Abstract
Tissue factor (TF) is the primary activator of the coagulation cascade. Under normal conditions, endothelial cells (ECs) and blood cells, such as monocytes, do not express TF. However, bacterial lipopolysaccharide (LPS) induces TF expression in monocytes and this leads to disseminated intravascular coagulation during endotoxemia and sepsis. A variety of stimuli induce TF expression in ECs in vitro, although it is unclear how much TF is expressed by the endothelium in vivo. LPS induction of TF gene expression in monocytic cells and ECs is mediated by various intracellular signaling pathways and the transcription factors NF-κB, AP-1 and Egr-1. In contrast, vascular endothelial cell growth factor (VEGF) induces TF gene expression in ECs via the transcription factors NFAT and Egr-1. Similarly, oxidized phospholipids (oxPAPC) induce TF expression in ECs and possibly monocytes via NFAT and Egr-1. Thromboxane (TX) A2 can now be added to the list of stimuli that induce TF gene expression in both monocytes and ECs. Interestingly, inhibition of the TX-prostanoid (TP) receptor also reduces TF expression in ECs stimulated with tumor necrosis factor (TNF)-α and monocytes stimulated with LPS, which suggests that TP receptor antagonist may be useful in reducing pathologic TF expression in the vasculature.
Keywords: tissue factor, expression, thromboxane A2, endothelial cells, monocytes
Introduction
TF is a transmembrane protein that functions as the primary initiator of the coagulation cascade1. Upon vascular damage, TF surrounding the vasculature comes into contact with blood. This leads to the formation of the TF:FVIIa complex that activates both FX and FIX, with subsequent thrombin generation, fibrin deposition and activation of platelets1. TF is constitutively expressed by cells within and surrounding the blood vessel wall, such as pericytes and adventitial fibroblasts2,3. It has been proposed that TF expressed by these cell types forms a hemostatic envelope that limits bleeding after vessel injury2. However, in pathologic conditions like sepsis, TF is also expressed by vascular cells, such as monocytes and ECs4. This expression can lead to disseminated intravascular coagulation (DIC) and thrombosis. TF expression by monocytes may be part of the innate immune response and is probably an attempt by the host to reduce the spread of pathogenic organisms. In atherosclerosis, TF is expressed by several cell types within atherosclerotic plaques, including macrophage-derived foam cells 5. After plaque rupture, TF likely contributes to the formation of a thrombus.
TF expression in monocytes and ECs
Under normal conditions TF is not expressed by circulating blood cells2. However, one study found low levels of TF expression in a few CD14-positive monocytes6. Stimulation of monocytes and monocytic cells with LPS induces TF expression in vitro and in vivo2,6–9. Furthermore, we and others have shown that TF expression by hematopoietic cells contributes to the activation of coagulation in endotoxemic mice10,11. In vitro studies demonstrated that a variety of agonists, including LPS, IL-1β, TNF-α, thrombin and VEGF, induce TF expression on ECs12–26. In contrast, only a limited number of studies have reported TF expression by ECs in vivo. One study found co-localization of TF and the EC marker von Willebrand factor within the splenic microvasculature of septic baboons but not in ECs of pulmonary vessels4. Another study found TF protein on ECs in LPS treated mice and rabbits27,28. More recently, TF protein was observed on ECs at branch points of the aorta of septic baboons29. TF protein co-localized with fibrin deposition, suggesting that it was functional29. However, TF present on ECs was restricted to granular structures some of which were also positive for the leukocyte marker P-selectin glycoprotein ligand-1 (PSGL-1)29. This suggests that leukocyte-derived microparticles may deliver TF to activated ECs in vivo. In contrast to these studies, we and others did not detect TF expression by ECs in LPS treated mice, rats, and rabbits30–33. These different results may be caused by the relative sensitivity of the various techniques used to detect TF expression. Furthermore, it is possible that TF expression on ECs contributes to signaling rather than activation of coagulation. We analyzed the effect of EC-specific deletion of the TF gene on the activation of coagulation in mouse models of endotoxemia and sickle cell disease. We found that a deficiency of TF in ECs did not decrease the activation of coagulation in either model34,35. However, in the sickle cell disease model we found a reduction of IL-6 expression35. Similar results were observed with a FXa inhibitor or protease-activated receptor (PAR)-2 deficiency in non-hematopoietic cells suggesting that TF on ECs contributes to the induction of IL-6 expression via FXa activation of PAR-2.
