Abstract
Polybrominated diphenyl ether (PBDE) flame retardants (FRs) have been ubiquitously detected at high concentrations in indoor environments; however, with their recent phase-out, more attention is being focused on measurements of exposure to alternative FRs such as organophosphate FRs (OPFRs). In our previous research, we found that PBDE residues measured on children’s handwipes were a strong predictor of serum PBDE levels. Here we build upon this research to examine longitudinal changes in PBDEs in indoor dust and children’s handwipes, and explore the associations between handwipes and dust for alternative FRs. Children from our previous study were re-contacted after approximately two years and new samples of indoor dust and handwipes were collected. PBDE dust-levels were significantly correlated between two different sampling rounds separated by two years; however, PBDE levels in handwipes were not correlated, perhaps suggesting that the sources of PBDEs remained relatively constant in the home, but that behavioral differences in children are changing with age and influencing handwipe levels. OPFRs [i.e. tris (1,3-dichloroisopropyl) phosphate (TDCPP), tris(2-chloroethyl) phosphate (TCEP), tris(2-chloroisopropyl) phosphate (TCIPP)], 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB, also known as TBB), di(2-ethylhexyl) tetrabromophthalate (BEH-TEBP, also known as TBPH), and 1,2,5,6,9,10-hexabromocyclododecane (HBCD) were also ubiquitously detected in house dust samples and geometric mean levels were similar to PBDE levels, or higher in the case of the OPFRs. Significant associations between handwipes and house dust were observed for these alternative FRs, particularly for EH-TBB (rs= 0.54; p<0.001). Increasing house dust levels and age were associated with higher levels of FRs in handwipes, and high hand washing frequency (>5 times/day) was associated with lower FR levels in handwipes. Overall these data suggest that exposure to these alternative FRs will be similar to PBDE exposure, and the influence of hand-to-mouth behavior in children’s exposure needs to be further examined to better estimate exposure potential.
Keywords: Flame Retardants, Dust, Handwipes, Children, PBDEs, OPFRs
1. Introduction
Flame retardants (FR) are a class of chemicals that are used to reduce the flammability of materials in both construction materials in homes and in some consumer products (e.g. furniture, electronics, etc). FRs can be classified as either reactive (i.e. covalently bound chemicals) or additive (i.e. chemicals mixed in with material). Additive FRs applied to consumer products are either halogen based, organophospate based, or halogenated organophosphates (Alaee et al. 2003; de Wit 2002; van der Veen and de Boer 2012). Polybrominated diphenyl ethers (PBDEs) are halogen based additive FRs that were historically marketed as three different commercial mixtures commonly referred to as PentaBDE, OctaBDE and DecaBDE (Hale et al. 2003).
PentaBDE was a commercial flame retardant mixture primarily applied to polyurethane foam used in furniture and baby products, often to meet California’s residential furniture flammability standard known as Technical Bulletin 117 (TB 117). This mixture contained a variety of tri- through hexabrominated diphenyl ethers that have well established persistence and bioaccumulation properties. PBDEs also have a chemical structure that is similar to endogenous thyroid hormones (e.g. thyroxine) and they have been demonstrated to disrupt thyroid hormone homeostasis in animal exposure studies (Fernie et al. 2005; Noyes et al. 2013; Tomy et al. 2004; Zhou et al. 2001). Exposure to PBDEs is also associated with effects on circulating levels of thyroid hormone levels in US adults, and adverse neurodevelopmental outcomes in children (Chevrier et al. 2010; Eskenazi et al. 2013; Herbstman et al. 2010; Hoffman et al. 2012; Stapleton et al. 2011a; Turyk et al. 2008). Due to mounting concerns over the exposure and potential health effects of the PentaBDE and OctaBDE mixtures, they were officially banned from use in the European Union in 2002, voluntarily phased out of use in the US in 2005, and were listed on the Stockholm Convention in 2009. DecaBDE is now scheduled to be phased-out by the end of 2013 (EPA 2009).
In our previous research we found that PentaBDE was often used in residential furniture and baby products to meet TB 117 prior to its phase-out (Stapleton et al. 2011b; Stapleton et al. 2012b). However, since the phase-out of PentaBDE, a wider variety of flame retardant chemicals have been used to meet flammability standards, particularly in residential furniture. The two most common flame retardants identified in polyurethane foam purchased post 2005 were tris (1,3-dichloroisopropyl) phosphate (TDCPP), and a mixture known as Firemaster 550 (FM 550). TDCPP is considered a probable human carcinogen (Babich 2006) and little is known about the health effects of FM 550. In a recent rodent study, we found that perinatal exposure to FM 550 resulted in obesity and early puberty in developing pups, suggesting that FM 550 is an endocrine disruptor (Patisaul et al. 2012). Given these facts, more information is needed on children’s exposure to these new flame retardant chemicals.
