with the current findings, these studies suggest that GLP-1-induced satiation is dependent on feeding status. Williams et al. [7] provided preliminary evidence that the inhibitory effect of GLP-1 on food intake is induced postprandially; IP administrations of GLP-1 significantly decreased food intake in rats fed ad libitum then fasted for 4 hours, but had no effect in overnight fasted rats. In the present study, the re-fed group was given a specific amount of food for a controlled period of time prior to a GLP-1 administration to ensure little variation in hunger and satiation. In addition, we designed a within subject study to reduce the amount of variation associated with individual differences. This study provides clear evidence that the satiating effect of GLP-1 is dependent on postprandial status.
Previous work on anorexigenic hormones has found that their ability to suppress food intake was independent of feeding status [20, 21]; GLP-1 only induces satiation postprandially and therefore differs from other gut peptides such as cholecystokinin (CCK) [7, 10]. It is possible that GLP-1 requires the presence of another gut peptide to reinforce its physiological effect on regulating energy intake. There is considerable evidence that the gut hormones leptin and cholecystokinin (CCK) act synergistically to inhibit food intake [22]. There is evidence that this interactions occurs at the level of VAN; sensitivity of VAN to CCK is increased in the presence of leptin [23]. Leptin induces the expression of early gene regulator 1 while CCK translocates it to the nucleus [23]. There is also some evidence to suggest that GLP-1 acts synergistically with other gut hormones to potentiate its satiating effects. For example, Williams et al demonstrate that sequential peripheral administrations of leptin and GLP-1 additively to reduce 24h food intake [7]. In humans, infusions of PYY and GLP-1 administered together significantly decreased energy intake whereas GLP-1 alone and GLP-1 at a higher dose had no effect [11]. Further work is needed to assess whether there are similar interactions in the inhibitory effects of gut-derived GLP-1 at the level of the vagus.
We used an acute peripheral administration of native GLP-1 in order to mimic endogenous GLP-1 release from the GI tract in response to a meal. Williams et al. [24] have previously demonstrated in rats that when central GLP-1Rs are blocked, peripheral administration of GLP-1 is still able to decrease food intake, showing a specific peripheral site of action for the inhibitory effects of GLP-1. Numerous studies demonstrate that peripheral administration of GLP-1 will decrease food intake [7, 25], though central administration will also suppress energy intake [26, 27]. It remains unclear which GLP-1R population mediates the satiating effects of gut-derived GLP-1. Studies using synthetic GLP-1R agonist, exendin-4 (Ex-4), demonstrate that GLP-1 inhibits food intake in a dose-dependently manner after IP and vena cava infusions [8, 28, 29]. Unlike endogenous GLP-1, Ex-4 has a prolonged half-life and crosses the blood brain barrier in its intact form resulting in a more potent energy intake suppressor than GLP-1 [30, 31]. Ex-4 acts on both peripheral and central sites [31, 32]; it is unclear how much endogenous GLP-1 reaches the central receptors in response to a meal. In this study, we used native GLP-1 instead of Ex-4 to produce a more physiological condition that reflects gut-derived GLP-1 release in response to a meal. Our study indicates that gut-derived GLP-1 may play a physiological role in regulating energy balance.
In the present study, we show that VAN express both mRNA and protein for GLP-1Rs. It has previously been shown that VAN express GLP-1R mRNA but there is little evidence to suggest protein expression. Vahl et al. provided preliminary immunocytochemical evidence that GLP-1R might be present on nodose neurons, however, the specificity of the antibody was not established [18]. The present study is the first to use appropriate controls to definitively show specificity of a GLP-1R antibody. There was GLP-1 immunoreactivity in tissue sections of kidney, known to express GLP-1Rs [17] but not in liver, which does not express the receptor. Moreover, immunoblots revealed a positive band at the correct molecular weight for the receptors [17] in lung, hindbrain and nodose ganglia, but not in liver, spleen and testes tissue. Finally, positive immunoreactivity was abolished by pre-incubation with the immunogenic peptide. Therefore, our data conclusively show protein expression of GLP-1Rs by VAN. Panjwani et al [33] have suggested that GLP-1R antibody is not specific as an immunoreactive band was observed in tissue from GLP-1R knockout mice. It is possible that difference in the protocol between the current study and that of Panjawani et al explain the differences in the results; alternatively, it is possible that this represents a species difference. There is a possibility that the GLP-1R knockout mice have an increase in a protein that cross reacts with the GLP-1R antibody, explaining immunoreactive bands in negative control tissue. It is worth noting that we were unable to obtain any immunoreactive product when we used our protocols for either Western blot or immunocytochemistry in mice (Ronveaux et al, unpublished observations).