Induction of TF gene expression in monocytes
i) LPS
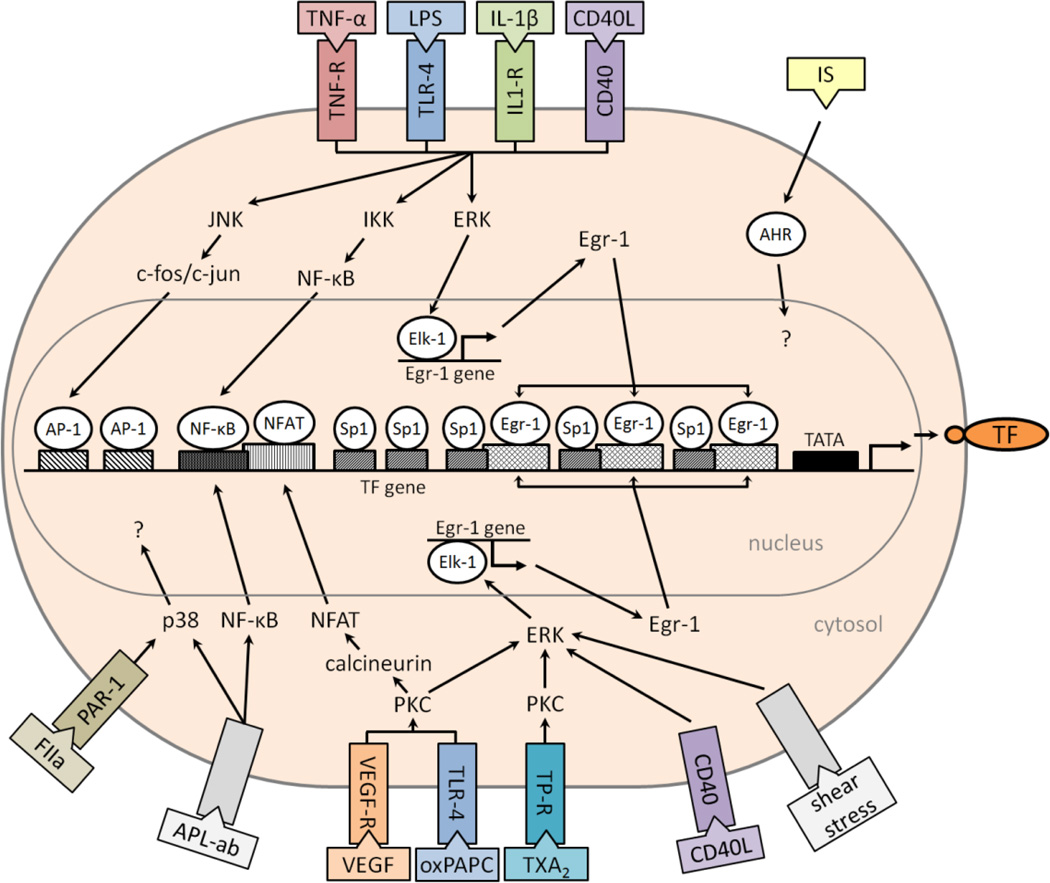
The THP-1 cell line has been used as a model to study the regulation of TF gene expression in monocytes. These cells are derived from an acute human monocytic leukemia. LPS stimulation of THP-1 increases the rate of TF gene transcription, TF mRNA and TF protein. The human TF promoter contains a NF-κB site and two AP-1 sites in a distal region (Figure 1)36. In addition, the proximal region of the promoter contains two Sp1 sites (−172 to −112) and three overlapping Sp1/Egr-1 sites (−111 to +14) (Figure 1)37. The proximal region of the promoter (−170 to −59 bp) is required for basal expression38.
Figure 1.
Induction of the human tissue factor (TF) promoter in endothelial cells. Shown are intracellular signaling pathways, transcription factors and DNA binding sites that regulate TF gene expression in response to different agonists. Receptor (R), oxidized phospholipids (oxPAPC), antiphospholipid antibody (APL-ab), aryl hydrocarbon receptor (AHR), thromboxane A2 (TXA2), thrombin (FIIa), indoxylsulfate (IS), vascular endothelial growth factor (VEGF), protease-activated receptor 1 (PAR-1).