Serum PBDE levels in the US population have been shown to be significantly associated with exposure to house dust (Johnson et al. 2010). In a recent study we found that PBDEs in handwipe samples were also predictive of serum levels, and was a better predictor than house dust, likely because it accounts for both indoor dust and behavioral aspects affecting the magnitude of exposure (Stapleton et al. 2012a). For example, residues of PentaBDEs measured on toddlers’ hands explained 32% of the serum variability, and were better predictors than levels measured in indoor dust alone (Stapleton et al. 2012a). PentaBDEs measured in handwipes were also predictors of serum PentaPBDE levels in a cohort of office workers (Watkins et al. 2011). These two studies suggest that handwipes may provide a useful measure of exposure to a wide range of flame retardant chemicals; however, no studies have examined these relationships in non-PBDE FRs to date. This is important considering the diversity of flame retardant chemicals that are now found in indoor environments and the lack of data on exposure levels and potential health effects.
In the present study, we have built upon our previous research and investigated toddlers’ exposure to a larger suite of FR additives. To accomplish this, we re-contacted our toddler cohort from North Carolina and went back to the homes to collect a second set of handwipe and dust samples. Our primary objectives in this study were to: 1) examine the correlations between flame retardant residues on children’s hands and levels measured in house dust; 2) examine longitudinal associations in PBDE levels measured in house dust and handwipes collected from the same set of children approximately two years apart, and 3) determine if age and hand washing frequency were predictors of FR levels in handwipes.
2. Materials and Methods
2.1 Sample Collection
Duke University’s Institutional Review Board authorized all aspects of this study, and all parents/guardians gave informed consent prior to sample collection. Parents and guardians of children enrolled in our previous study (Stapleton et al. 2012a) were re-contacted and asked to participate in a follow-up study. Of the 83 families that participated in the original study, 30 lived in the same home, responded to our requests for a follow-up visit, and agreed to be involved in this new study. A research team traveled to the homes of all participants interested in this study to collect a house dust sample, and a handwipe sample from the same child that participated in our previous study. Handwipe samples were also collected from any siblings in the home that were between 2–5 years of age (these siblings had not been sampled previously). Samples were collected over several weeks during the spring of 2012. Handwipe and dust collections were identical to the methods used in our previous study (Stapleton et al. 2012a).
A short questionnaire was also administered during the home visit, which collected information on the time the child last washed their hands, how often they washed their hands, and where the child spends most of his/her time. Once completed, the child’s height and weight were also recorded. Floor dust samples were taken in the room identified by the parent as where the child spends most of his/her time. The dust was collected on both hardwood and carpeted floor using a vacuum cleaner with a cellulous thimble inserted in the hose attachment (as described in Stapleton et. al 2012a). Dust was collected until a sufficient amount (at least 100 mg) had accumulated, as determined by the team member. Dust samples were wrapped in aluminum foil and placed in small individual plastic bags. Handwipe samples from both hands were placed into small pre-cleaned glass vials, wrapped in aluminum foil within a plastic bag, and transported back to the laboratory where they were stored at −20°C until analysis. Dust samples were stored at room temperature and were sieved to <500 microns prior to analysis.
2.2 Sample Processing
Handwipe and dust samples were extracted in the laboratory and analyzed for a suite of brominated and organophosphate FRs including BDE-28, -47, -66, -85/155, -99, -100, -153, 154, 183, 209, 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB, also known as TBB), bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (BEH-TEBP, also known as TBPH)), tetrabromobisphenol A (TBBPA), 1,2,5,6,9,10-hexabromocyclododecane (HBCD; α-HBCD, β-HBCD, γ-HBCD), tris(2-chloroethyl) phosphate (TCEP), tris(2-chloroisopropyl) phosphate (TCIPP), and TDCPP. Each handwipe sample was extracted using a Soxhlet apparatus. Prior to Soxhlet extraction, each handwipe sample was spiked with three internal standards, d15-TDCPP (155 ng), a monofluorinated tetrabrominated diphenyl ether (F-BDE-69; 50 ng) and 13C-BDE-209 (100 ng). To serve as laboratory blanks, three new sterile gauze pads were taken through the same procedure and run next to the handwipe samples. After Soxhlet extraction, each extract was concentrated using an automated nitrogen evaporation system (Turbo Vap II, Zymark Inc.) and transferred to a 4.0 mL amber vial, stored in a −20 degrees Celsius freezer. Extracts were then cleaned using Florisil solid-phase extraction (Supelclean ENVI-Florisil, 6mL, 500mg bed weight, Supelco), eluting the F1 fraction with 10mL hexane (PBDEs) and the F2 fraction with 10mL ethyl acetate (OPFRs), based on the method developed by Van den Eede et al (Van den Eede et al. 2012). Each fraction was then concentrated to approximately 1ml using a nitrogen concentration system and transferred to an autosampler vial (ASV) for gas chromatography–mass spectrometry (GC/MS) analysis. Dust samples (~100 mg) were extracted with 10 mL of 50:50 dichloromethane (DCM):hexane using sonication. This process was repeated three times and the combined extract (~30 mL) was concentrated using an automated nitrogen evaporation system (Turbo Vap II, Zymark Inc.) and transferred to a 4.0 mL amber vial, stored in a −20 degrees Celsius freezer. The dust extracts were cleaned using the same method as described for the handwipe samples above. To measure recovery of the brominated internal standards, the extracts were spiked with 2, 2', 3, 4, 5, 5'-hexachloro[13C12]diphenyl ether (13C-CDE 141), while d9-TCEP was spiked into each sample to measure recovery of d15-TDCPP. Recoveries of F-BDE-69, and d15-TDCPP averaged 84 ±19%, and 76 ±21%, respectively, in all samples. Recovery of 13C-BDE-209 averaged 116 ± 25% in the house dust samples and averaged 17% in the handwipes, with 26 of the 45 samples having a recovery of zero. The low recovery is likely attributable to low organic matter content in the handwipe extracts combined with loss to the glassware during extraction. 13C-BDE-209 is used to assess recovery of BDE-209 alone, and all values were adjusted for internal standard recovery. Analysis of laboratory blanks (n=4) and an indoor dust Standard Reference Materials (SRM 2585, NIST, Gaithersburg, MD) were also employed for quality assurance and quality control. FR measurements in handwipes were blank subtracted using the average mass of FR measured in the field blanks. Method detection limits were calculated using three times the standard deviation of the appropriate blank (i.e. dust or handwipe). Measured PBDE levels in SRM 2585 ranged from 73 to 117 % of certified values. Measurements of TCEP, TCIPP and TDCPP in SRM 2585 were 839 ± 69, 791 ± 55, 2180 ± 62 ng/g, respectively. These values are very similar to reports published by Van den Eede et al. (van den Eede et al. 2011), and Bergh et al. (Bergh et al. 2012).
