Abstract
A member of a family with an autosomal dominant pattern of frontotemporal dementia (FTD) with a TDP-43 pathological substrate in other members and no mutations in FTD-associated genes, developed behavioral variant FTD followed by Progressive Supranuclear Palsy. Autopsy revealed a pure tauopathy of PSP pattern. Conclusions: The findings raise the possibility of shared pathogenic pathways and a proximal genetic abnormality between PSP and FTLD-43.
Search terms: Frontotemporal dementia, Progressive Supranuclear Palsy, Genetics
INTRODUCTION
Frontotemporal dementia (FTD) is a clinical syndrome with three classical presentations: Behavioural variant (bvFTD), Non fluent progressive aphasia (NFPA) and semantic dementia. Frontotemporal lobar degeneration (FTLD), the pathological substrate of FTD, is characterized by focal atrophy in the distribution appropriate to the signs and symptoms and the presence of neuronal cytoplasmic inclusions (NCI). In approximately 40% of cases tau is the protein forming the NCI; in the remainder the NCI, labeled by antibodies to ubiquitin, contain either TDP-43 or FUS. While the association of each pathological form with the clinical presentations is variable, the pathological varieties are considered separate diseases 1. We present a family with an autosomal dominant pattern of inheritance of FTD, and multiple documented cases of ubiquitin positive (FTLD-U)/TDP-43 pathology and coincident tau positive PSP pathology
The propositus described in this paper is a member presenting with bv-FTD evolving to a Progressive Supranuclear Palsy (PSP) syndrome2, in whom autopsy demonstrated a pure tauopathy. This family raises the possibility of upstream associations between tau and TDP-43 pathologies.
CASE REPORT
The propositus, a right handed, previously well Caucasian woman, became ill approximately at the age of 59. Her husband noted she was disorganized and accomplished relatively little around the house. She also began to make impulsive phone calls, calling him at the office several times a day without reason. She was emotionally flat and she was laughing inappropriately about the death of her aunt. She also became impulsive, outspoken and compulsively repetitive in her actions. She lost initiative and became dependent on her husband and her children a couple of years after the onset. Other personality changes included increasing impatience; she would lose her temper when she couldn’t find something. Her conversations became very concrete. She couldn’t get a joke or the point of a conversation. She became inappropriate in public, shouting repetitively.
Her speech pattern also changed. She started to speak less and less, not initiating conversations, even though she used to be the centre of any company in the past. At times, she would have fluent, childish, sing-song speech, saying things like “I got the ball and you did not”. She had difficulty comprehending others and her speech output became nonsensical. She neglected herself and her table manners deteriorated. She would pick up food by hand (partly because she had trouble cutting up meat) and she would sit down before anyone else and started to gobble up the food, eating everything that was in front of her, particularly sweets. She developed compulsive shopping at certain stores and insisted on returning to the same places. She got into the habit of ordering things she did not need through the internet. She also became disinhibited and would undress in front of her children. Although her children recognized the pattern of her illness resembled other affected members of the family, her husband refused to have her evaluated until she developed a movement disorder.