GLP-1 has been demonstrated to activate a vagal afferent pathway suggesting the presence of GLP-1R on VAN [34, 35]. Peripheral GLP-1 increases electrophysiological activity on both in vivo and in vitro preparations of the vagus nerve [13, 14, 35]. Vagal afferent activation in response to peripheral GLP-1 injection was reduced by vagotomy [34]. In clinical trials, the effect of inhibitory GLP-1 on energy intake was attenuated in vagotomized subjects indicating a possible functional role for the vagal afferent pathway in mediating the effects of GLP-1 [36]. Nakagawa et al. [37] found GLP-1R mRNA expression in cell bodies of VAN.
VAN constitute an important pathway in the regulation of food intake. These neurons exhibit plasticity and switch their phenotypes between a food-deprived and a postprandial state [15]. Previous data has shown that anorexigenic Y2 receptor expression by vagal afferent neurons is high following re-feeding, and conversely is decreased by 50% after a 13h fast [38]. Since GLP-1-induced satiation was found to be dependent on nutrient availability, we hypothesized that, similarly to Y2 receptor, GLP-1R expression would change according to feeding status. In contrast, in the present study, we found that the GLP-1R are constitutively expressed on VAN, regardless of feeding status. We found no evidence of changes in GLP-1R mRNA or protein expression in response to feeding or fasting. Instead we found that GLP-1R translocates to the plasma membrane postprandially when endogenous GLP-1 is released from the gastrointestinal tract. Importantly, the rapid translocation of GLP-1R to the plasma membrane occurs within a time frame that coincides with the behavioral changes we observe in response to exogenous GLP-1. Therefore we hypothesize that this mechanism is driving the behavioral changes. Future in vitro studies will be needed to be able to link the behavioral changes to the change in receptor expression. Although we do not provide direct evidence of the mechanism at the vagal terminals where GLP-1 is released, we can speculate that changes in localization of the receptor in cell bodies of VAN in the nodose ganglia action is also seen at the terminals in the intestine.
In conclusion, we demonstrate that GLP-1Rs are located on the VAN providing further evidence that gut-derived GLP-1 may signal via the vagal afferent pathway to alter function. Our findings indicate that GLP-1R on VAN play a functional role in regulating food intake by a change in cellular localization which is driven by feeding status. Given that the obesity epidemic is progressively growing worldwide, it is important to under mechanisms regulating energy balance. For example, in obese subjects there are diminished levels of GLP-1 concentrations in response to a meal [39]; moreover, there is evidence to suggest that bariatric surgery will restore GLP-1 signaling in obese subjects [40]. In this study, we have furthered our understanding of the potential mechanism by which peripheral GLP-1-induces satiation. Furthermore we have demonstrated a novel mechanism regulating the expression of biological activity of a gut peptide on VAN which may play a role in the integration of gut signals and the regulation of systemic hormonal signaling to the brain.
Highlights.