2.3 Statistical Analyses
Descriptive statistics were calculated for FRs measured on handwipes and in house dust samples. These data indicated that the distributions of FR levels were considerably skewed. Thus log10-transformed values were used in statistical analyses. A value of MDL/2 was used for all values <MDL. Using a paired t-test, we investigated differences in the geometric mean PBDE levels in samples collected at both time points. Spearman correlation coefficients were calculated to determine associations between continuous measures of FRs in handwipes and dust samples. Similarly, Spearman correlations were used to assess temporal relationships in the levels of FRs in house dust and handwipes from the same children taken approximately two years apart. Generalized estimating equations (GEEs) were used to examine relationships between continuous measures of FRs on handwipes and children’s age and hand washing practices. GEEs are an extension of linear regression models that account for potential residual within-family correlations that may arise from including multiple children from the same family in analyses. To further investigate the associations between dust and handwipes while minimize the effect of skewed data and outliers, dust concentrations were dichotomized (above and below the median) and entered into GEEs as predictors of handwipe FR levels. Beta coefficients from GEEs were exponentiated (10β), producing an estimate of the multiplicative change in handwipe FR levels associated with a unit change in each explanatory factor. To assess the degree of similarity in handwipe levels collected from children within the same family, intraclass correlation coefficients (ICCs) were calculated (Hamer 1995; Shrout and Fleiss 1979). ICC values range from 0, indicating no consistence, to 1, indicating perfect agreement in handwipes from children in the same family. All statistical analyses were performed in SAS (version 9.2; SAS Institute Inc, Cary, NC), with statistical significance defined as α=0.05.
3. Results
A total of 43 children from 30 families participated in the follow-up study (siblings of the original cohort were only sampled during the follow-up study). Children ranged in age from 26.9 to 68.0 months of age (mean=45.9 months) at the time of the home visit. As in the original cohort, children were evenly split by gender (female n=23 (53.5%); male n=20 (46.5%)).
3.1 FR Levels in House Dust and Handwipes
Summary statistics for FRs measured in handwipes and house dust are presented in Table 1. FRs were detected in all house dust samples. TCIPP, TDCPP, BDE-209, ΣPentaBDE, and BEH-TEBP were detected in the highest concentrations with geometric mean levels of 3440, 2730, 1720, 1400, and 604 ng/g, respectively. TCEP, HBCD, TBB, and TBBPA were also detected frequently, but at lower levels overall. FRs were also quite frequently detected in the children’s handwipes. Again, TDCPP, TCIPP, and ΣPentaBDE were the most abundant FRs measured in handwipes with geometric mean levels of 74.2, 31.3, and 49.0 ng, respectively.
Table 1.
Descriptive statistics for all flame retardants measured in handwipe and house dust samples.