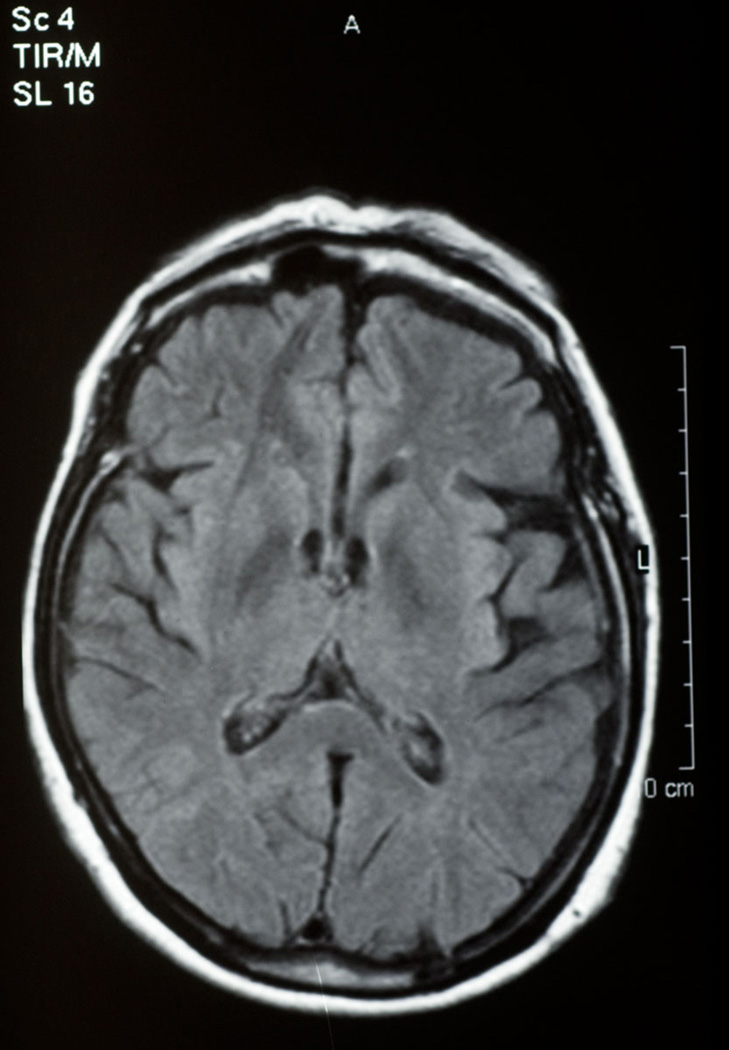
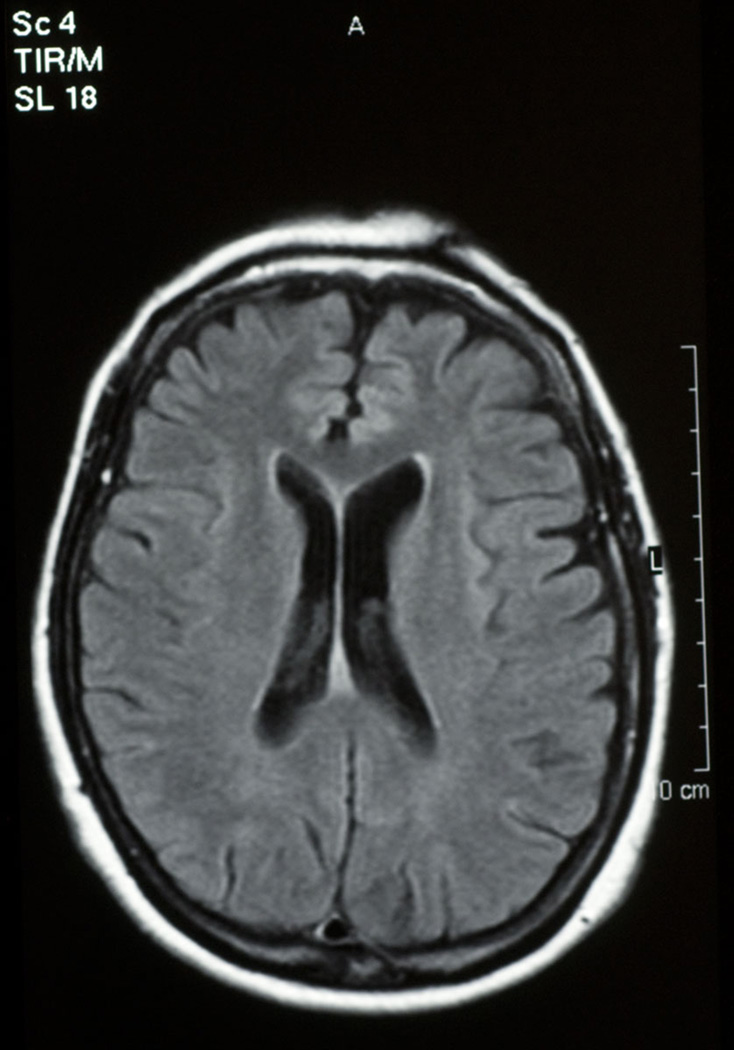
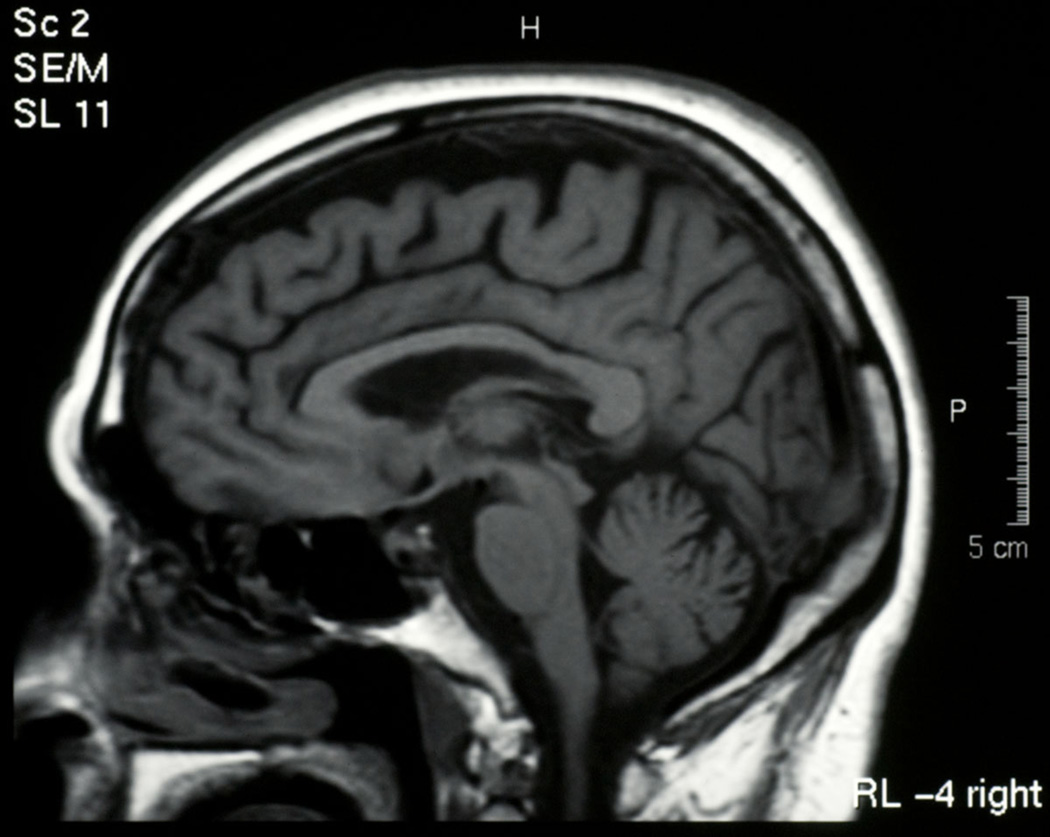
She was first evaluated neurologically at the age of 63, following a diagnosis of progressive supranuclear palsy (PSP) made by a neuro-ophthalmologist. Her movement disorder began approximately three years after her behavioural problems. Her gait became stiff and unsteady. She would fall off her bicycle, and the sidewalk. Neuroimaging with MRI showed left sided frontotemporal (Figure 1) and some parietal (Figure 2) atrophy and the sagittal brainstem images suggested tegmental atrophy (“hummingbird sign”) (Figure 3). SPECT scan, CSF cells, glucose and protein were normal. Cognitive testing showed her category fluency was within normal range but letter fluency was poor. She had difficulty on the Stroop test and on the Wisconsin Card Sorting task. She did poorly on similarities and estimating quantities. She showed compulsive, perseverative behavior. Levodopa didn’t help her and she shuffled even more.