Satiation induced by peripheral glucagon-like peptide-1 dependent on feeding status
GLP-1R protein expressed by vagal afferent neurons
Feeding increases localization of GLP-1Rs to neuronal membrane
Provides a novel mechanism regulating biological activity of gut hormone
Acknowledgements
Research was supported by NIH DK41004 (HER). The authors would like to thank UC Davis core facility for the qPCR analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54(Suppl 4):9–17. [PubMed] [Google Scholar]
- 2.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 3.Hansotia T, Drucker DJ. GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept. 2005;128(2):125–34. doi: 10.1016/j.regpep.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Scrocchi LA, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2(11):1254–8. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 5.Elliott RM, et al. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–66. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 6.Gutzwiller JP, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44(1):81–6. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55(12):3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 8.Ruttimann EB, et al. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–81. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgartner I, et al. Hepatic-portal vein infusions of glucagon-like peptide-1 reduce meal size and increase c-Fos expression in the nucleus tractus solitarii, area postrema and central nucleus of the amygdala in rats. J Neuroendocrinol. 2010;22(6):557–63. doi: 10.1111/j.1365-2826.2010.01995.x. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval D, et al. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS One. 2012;7(12):e51870. doi: 10.1371/journal.pone.0051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neary NM, et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146(12):5120–7. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 12.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–96. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 13.Bucinskaite V, et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21(9):978–e78. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 14.Kakei M, et al. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102(1-2):39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 15.Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201(3):313–21. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 16.Freeman SL, et al. Ligand-induced 5-HT3 receptor internalization in enteric neurons in rat ileum. Gastroenterology. 2006;131(1):97–107. doi: 10.1053/j.gastro.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Dunphy JL, Taylor RG, Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol. 1998;141(1-2):179–86. doi: 10.1016/s0303-7207(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 18.Vahl TP, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965–73. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 19.Izumi H, Kaneko Y. Evidence of asymmetric cell division and centrosome inheritance in human neuroblastoma cells. Proc Natl Acad Sci U S A. 2012;109(44):18048–53. doi: 10.1073/pnas.1205525109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naslund E, Hellstrom PM. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol Behav. 2007;92(1-2):256–62. doi: 10.1016/j.physbeh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Barrachina MD, et al. Leptin-induced decrease in food intake is not associated with changes in gastric emptying in lean mice. Am J Physiol. 1997;272(3 Pt 2):R1007–11. doi: 10.1152/ajpregu.1997.272.3.R1007. [DOI] [PubMed] [Google Scholar]
- 22.Barrachina MD, et al. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94(19):10455–60. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lartigue G, et al. EGR1 Is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology. 2010;151(8):3589–99. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–7. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poleni PE, et al. Possible involvement of melanocortin-4-receptor and AMP-activated protein kinase in the interaction of glucagon-like peptide-1 and leptin on feeding in rats. Biochem Biophys Res Commun. 2012;420(1):36–41. doi: 10.1016/j.bbrc.2012.02.109. [DOI] [PubMed] [Google Scholar]
- 26.Barrera JG, et al. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes. 2009;58(12):2820–7. doi: 10.2337/db09-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang-Christensen M, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271(4 Pt 2):R848–56. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 28.Hayes MR, et al. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 2011;19(7):1342–9. doi: 10.1038/oby.2011.50. [DOI] [PubMed] [Google Scholar]
- 29.Kanoski SE, et al. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62(5-6):1916–27. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27(3):313–8. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 31.Jessen L, et al. Suppression of food intake by glucagon-like peptide-1 receptor agonists: relative potencies and role of dipeptidyl peptidase-4. Endocrinology. 2012;153(12):5735–45. doi: 10.1210/en.2012-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baraboi ED, et al. Brain activation following peripheral administration of the GLP-1 receptor agonist exendin-4. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1011–24. doi: 10.1152/ajpregu.00424.2010. [DOI] [PubMed] [Google Scholar]
- 33.Panjwani N, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 2013;154(1):127–39. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- 34.Abbott CR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem- hypothalamic pathway. Brain Res. 2005;1044(1):127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Gaisano GG, et al. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol Motil. 2010;22(4):470–9. e111. doi: 10.1111/j.1365-2982.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 36.Plamboeck A, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1117–27. doi: 10.1152/ajpgi.00035.2013. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa A, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose- dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110(1):36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Burdyga G, et al. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008;28(45):11583–92. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranganath LR, et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38(6):916–9. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morinigo R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16(12):1594–601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]