| Handwipes (ng), n=43 | House Dust (ng/g), n=30 | |||||
|---|---|---|---|---|---|---|
| Congener | % Detect | GM | Range | % Detect | GM | Range |
| BDE 28 | 100 | 1.05 | 0.45 – 4.8 | 97 | 0.77 | <0.07– 30.9 |
| BDE 47 | 89 | 19.2 | <3.0 – 249 | 100 | 452 | 55.0 – 24,720 |
| BDE 66 | 96 | 0.54 | <0.17 – 4.7 | 97 | 1.6 | <0.07 – 26.9 |
| BDE 85/155 | 96 | 0.60 | <0.05 – 5.0 | 95 | 26.7 | <0.70 – 1860 |
| BDE 99 | 96 | 18.8 | <1.1 – 330 | 100 | 741 | 8.0 – 36,210 |
| BDE 100 | 91 | 3.0 | <0.40 – 31.1 | 100 | 98.6 | 9.0 – 10,230 |
| BDE 153 | 98 | 0.80 | <0.05 – 7.3 | 100 | 40.6 | 7.0 – 3407 |
| BDE 154 | 98 | 0.90 | <0.05 – 7.0 | 100 | 56.8 | 5.0 – 3061 |
| BDE 183 | 7 | NA | <0.05 – 0.46 | 97 | 1.0 | <0.06 – 4.5 |
| BDE 209a | 24a | NA | <5 | 100 | 1720 | 441 – 76,130 |
| ΣPentaBDEb | 49.0 | <5.3 – 397 | 1400 | 152 – 74,560 | ||
| EH-TBB | 93 | 4.1 | <0.60 – 154 | 100 | 97.0 | 6.0 – 2,430 |
| BEH-TEBP | 53 | 2.5 | <0.70 – 116 | 100 | 604 | 82.9 – 20,960 |
| TBBPAc | 70 | 0.40 | <0.02 – 35.0 | 76 | 7.9 | <0.20 – 245 |
| α-HBCDc | 53 | 0.35 | <0.10 – 5.2 | 100 | 214 | 44.4 – 2210 |
| β-HBCDc | 47 | 0.11 | <0.05 – 5.1 | 100 | 27.8 | 4.4 – 274 |
| γ_HBCDc | 40 | 0.32 | <0.15 – 5.6 | 100 | 70.0 | 11.4 – 823 |
| ΣHBCDd | 0.97 | <0.05 – 10.8 | 338 | 77.6 – 2658 | ||
| TCEP | 47 | NA | <24 – 197 | 100 | 348 | 20.0 – 6,920 |
| TCIPP | 69 | 31.3 | <13 – 532 | 100 | 3440 | 217 – 67,810 |
| TDCPP | 96 | 74.2 | <7.0 – 530 | 100 | 2730 | 621 – 13,110 |
N/A- indicates not available
- Detection compromised by low recovery of 13C BDE 209 during extraction.
- ΣPentaBDE represents the sum total of BDE congeners 28,47, 66, 99, 100, 85/155, 154 and 153.
- Only 30 handwipe samples were analyzed for TBBPA and HBCD.
- Sum of alpha, beta and gamma HBCD isomers.
3.2 Longitudinal Trends in House Dust and Handwipe PBDEs
Homes sampled in this study were the same homes sampled in an earlier study examining children’s exposure to PBDEs. Here, we examined the correlation in PBDEs in house dust and handwipes collected during the two different sampling campaigns. The time frame between collection of the two sets of samples ranged from 18.9 to 34.7 months and was on average 24.1 months apart. Table 2 presents the Spearman correlation coefficients for individual PBDE congeners in handwipes and house dust samples. PBDEs in dust were significantly correlated between the two sample collection points; however, levels in handwipes collected from the same children approximately 24 months apart were not significantly associated. We also examined the data to determine if there were any differences in the geometric mean of PBDEs in dust and handwipes between the two sampling points, but no significant differences were observed (data not shown).
Table 2.
Spearman correlation coefficients for PBDEs measured in house dust (n=30) and handwipes collected (n=31*) at two different time points (approximately 2 years apart).
| House Dust | Handwipes | |||
|---|---|---|---|---|
| Congener | rs | p-value | rs | p-value |
| BDE 47 | 0.61 | 0.004 | 0.10 | 0.57 |
| BDE 99 | 0.47 | 0.01 | 0.09 | 0.61 |
| BDE 100 | 0.66 | 0.0001 | 0.15 | 0.42 |
| BDE 153 | 0.35 | 0.06 | 0.08 | 0.67 |
| BDE 209 | 0.43 | 0.02 | N/A | N/A |
Handwipes were collected from two children from the same family at both time points.
3.3 FR Correlations Within Dust and Handwipes
Correlation analyses were conducted and are presented in Tables S1 (within dust) and S2 (within handwipes). Generally there was a high degree of correlation among the PBDEs congeners measured in dust, with correlation coefficients ranging from 0.33 to 0.96 (range excludes BDE-209). However, BDE-209 was not significantly associated with the other PBDE congeners measured. It’s also interesting to note that EH-TBB and BEH-TEBP were also commonly correlated with many of the PBDE congeners. TCEP was correlated to TCIPP, and TCIPP was correlated to TDCPP. BDE-209 was significantly associated with levels of TCEP in dust (rs = 0.64, p<0.001).
Correlation analyses were conducted on handwipe data in which detection frequency was >70%. Within the handwipe samples several FRs were significantly correlated. All PBDEs measured in handwipes were significantly correlated with one another, with correlation coefficients ranging from 0.55 to 0.95. EH-TBB was also significantly associated with BDE-47, BDE-66, BDE-99, and TDCPP. TDCPP was not associated with any of the PBDE congeners measured in handwipes.
3.4 FR Correlations Between Dust and Handwipes
Correlation analyses were also conducted between FRs in handwipes and FRs measured in house dust. As seen in Table 3, continuous measures of EH-TBB and most PBDEs in handwipes and dust were significantly and positively correlated, with correlation coefficients ranging from 0.30 to 0.54 (range excludes BDE-28, -66, and -153, for which correlations were not statistically significant). The strongest correlation between handwipes and dust was observed for EH-TBB (rs=0.54, p<0.001), as seen in Figure S1.