An LPS response element (LRE) in the human TF promoter was identified by analyzing a series of plasmids containing different lengths of the promoter cloned upstream of the luciferase reporter gene. This element spans 56-bp (−227 to −172) and contains a NF-κB site and two AP-1 sites36. The NF-κB site is essential for full functionality of the LRE36. Interestingly, the NF-κB site does not match the κB consensus sequence due to a C instead of a G at position 139 and binds c-Rel-p65 heterodimers and not the prototypic p50-p65 heterodimers40. It was found that the transcriptional activation of the TF gene involves functional interactions between c-fos/c-jun and c-Rel-p65 heterodimers14. In addition, LPS induction of the TF gene was sensitive to nucleotide spacing between the proximal AP-1 and κB sites. Conservation of this 15-bp spacing in the human, murine, and porcine promoters may be required for physical association between c-fos/c-jun and c-Rel/p65 heterodimers41. Alternatively, the conserved spacing and defined DNA bending between the AP-1 and κB sites may be important for allowing the interaction of c-fos/c-jun and c-Rel/p65 with the TATA box binding protein and transcription factor IIB within the basal transcriptional machinery41. Additional studies showed that Egr-1 is required for maximal LPS induction of the TF promoter42. Mutation of the Egr-1 sites in the TF promoter or inhibition of the ERK 1/2 pathway, which induces Egr-1 gene expression, reduced the level of LPS induction of TF gene expression42.
ii) Oxidized low-density lipoprotein (oxLDL)
We recently showed that oxLDL, but not LDL, increased TF expression in THP-1 and human peripheral blood mononuclear cells (PBMCs)43. Preincubation of the cells with a TLR-4 inhibitor (CLI-095) or simvastatin reduced the induction of TF expression43. We are currently analyzing the different signaling pathways and transcription factors that mediate oxLDL induction of TF expression.
Induction of TF gene expression in ECs
i) LPS, IL-1β and TNF-α
We found that LPS induction of TF gene expression in ECs was mediated by the LRE and Egr-1 sites (Figure 1), indicating that a common mechanism regulates TF gene expression in both human monocytes and ECs14,42. Furthermore, TNF-α and IL-1β also activated AP-1 and NF-κB in human umbilical vein ECs (HUVECs)14. A study that subjected human pulmonary artery ECs to inhibitors of several intracellular signaling pathways demonstrated a critical role for protein kinase C (PKC) and for p38 in the induction of TF expression44.
ii) CD40L
CD40L induces TF expression through a variety of pathways in ECs, ultimately involving the transcription factors AP-1, NF-κB and Egr-1 that all appear to be necessary in order to achieve a maximal response (Figure 1)19–22.
iii) Antiphospholipid antibodies
Antiphospholipid syndrome is an autoimmune disease caused by antiphospholipid antibodies. Patients are hypercoagulable particularly during pregnancy45,46. Anti-phospholipid antibodies have been shown to induce TF expression on HUVECs through an unknown receptor but involving the NF-κB and p38 intracellular pathways (Figure 1)24. Similar results were observed using PBMCs47. Induction of TF expression in monocytes and ECs may explain the prothrombotic state caused by these antibodies and it may lead to the development of more directed antithrombotic/anti-inflammatory therapy in these patients, for example by inhibition of p38 (Figure 1).
iv) VEGF
VEGF has been shown to induce TF gene expression in HUVECs via two distinct pathways. First, it triggers NFAT dephosphorylation by calcineurin, which allows nuclear translocation of NFAT, binding to a site in the TF promoter (−197 to −183) and induction of TF gene expression16. There is some evidence that it also increases the transcriptional activity of AP-116,48. Secondly, VEGF induces TF gene expression via a PKC-dependent pathway that leads to activation of ERK 1/2 and Egr-1 gene expression17. Importantly, NFAT and Egr-1 synergistically cooperate in VEGF induction of the TF promoter (Figure 1)49.
v) oxPAPC
OxLDL and oxPAPC induce TF expression in HUVECs50. Interestingly, oxPAPC induction of TF gene expression involved both NFAT and Egr-1 in a similar manner to VEGF (Figure 1)18.
vi) Shear stress
Two studies have reported that induction of the TF gene in ECs by laminar shear stress was mediated by a GC-rich region (−111 to +14) containing three copies each of the Egr-1 and Sp1 sites15,51. These Egr-1 and Sp1 binding sites are overlapping which precludes binding of both transcription factors at the same time15. One study concluded that the induction was mediated by modifying Sp1 bound to the promoter51. However, a second study concluded that shear stress induced the expression of Egr-1 and that this leads to increased TF gene expression (Figure 1)15, which is a more plausible mechanism.
vii) Indolic uremic solutes
Uremic solutes are increased in patients with chronic kidney disease and could contribute to their prothrombotic phenotype and high cardiovascular mortality. Recently, a study reported that the indolic uremic solutes indoxyl sulfate and indole-3-acetic acid induce TF expression in HUVECs23. Interestingly, this induction was mediated by the aryl hydrocarbon receptor (AHR) (Figure 1)23. After activation, AHR translocates to the nucleus and acts as a transcription factor. However, there is no consensus sequence for AHR binding in the TF promoter, although it may bind to a non-consensus sequence. Alternatively, AHR may enhance signaling pathways or interact with transcription factors that regulate TF gene expression52–54.