Figure 1.
Fontotemporal atrophy, propositus
Figure 2.
frontoparietal atrophy, propositus
Figure 3.
Brainstem, propositus
On examination, seven years after the onset, she had decreased, hesitant speech output, vertical gaze palsy on pursuit and saccades, mask-like face and bradykinesia with difficulty getting out of the chair and adjusting her posture on the examining table. She raised her arm to her face to look at her watch, because she could not look down. She also had a retropulsion and a tendency to fall backwards. Her gait was slow and stiff and she had significant apraxia, with difficulty imitating alternating movements or copying the “fist, edge, palm” sequence. She had micrographia and a mild, rapid action tremor in the right hand; previously diagnosed as essential tremor (she had it since her teenage years).
Neuropsychological testing, seven years post onset showed logopenia, but her sentences were connected and grammatical. Her aphasia quotient was still 95.2% but her semantic fluency for animal categories was low at 8/20. Her drawings were oversimplified, small and crowded, lacked detail and perspective. The numbers on the clock overlapped and were difficult to read and the hands were set poorly. She did well on the block design and calculation subtest and her Raven's Progressive Matrices were 22/37.
About 10 years after onset she was in a wheelchair, unable to propel herself, chocking on food and biting on a spoon. She whispered “Yes” or “No” and recognized people. Her limbs and body were rigid, her head was tiled backwards. She died at the age of 69 after 10 years of illness.
FAMILY HISTORY
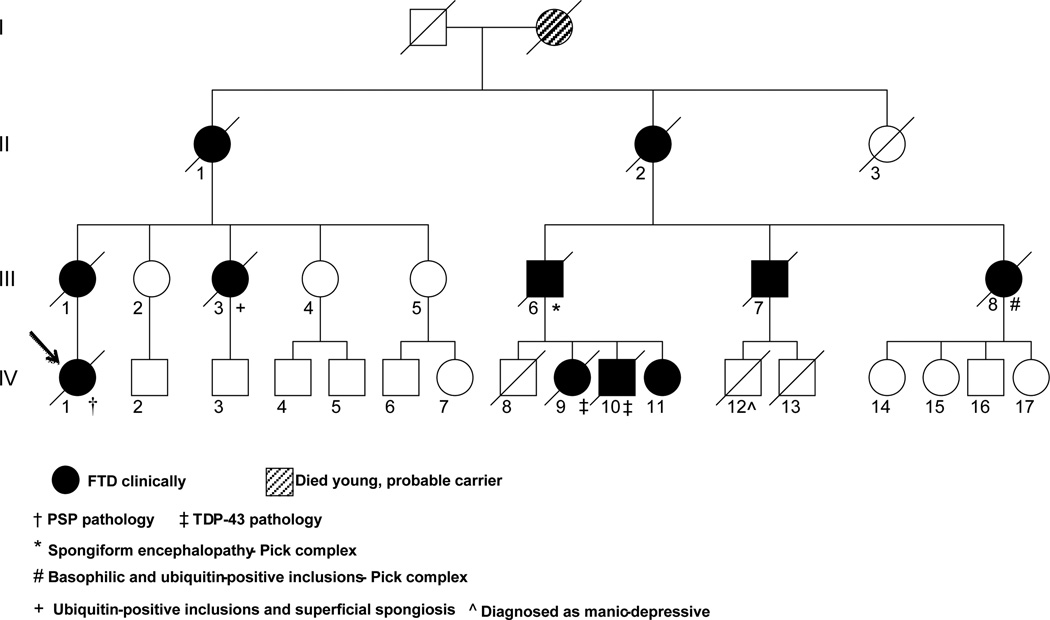
The family history is significant, with the updated pedigree displayed on Fig 4. The propositus is IV-1 (previously she was shown as unaffected3). Her mother was affected with a similar behavioral illness and died in 1958 without autopsy (III-1). Her maternal aunt had bvFTD with FTLD-U (III-3). One of the mother’s cousins (III-7) had PSP like symptoms and signs on examination, but autopsy was not available. Two other maternal cousins (III-6 and III-8) had bvFTD with FTLD-U and so had two of III-7’s autopsied children, IV-1’s second cousins (IV-9 and IV-10) Thus, the histology of ubiquinated tau negative FTD was found in four autopsied members of the family(3) Chromosome-17 linkage was not conclusively shown and no mutation was found in MAPT in several other individuals in the family(IV-9 IV-10 and IV-11) at the time of the first publication of this family 13 years ago. Subsequently two of the family members (IV-9 and IV-10) were found to have TDP-43 positive neuronal cytoplasmic inclusions on their autopsy material. (Figure 5)
Figure 4.