Table 3.
Correlation matrix for flame retardants levels measured in paired handwipes and indoor dust. Correlation analyses were conducted on handwipe data in which detection frequency was >70%.
| Congener | Dust | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE 28 | BDE 47 | BDE 66 | BDE 85/155 |
BDE 99 | BDE 100 | BDE 153 | BDE 154 | ΣPenta BDE |
EH-TBB | TDCPP | ||
| Handwipes | BDE 28 | 0.15 | 0.06 | −0.02 | 0.06 | −0.07 | 0.05 | −0.04 | −0.07 | 0.03 | −0.22 | −0.01 |
| BDE 47 | 0.44# | 0.33* | 0.32* | 0.30* | 0.16 | 0.31* | 0.11 | 0.22 | 0.29 | −0.08 | 0.03 | |
| BDE 66 | 0.42# | 0.25 | 0.24 | 0.22 | 0.12 | 0.23 | 0.06 | 0.13 | 0.23 | −0.05 | 0.02 | |
| BDE 85/155 | 0.43# | 0.39# | 0.44# | 0.39* | 0.20 | 0.40** | 0.22 | 0.33 | 0.35* | −0.12 | 0.14 | |
| BDE 99 | 0.50† | 0.34* | 0.41# | 0.26 | 0.30* | 0.30* | 0.10 | 0.25 | 0.35* | 0.15 | 0.00 | |
| BDE 100 | 0.42# | 0.35* | 0.34* | 0.34* | 0.17 | 0.35* | 0.18 | 0.26 | 0.31* | −0.12 | 0.10 | |
| BDE 153 | 0.31* | 0.36* | 0.40# | 0.40# | 0.19 | 0.39# | 0.28 | 0.33* | 0.32* | −0.19 | 0.06 | |
| BDE 154 | 0.40# | 0.39# | 0.42# | 0.38* | 0.22 | 0.39# | 0.24 | 0.33* | 0.35* | −0.08 | 0.15 | |
| ΣPentaBDEa | 0.48# | 0.32* | 0.35* | 0.27 | 0.22 | 0.29 | 0.09 | 0.23 | 0.31* | 0.03 | 0.00 | |
| EH-TBB | 0.39* | 0.26 | 0.37* | 0.18 | 0.40# | 0.20 | −0.09 | 0.25 | 0.33* | 0.54† | 0.19 | |
| TDCPP | −0.05 | −0.11 | −0.03 | −0.13 | −0.04 | −0.16 | −0.12 | −0.11 | −0.06 | 0.12 | 0.24 | |
ΣPentaBDE represents the sum total of BDE congeners 28,47, 66, 99, 100, 85/155, 154 and 153
<0.05;
<0.01;
<0.001
3.5 Predictors of FR Levels in Handwipes
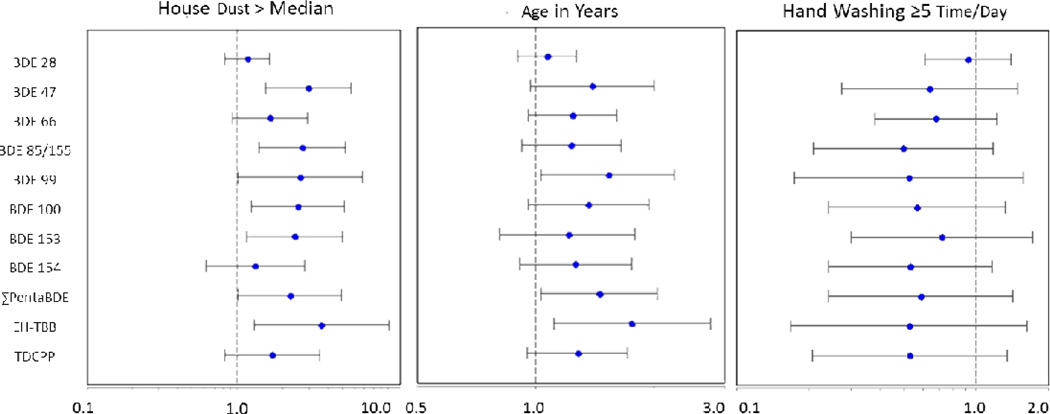
Using GEEs we investigated bivariate associations between handwipe FR levels and dichotomized measures of FRs in house dust (above vs. below the median), hand washing frequency (< vs. ≥5times/day), and with a continuous measure of age (in months). As observed in Figure 1, higher levels of FRs in house dust levels were consistently associated with higher handwipe levels. For example, children living in homes with higher levels of EH-TBB in dust had 3.62 times the mass of EH-TBB on their hands (95% CI: 1.30, 10.10). Age was a significant predictor of FR levels for ΣPentaBDE and EH-TBB, with an estimated increase of 3% and 5% for each monthly increase in age (between 26.9–68.0 months). Although associations were imprecisely estimated, hand-washing frequency was consistently associated with the levels of FRs measured on participants’ hands. On average, children who washed their hands at least 5 times per day had 30 to 50% lower levels of FRs on their hands.
Figure 1.