A summary of the different intracellular signaling pathways and transcription factors involved in the induction of TF gene expression in monocytes and ECs is shown in Table 1.
Table 1.
TF gene expression in monocytes and endothelial cells Monocytes
| Monocytes | |||||
|---|---|---|---|---|---|
| Agonist | Signaling pathways | Transcription factor | Promoter region | Cell type | References |
| LPS | AP-1, NF-κB | −227 to −172 | THP-1 | 36,40 | |
| LPS | MEK 1/2, ERK 1/2, Elk-1 | Egr-1 | −111 to +14 | THP-1 | 42 |
| LPS | AP1, NF-κB, Egr-1 | −227 to −172, −111 to +14 | THP-1 | 38 | |
| LPS | p38; ERK 1/2 | THP-1 | 65 | ||
| oxLDL | THP-1, PBMC | 43 | |||
| IS, IAA | AHR | PBMC | 23 | ||
| APL Ab | p38 | NF-κB | THP-1 | 47 | |
| TXA2 | ERK 1/2 | PBMC | 61,63 | ||
| Endothelial cells | |||||
|---|---|---|---|---|---|
| Agonist | Signaling pathways | Transcription factor | Promoter region | Cell type | References |
| LPS, TNF-α, IL-1β | AP-1, NF-κB | −227 to −172 | HUVEC | 14 | |
| Thrombin | PKC, p38 | HPAEC | 44 | ||
| Shear stress | Egr-1 | −111 to +14 | HUVEC | 15 | |
| VEGF | calcineurin | NFAT | −197 to −183 | HUVEC | 16 |
| VEGF | PKC, ERK 1/2 | Egr-1 | HUVEC | 17 | |
| oxPAPC | PKC, ERK 1/2; calcineurin | Egr-1; NFAT | HUVEC | 18 | |
| CD40L | AP1, NF-κB, Egr-1 | −278 to +121 | HSVEC, HUVEC | 22 | |
| IS, IAA | AHR | HUVEC | 23 | ||
| APL Ab | p38 | NF-κB | HUVEC | 24 | |
| PAF | HUVEC | 66 | |||
| TXA2 | PKC, ERK 1/2, JNK | HUVEC | 25 | ||
Bacterial lipopolysaccharide (LPS), oxidized low-density lipoprotein (oxLDL), peripheral blood mononuclear cell (PBMC), indoxyl sulfate (IS), indole-3-acetic acid (IAA), aryl hydrocarbon receptor (AHR), antiphospholipid antibody (ABL Ab), thromboxane A2 (TXA2), human umbilical vein endothelial cell (HUVEC), human pulmonary artery endothelial cell (HPAEC), vascular endothelial growth factor (VEGF), oxidized phospholipids (oxPAPC), human saphenous vein endothelial cell (HSVEC), platelet activating factor (PAF)
TXA2 and TF expression in monocytes and ECs
The eicosanoid TXA2 is a proinflammatory mediator. It activates a variety of cell types, including monocytes and ECs, by binding to the TP receptor55. A paper in this issue of Vascular Pharmacology found that a TP receptor agonist (U46619) induced TF expression in ECs25. A previous study showed that U46619 induces MCP-1 expression in ECs56. The TP receptor activates a PKC dependent pathway that leads to the activation of AP-1 and NF-κB56. In a mouse model of microcirculatory dysfunction in the liver, TNF-α induced leukocyte adhesion was significantly reduced by administration of a TXA2 synthase inhibitor (OKY-046) and in TP receptor knockout mice, suggesting TP receptor signaling may promote hepatic dysfunction elicited by TNF-α57. The phenotype of TP deficient mice was more pronounced than that of TX synthase deficient mice suggesting that ligands other than TXA2 may activate the TP receptor55. This study indicated that TXA2 stimulation of the TP receptor contributes to the effects of TNF-α in vivo. Interestingly, Del Turco and colleagues found that inhibition of the TP receptor reduced TNF-α induction of TF expression in ECs25. Importantly, TXA2 production is enhanced in HUVECs by TNF-α or platelet-activating factor (PAF) stimulation58–60. However, Del Turco and colleagues concluded that the reduction of TNF-α induction of TF expression by blocking the TP receptor was not due to the production of TXA2 or prostanoids by the ECs since they did not observe any effect after treating the cells with acetylsalicylic acid (ASA) or indomethacin25. One concern is that levels of the TXA2 metabolic product TXB2 were only measured at 24 hours. Moreover, the cells may express other ligands that activate the TP receptor55.
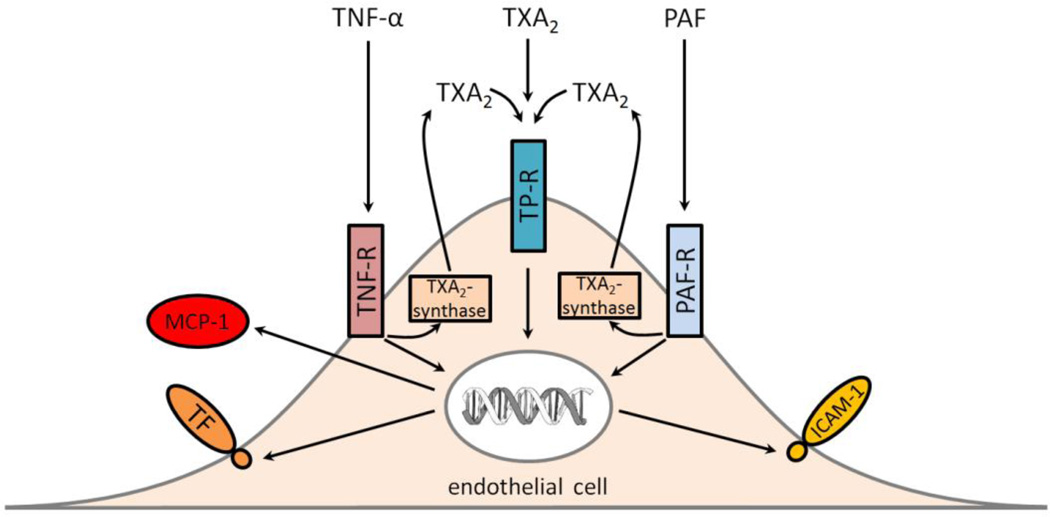
An alternative explanation for the effect of the TP antagonist on TNF-α is that the activated cells express TXA2 and this activates the TP receptor and enhances the induction of TF expression (Figure 2). If this notion is correct one would predict that the effect of the TP antagonist would be more pronounced at later times. Unfortunately, Del Turco and colleague only analyzed TF expression at 6 hours25. Another study found that TNF-α or PAF induction of ICAM-1 expression in ECs was decreased with a TXA2 synthesis inhibitor (DP-1904)58. Similarly, treatment of ECs with a TP receptor antagonist (SQ29 548 or BAYu3405) reduced TNF-α or PAF induction of ICAM-1 and MCP-1 expression56,59. Taken together, these results suggest that TNF-α and PAF stimulation of ECs leads to production of TXA2 that is secreted and then activates intracellular pathways through the TP receptor (Figure 2).
Figure 2.
Proposed mechanism by which the TP receptor (TP-R) contributes to gene expression in endothelial cells. TP-R can directly be activated by TXA2 or receptor agonists and induce the expression of tissue factor (TF), ICAM-1 and MCP-1 In addition, the presence of the TP-R enhances TNF-α and PAF induction of gene expression by increasing TXA2 expression by TXA2-synthase. TNF-α receptor (TNF-R), PAF receptor (PAF-R).
Consistent with the above results in ECs, TF expression is reduced in LPS stimulated human monocytes by a TP receptor antagonist (SQ29 548) and by indobufen, a cyclooxygenase (COX)-1/2 inhibitor, which decreases TXA2 production61,62. Treatment with ASA, a COX-1 inhibitor, does not reduce TF expression, suggesting that COX-2 metabolites, such as TXA2, are regulators of TF expression61. Indobufen also led to reduced ERK 1/2 phosphorylation, suggesting an involvement of this pathway in induction of TF expression61. In another study examining the effect of a variety of inhibitors on the LPS induced monocyte TF expression in human whole blood, the TP receptor and PAF receptor were shown to be necessary for full induction of TF activity63.
Conclusions
TF is a cellular receptor that initiates blood coagulation. It is constitutively expressed in some extravascular cell types and its expression is inducible in several vascular cell types, including monocytes and ECs. Further studies are needed to clarify the exact mechanism of TNF-α induced TP receptor activation and to assess the effects of this activation in different cell types and in vivo in different pathologic settings. The observation that the TP receptor is an important inducer of TF expression in ECs is intriguing because antagonization of the TP receptor may represent a new treatment of acute and chronic inflammatory conditions that involve TF expression, such as sepsis and atherosclerosis. Terutroban, the TP receptor antagonist used by Del Turco and colleagues has already been compared to ASA in a randomized controlled trial (PERFORM)64 on patients with recent ischemic stroke or transient ischemic attacks. No significant difference was found for the primary endpoint which was a composite of fatal or non-fatal ischemic stroke, fatal or non-fatal myocardial infarction, or other vascular death. One possible explanation for the negative result, with the notion that a major effect of the drug is the inhibition of TF expression, is that there is little benefit to be gained after the ischemic event. It would be interesting, however, to see TP receptor antagonists evaluated in the primary prevention of stroke or coronary artery disease and in the treatment of DIC or other thrombotic conditions associated with monocyte TF expression.