Predictors of FR levels in children’s handwipes. Values represent the multiplicative increase or decrease in FR levels relative to either a reference group (house dust, hand washing frequency) or with a continuous increase in age. Analyses were conducted on handwipe data in which detection frequency was >70%.
3.6 FR Associations Within and Among Siblings
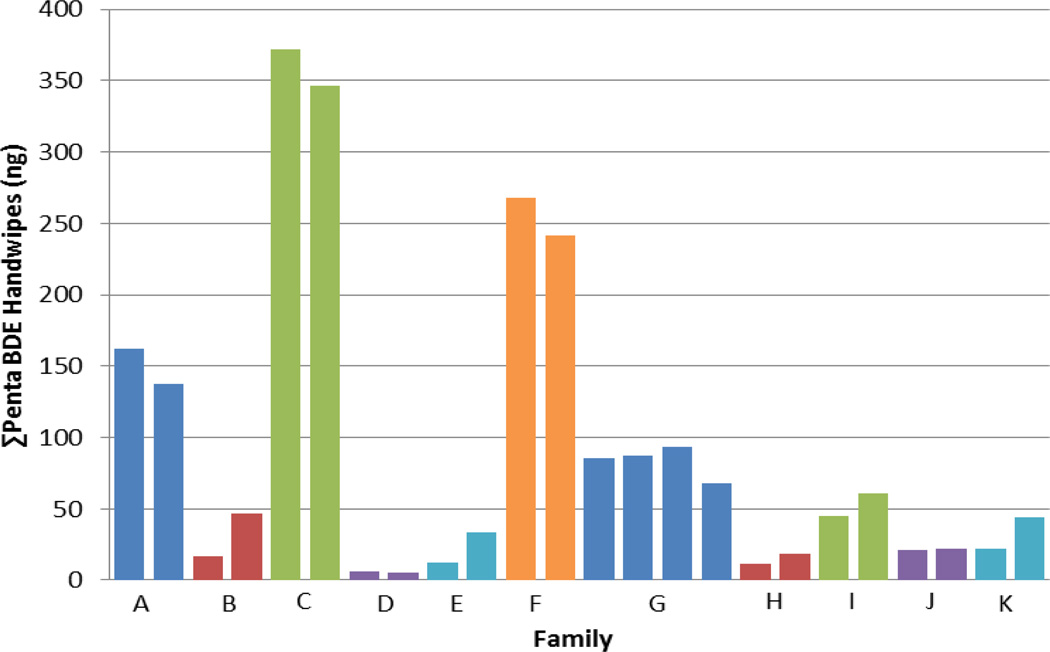
As part of this follow-up study, we collected handwipes from some of the children in our original cohort, but also collected handwipes from any siblings living in the home that were between the ages of 2–5 years. Intraclass correlation coefficients were calculated for FRs measured in handwipes to determine how variable levels are within sibling sets relative to variability in handwipes from children living in different homes. As seen in Figure 2, levels of ΣPentaBDE within sibling sets were very similar. ICCs for the PBDE congeners ranged from 0.49 (95% CI: 0.31, 0.63) for BDE-28 to 0.86 (95% CI: 0.78, 0.91) for BDE-99. ICCs for EH-TBB and TDCPP were 0.75 (95% CI: 0.62, 0.84) and 0.49 (95% CI: 0.31, 0.63), respectively (Table S3).
Figure 2.
ΣPentaBDE in siblings handwipes.
4. Discussion
In this study we were able to examine longitudinal changes in PBDE levels in house dust and handwipes collected from the same homes and the same children at an average interval of two years. Despite this relatively long time period between sampling, the PBDE concentrations in dust remained surprisingly similar between the two time points. The correlation of FR levels between sampling rounds was not only apparent for PBDEs associated with the PentaBDE mixture, but also with BDE-209, which is associated with the DecaBDE mixture. This suggests that PBDE levels were fairly constant in the household over this time period as we observed in an earlier study examining PBDE trends over a 6–8 month period (Allen et al. 2008). A study investigating PBDEs in house dust collected 3–8 years apart also found similar correlations over time (Whitehead et al. 2013). We also examined the correlation in PBDEs measured in children’s handwipes collected from the same children, but again approximately two years apart. In contrast to the dust samples, there were no significant correlations in the handwipe samples over time. This suggests that the sources in the home remain relatively constant over this time frame, but the behavior of the children likely changes as they age, and thus may be contributing to differences in their exposure pathways. Further, we observed increasing handwipe FR levels with age, again suggesting that behavior may play a role in children’s exposure to FRs; however, data are not available to investigate behavioral changes in this cohort. Increases in hand surface area with age may also explain the association we observed between handwipe levels and age. Further data are needed to investigate the factors driving age and FR relationships
As part of this follow-up study we also measured the concentrations of several non- PBDE flame retardants in both the dust and handwipes. Dust concentrations of the organophosphate flame retardants (TCEP, TCIPP and TDCPP) were equivalent to, or higher than, levels of PBDEs in most cases. These levels are very similar to house dust levels reported recently from a study in California (Dodson et al. 2012). Levels of EH-TBB and BEH-TEBP, components of FM 550, were found at levels comparable to PBDEs in house dust but slightly lower than PBDEs in handwipes. It’s also interesting to note that the ratio of EH-TBB:BEH-TEBP was very different in the dust (~0.16) than in the handwipes (~1.6). The ratio observed in the handwipes is more similar to the ratio observed in FM550 (~3), and may suggest that EH-TBB and BEH-TEBP on children’s hands comes from contact from products containing FM550. It’s also possible that differences in physic-chemical properties between the two could affect partitioning to the skin surface. Other sources in the home may also be contributing to levels of BEH-TEBP in the dust. For example, BEH-TEBP is the primary ingredient in a flame retardant mixture known as DP-45, which is applied to polyvinyl chloride plastics and neoprene rubber, and may have some applications also as a plasticizer (Andersson et al. 2006).