Acknowledgements
This work was supported by the National Institutes of Health grant HL 006350. We would like to thank Silvio Antoniak, Julia Geddings and Nicole Fleming for critical reading of the manuscript, and Weeranun Bode for assisting with the design of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24(6):1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 2.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134(5):1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 3.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 4.Drake TA, Cheng J, Chang A, Taylor FB. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142(5):1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egorina EM, Sovershaev MA, Bjørkøy G, Gruber FXE, Olsen JO, Parhami-Seren B, Mann KG, Østerud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25(7):1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9(6):2752–2755. doi: 10.1128/mcb.9.6.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco RF, de Jonge E, Dekkers PE, Timmerman JJ, Spek CA, van Deventer SJ, van Deursen P, van Kerkhoff L, van Gemen B, ten Cate H, van der Poll T, Reitsma PH. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96(2):554–559. [PubMed] [Google Scholar]
- 9.Brand K, Fowler BJ, Edgington TS, Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991;11(9):4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlinski R, Pedersen B, Schabbauer G, Tencati M, Holscher T, Boisvert W, Andrade-Gordon P, Frank RD, Mackman N. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103(4):1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoenmakers SHHF, Groot AP, Florquin S, Reitsma PH, Spek CA. Blood cell-derived tissue factor influences host response during murine endotoxemia. Blood Cells Mol Dis. 32(2):325–333. doi: 10.1016/j.bcmd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Colucci M, Balconi G, Lorenzet R, Pietra A, Locati D, Donati MB, Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983;71(6):1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA. Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15(5):612–621. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 15.Houston P, Dickson MC, Ludbrook V, White B, Schwachtgen JL, McVey JH, Mackman N, Reese JM, Gorman DG, Campbell C, Braddock M. Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. Arterioscler Thromb Vasc Biol. 1999;19(2):281–289. doi: 10.1161/01.atv.19.2.281. [DOI] [PubMed] [Google Scholar]
- 16.Armesilla AL, Lorenzo E, Gómez del Arco P, Martínez-Martínez S, Alfranca A, Redondo JM. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol Cell Biol. 1999;19(3):2032–2043. doi: 10.1128/mcb.19.3.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, De Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J. 2001;15(1):230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- 18.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99(1):199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Stordeur P, de Lavareille A, Thielemans K, Capel P, Goldman M, Pradier O. CD40 engagement on endothelial cells promotes tissue factor-dependent procoagulant activity. Thromb Haemost. 1998;79(5):1025–1028. [PubMed] [Google Scholar]
- 20.Miller DL, Yaron R, Yellin MJ. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J Leukoc Biol. 1998;63(3):373–379. doi: 10.1002/jlb.63.3.373. [DOI] [PubMed] [Google Scholar]
- 21.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Müller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80(6):1008–1014. [PubMed] [Google Scholar]
- 22.Bavendiek U, Libby P, Kilbride M, Reynolds R, Mackman N, Schönbeck U. Induction of tissue factor expression in human endothelial cells by CD40 ligand is mediated via activator protein 1, nuclear factor kappa B, and Egr-1. J Biol Chem. 2002;277(28):25032–25039. doi: 10.1074/jbc.M204003200. [DOI] [PubMed] [Google Scholar]
- 23.Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat-George F, Burtey S. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84(4):733–744. doi: 10.1038/ki.2013.133. [DOI] [PubMed] [Google Scholar]
- 24.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52(5):1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 25.Del Turco S, Basta G, Lazzerini G, Chancharme L, Lerond L, De Caterina R. Involvement of the thromboxane-prostanoid receptor in TNF-α induced endothelial tissue factor expression. Vascul Pharmacol. 2014 doi: 10.1016/j.vph.2014.03.007. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Szotowski B, Antoniak S, Poller W, Schultheiss H-P, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96(12):1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 27.Song D, Ye X, Xu H, Liu SF. Activation of endothelial intrinsic NF-{kappa}B pathway impairs protein C anticoagulation mechanism and promotes coagulation in endotoxemic mice. Blood. 2009;114(12):2521–2529. doi: 10.1182/blood-2009-02-205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semeraro N, Triggiani R, Montemurro P, Cavallo LG, Colucci M. Enhanced endothelial tissue factor but normal thrombomodulin in endotoxin-treated rabbits. Thromb Res. 1993;71(6):479–486. doi: 10.1016/0049-3848(93)90121-4. [DOI] [PubMed] [Google Scholar]