Even though the PBDE levels in handwipes measured from the same children over a two-year period were not correlated, on average the levels of PBDEs measured in the handwipes were similar in both sampling rounds. This would suggest that exposure levels during the approximate two year time frame have remained relatively constant. Generally, the flame retardants in highest abundance in the dust were detected frequently in the children’s handwipes samples, suggesting that dust may be the source to the hands. Similar to dust the FRs detected in highest abundance in the handwipes were PBDEs, TDCPP, and TCIPP. But in contrast to dust, EH-TBB was detected at higher levels than BEH-TEBP in handwipes. Although α-HBCD was ubiquitously detected and relatively abundant in house dust samples (geomean = 214 ng/g), it was not frequently detected in handwipes.
Our group previously observed significant associations between PBDE levels measured on handwipes with both dust and serum PBDE levels (Stapleton et al. 2012a; Watkins et al. 2011). Particularly in our previous toddler cohort, handwipe levels were the strongest predictor of serum PBDE levels, suggesting that hand –to-mouth contact may be a significant exposure pathway. Here we built upon this finding to investigate associations between handwipes and dust for other FRs. As seen in Table 3, many of the FRs measured in both handwipes and dust were significantly and positively associated. The correlation coefficients for PBDEs were very similar to our observations in the previous study (Stapleton et al. 2012a). The relationship was strongest for EH-TBB and weakest for TDCPP. These relative differences may reflect differences in the physicochemical properties of the FRs. For example, TDCPP is a smaller compound and has a higher vapor pressure than the brominated FRs. Recent research by Weschler and Nazaroff (Weschler and Nazaroff 2012) speculates that SVOCs present in indoor air may be sorbing to our skin, and that long contact times in indoor absorptions can lead to significant dermal absorption over time, despite predicted slow dermal uptake rates. Therefore, the weaker association for TDCPP between handwipes and dust may reflect a greater source of TDCPP in handwipes from the indoor air, as opposed to house dust. Alternatively, it’s possible that TDCPP with its higher vapor pressure may evaporate from the skin surface due to higher body temperatures relative to room temperatures, thus leading to a weaker association with dust. Further studies are needed to explore these potential hypotheses.
The moderate to high intraclass correlation coefficients (ICCs – Table S3 calculated for FRs on handwipes) suggest that children living in the same home have the potential to receive the same levels of exposure; however, their overall exposure may be influenced more by their specific hand-to-mouth behavior, which will be age dependent. This is not necessarily surprising since house dust is predicted to be a strong source of exposure. As seen in Figure 2, there was very little variability in handwipe PBDE levels among siblings living in the same home, relative to the variability among households. Some of these differences among households may also be driven by differences in hand washing behavior/habits among different families as our data also suggested that frequent hand washing is associated with lower FR levels on the hands (Figure 1).
5. Conclusion
This study represents the first examination of US children’s exposure to a suite of FR chemicals in indoor environments. While exposure to some of these FRs is expected to decrease over time (e.g. PBDEs), exposure may be increasing for some of these newer formulations (e.g. FM 550). In particular, TDCPP and FM550 components were detected ubiquitously in these samples and exposure is equivalent to (FM550) or higher than (TDCPP) PBDE exposure. Given the reported toxicity for TDCPP (Dishaw et al. 2011; Gold et al. 1978) and FM550 (Patisaul et al. 2012; Springer et al. 2012) in particular, more studies are warranted to determine if these levels of exposure are leading to long term health effects in children.
Supplementary Material
Highlights.
A suite of flame retardants were measured in paired samples of handwipes and dust
FR levels in handwipes were significantly correlated with house dust levels
PBDE levels in house dust was significantly correlated over a 2 year time frame
Children’s age, handwashing behavior and dust levels predicted handwipe levels
Siblings living in the same home had very similar FR exposure levels
Acknowledgements
Funding for this study was provided by a research grant from the National Institute of Environmental Health Sciences (NIEHS; grant R01 ES016099). Dr. Webster is supported in part by R01 ES015829. Additional thanks are extended to all the study participants.
Abbreviations
- BEH-TEBP
di(2-ethylhexyl) tetrabromophthalate
- EH-TBB
2-ethylhexyl-2-3,4,5-tetrabromobenzoate
- FR
flame retardant
- GEE
generalized estimating equations
- HBCD
1,2,5,6,9,10-Hexabromocyclododecane
- ICC
intraclass correlation coefficient
- OPFR
organophosphate flame retardant
- PBDE
polybrominated diphenyl ether
- TBBPA
tetrabromobisphenol A
- TCEP
tris(chloroethyl) phosphate
- TCIPP
tris(2-chloroisopropyl) phosphate
- TDCPP
tris (1,3-dichloroisopropyl) phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaee M, Arias P, Sjodin A, Bergman A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environment International. 2003;29:683–689. doi: 10.1016/S0160-4120(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to pbdes via house dust. Environment International. 2008;34:1085–1091. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Andersson PL, Oberg K, Orn U. Chemical characterization of brominated flame retardants and identification of structurally representative compounds. Environmental Toxicology and Chemistry. 2006;25:1275–1282. doi: 10.1897/05-342r.1. [DOI] [PubMed] [Google Scholar]
- Babich MA. Preliminary risk assessment of flame retardant (fr) chemicals in upholstered furniture foam. 2006 [Google Scholar]
- Bergh C, Luongo G, Wise S, Oestman C. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Analytical and Bioanalytical Chemistry. 2012;402:51–59. doi: 10.1007/s00216-011-5440-2. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjoedin A, Eskenazi B. Polybrominated diphenyl ether (pbde) flame retardants and thyroid hormone during pregnancy. Environmental Health Perspectives. 2010;118:1444–1449. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, et al. Is the pentabde replacement, tris (1,3-dichloropropyl) phosphate (tdcpp), a developmental neurotoxicant? Studies in pc12 cells. Toxicology and Applied Pharmacology. 2011;256:281–289. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. After the pbde phase-out: A broad suite of flame retardants in repeat house dust samples from california. Environmental Science & Technology. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Decabde phase-out initiative. [accessed September 23, 2013];2009 Available: http://www.epa.gov/opptintr/existingchemicals/pubs/actionplans/deccadbe.html.
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. In utero and childhood polybrominated diphenyl ether (pbde) exposures and neurodevelopment in the chamacos study. Environmental Health Perspectives. 2013;121:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouillard KG, et al. Exposure to polybrominated diphenyl ethers (pbdes): Changes in thyroid, vitamin a, glutathione homeostasis, and oxidative stress in american kestrels (falco sparverius) Toxicological Sciences. 2005;88:375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Gold MD, Blum A, Ames BN. Another flame-retardant, tris-(1,3-dichloro-2-propyl)-phosphate, and its expected metabolites are mutagens. Science. 1978;200:785–787. doi: 10.1126/science.347576. [DOI] [PubMed] [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the north american environment. Environment International. 2003;29:771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hamer R. Intracc.Sas. Macro to calculate reliabilities for intraclass correlations. 1995 Available: http://support.sas.com/documentation/onlinedoc/stat/ex_code/121/intracc.html. [Google Scholar]
- Herbstman JB, Sjoedin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to pbdes and neurodevelopment. Environmental Health Perspectives. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Adgent M, Goldman BD, Sjodin A, Daniels JL. Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers. Environ Health Perspect. 2012;120:1438–1442. doi: 10.1289/ehp.1205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ Sci Technol. 2010;44:5627–5632. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant bde-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environmental science & technology. 2013;47:10012–10021. doi: 10.1021/es402650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul H, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Developmental exposure to the flame retardant mixture firemaster 550 in rats: Accumulation, metabolism and endocrine disrupting effects. Journal of Biochemical and Molecular Toxicology. 2012;27:124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intra-class correlationsuses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Springer C, Dere E, Hall SJ, McDonnell EV, Roberts SC, Butt CM, et al. Rodent thyroid, liver, and fetal testis toxicity of the monoester metabolite of bis-(2-ethylhexyl) tetrabromophthalate (tbph), a novel brominated flame retardant present in indoor dust. Environmental Health Perspectives. 2012;120:1711–1719. doi: 10.1289/ehp.1204932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (pbde) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environmental Health Perspectives. 2011a;119:1454–1459. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environmental Science & Technology. 2011b;45:5323–5331. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjoedin A, Webster TF. Serum pbdes in a north carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environmental Health Perspectives. 2012a;120:1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and high volume use flame retardants in us couches reflective of the 2005 pentabde phase out. Environmental Science & Technology. 2012b;46:13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, et al. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (salvelinus namaycush) Environmental Science & Technology. 2004;38:1496–1504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by pbdes in adult male sport fish consumers. Environmental Health Perspectives. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environment International. 2011;37:454–461. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta. 2012;89:292–300. doi: 10.1016/j.talanta.2011.12.031. [DOI] [PubMed] [Google Scholar]
- van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88:1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjoedin A, et al. Exposure to pbdes in the office environment: Evaluating the relationships between dust, handwipes, and serum. Environmental Health Perspectives. 2011;119:1247–1252. doi: 10.1289/ehp.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW. Svoc exposure indoors: Fresh look at dermal pathways. Indoor Air. 2012;22:356–377. doi: 10.1111/j.1600-0668.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Brown FR, Metayer C, Park J-S, Does M, Petreas MX, et al. Polybrominated diphenyl ethers in residential dust: Sources of variability. Environment International. 2013;57–58:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicological Sciences. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.