- 29.Lupu C, Westmuckett AD, Peer G, Ivanciu L, Zhu H, Taylor FB, Lupu F. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am J Pathol. 2005;167(4):1161–1172. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlinski R, Pedersen B, Kehrle B, Aird WC, Frank RD, Guha M, Mackman N. Regulation of tissue factor and inflammatory mediators by Egr-1 in a mouse endotoxemia model. Blood. 2003;101(10):3940–3947. doi: 10.1182/blood-2002-07-2303. [DOI] [PubMed] [Google Scholar]
- 31.Erlich J, Fearns C, Mathison J, Ulevitch RJ, Mackman N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect Immun. 1999;67(5):2540–2546. doi: 10.1128/iai.67.5.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara S, Asada Y, Hatakeyama K, Marutsuka K, Sato Y, Kisanuki A, Sumiyoshi A. Expression of tissue factor and tissue factor pathway inhibitor in rats lungs with lipopolysaccharide-induced disseminated intravascular coagulation. Lab Invest. 1997;77(6):581–589. [PubMed] [Google Scholar]
- 33.Mackman N, Sawdey MS, Keeton MR, Loskutoff DJ. Murine tissue factor gene expression in vivo. Tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol. 1993;143(1):76–84. [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlinski R, Mackman N. Tissue factor and heart inflammation. J Thromb Haemost. 2009;7(2):288–289. doi: 10.1111/j.1538-7836.2008.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparkenbaugh EM, Chantrathammachart P, Mickelson J, van Ryn J, Hebbel RP, Monroe DM, Mackman N, Key NS, Pawlinski R. Differential contribution of FXa and thrombin to vascular inflammation in a mouse model of sickle cell disease. Blood. 2014;123(11):1747–1756. doi: 10.1182/blood-2013-08-523936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackman N, Brand K, Edgington TS. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackman N. Regulation of the tissue factor gene. FASEB J. 1995;9(10):883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- 38.Oeth P, Parry GC, Mackman N. Regulation of the tissue factor gene in human monocytic cells. Role of AP-1, NF-kappa B/Rel, and Sp1 proteins in uninduced and lipopolysaccharide-induced expression. Arterioscler Thromb Vasc Biol. 1997;17(2):365–374. doi: 10.1161/01.atv.17.2.365. [DOI] [PubMed] [Google Scholar]
- 39.Grilli M, Chiu JJ, Lenardo MJ. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 40.Oeth PA, Parry GC, Kunsch C, Nantermet P, Rosen CA, Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol Cell Biol. 1994;14(6):3772–3781. doi: 10.1128/mcb.14.6.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glover JN, Harrison SC. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature. 1995;373(6511):257–261. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- 42.Guha M, O’Connell MA, Pawlinski R, Hollis A, McGovern P, Yan SF, Stern D, Mackman N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood. 2001;98(5):1429–1439. doi: 10.1182/blood.v98.5.1429. [DOI] [PubMed] [Google Scholar]
- 43.Owens AP, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, Williams JC, Hubbard BK, Dutton J, Wang J, Tobias PS, Curtiss LK, Daugherty A, Kirchhofer D, Luyendyk JP, Moriarty PM, Nagarajan S, Furie BC, Furie B, Johns DG, Temel RE, Mackman N. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122(2):558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Aird WC. Thrombin, TNF-alpha, and LPS exert overlapping but nonidentical effects on gene expression in endothelial cells and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289(2):H873–H885. doi: 10.1152/ajpheart.00993.2004. [DOI] [PubMed] [Google Scholar]
- 45.Hughes GR, Harris NN, Gharavi AE. The anticardiolipin syndrome. J Rheumatol. 1986;13(3):486–489. [PubMed] [Google Scholar]
- 46.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RHWM, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 47.Lambrianides A, Carroll CJ, Pierangeli SS, Pericleous C, Branch W, Rice J, Latchman DS, Townsend P, Isenberg Da, Rahman A, Giles IP. Effects of polyclonal IgG derived from patients with different clinical types of the antiphospholipid syndrome on monocyte signaling pathways. J Immunol. 2010;184(12):6622–6628. doi: 10.4049/jimmunol.0902765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15(6):274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 49.Schabbauer G, Schweighofer B, Mechtcheriakova D, Lucerna M, Binder BR, Hofer E. Nuclear factor of activated T cells and early growth response-1 cooperate to mediate tissue factor gene induction by vascular endothelial growth factor in endothelial cells. Thromb Haemost. 2007;97(6):988–997. doi: 10.1160/th07-01-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drake Ta, Hannani K, Fei HH, Lavi S, Berliner Ja. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol. 1991;138(3):601–607. [PMC free article] [PubMed] [Google Scholar]
- 51.Lin MC, Almus-Jacobs F, Chen HH, Parry GC, Mackman N, Shyy JY, Chien S. Shear stress induction of the tissue factor gene. J Clin Invest. 1997;99(4):737–744. doi: 10.1172/JCI119219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogel CFA, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007;363(3):722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu D, Li W, Lok P, Matsumura F, Vogel CFA. AhR deficiency impairs expression of LPS-induced inflammatory genes in mice. Biochem Biophys Res Commun. 2011;410(2):358–363. doi: 10.1016/j.bbrc.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89(6):695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricciotti E, FitzGerald Ga. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishizuka T, Sawada S, Sugama K, Kurita A. Thromboxane A2 (TXA2) receptor blockade suppresses monocyte chemoattractant protein-1 (MCP-1) expression by stimulated vascular endothelial cells. Clin Exp Immunol. 2000;120(1):71–78. doi: 10.1046/j.1365-2249.2000.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katagiri H, Ito Y, Ito S, Murata T, Yukihiko S, Narumiya S, Watanabe M, Majima M. TNF-alpha induces thromboxane receptor signaling-dependent microcirculatory dysfunction in mouse liver. Shock. 2008;30(4):463–467. doi: 10.1097/SHK.0b013e3181673f54. [DOI] [PubMed] [Google Scholar]
- 58.Ishizuka T, Suzuki K, Kawakami M, Kawaguchi Y, Hidaka T, Matsuki Y, Nakamura H. DP-1904, a specific inhibitor of thromboxane A2 synthesizing enzyme, suppresses ICAM-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1994;262(1 – 2):113–123. doi: 10.1016/0014-2999(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 59.Ishizuka T, Suzuki K, Kawakami M, Hidaka T, Matsuki Y, Nakamura H. Thromboxane A2 receptor blockade suppresses intercellular adhesion molecule-1 expression by stimulated vascular endothelial cells. Eur J Pharmacol. 1996;312(3):367–377. doi: 10.1016/0014-2999(96)00478-5. [DOI] [PubMed] [Google Scholar]
- 60.Ishizuka T, Kawakami M, Hidaka T, Matsuki Y, Takamizawa M, Suzuki K, Kurita A, Nakamura H. Stimulation with thromboxane A2 (TXA2) receptor agonist enhances ICAM-1, VCAM-1 or ELAM-1 expression by human vascular endothelial cells. Clin Exp Immunol. 1998;112(3):464–470. doi: 10.1046/j.1365-2249.1998.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eligini S, Violi F, Banfi C, Barbieri SS, Brambilla M, Saliola M, Tremoli E, Colli S. Indobufen inhibits tissue factor in human monocytes through a thromboxane-mediated mechanism. Cardiovasc Res. 2006;69(1):218–226. doi: 10.1016/j.cardiores.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Gaussem P, Reny J-L, Thalamas C, Chatelain N, Kroumova M, Jude B, Boneu B, Fiessinger J-N. The specific thromboxane receptor antagonist S18886: pharmacokinetic and pharmacodynamic studies. J Thromb Haemost. 2005;3(7):1437–1445. doi: 10.1111/j.1538-7836.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 63.Eilertsen K-E, Østerud B. The central role of thromboxane and platelet activating factor receptors in ex vivo regulation of endotoxin-induced monocyte tissue factor activity in human whole blood. J Endotoxin Res. 2002;8(4):285–293. doi: 10.1179/096805102125000498. [DOI] [PubMed] [Google Scholar]
- 64.Bousser M-G, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, Hennerici MG, Mattle HP, Rothwell PM, de Cordoüe A, Fratacci M-D. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet. 2011;377(9782):2013–2022. doi: 10.1016/S0140-6736(11)60600-4. [DOI] [PubMed] [Google Scholar]
- 65.Chu aJ, Wang ZG, Walton Ma, Seto a. Involvement of MAPK activation in bacterial endotoxin-inducible tissue factor upregulation in human monocytic THP-1 cells. J Surg Res. 2001;101(1):85–90. doi: 10.1006/jsre.2001.6271. [DOI] [PubMed] [Google Scholar]
- 66.Herbert JM, Corseaux D, Lale A, Bernat A. Hypoxia primes endotoxin-induced tissue factor expression in human monocytes and endothelial cells by a PAF-dependent mechanism. J Cell Physiol. 1996;169(2):290–299. doi: 10.1002/(SICI)1097-4652(199611)169:2<290::AID-JCP8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]