(the legend is part of the figure)
Figure 5.
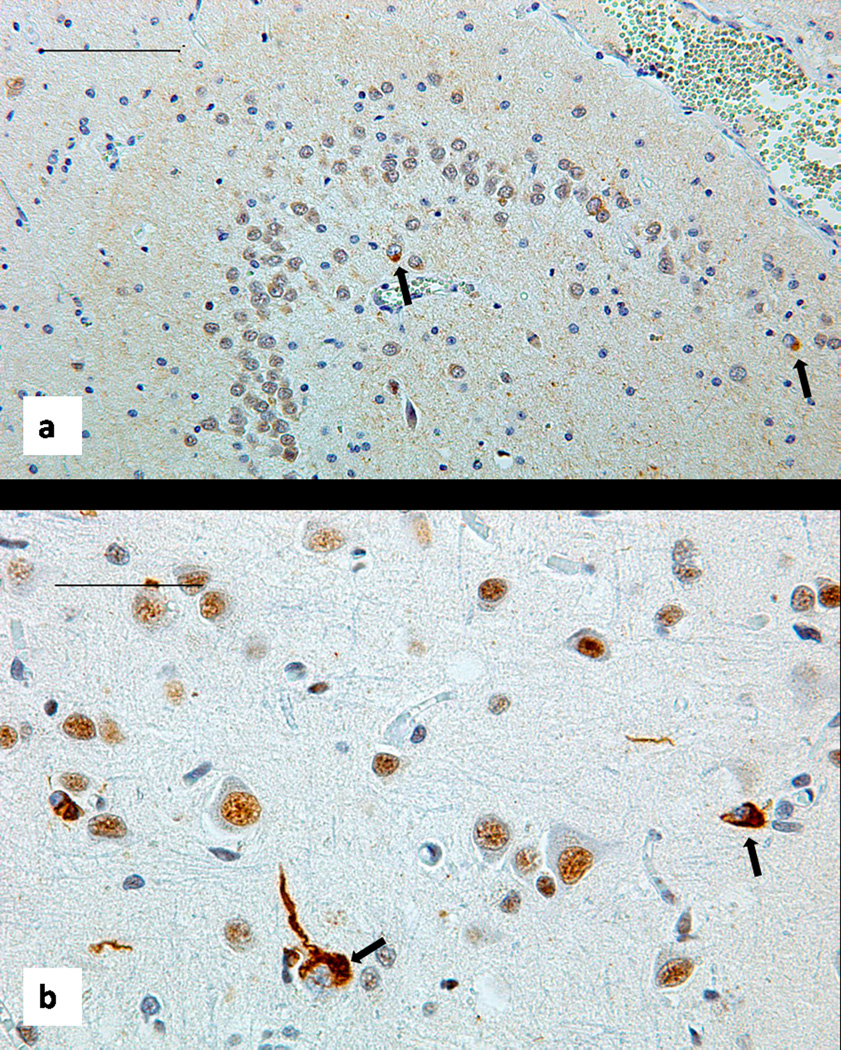
Brain histology. Sections stained with antibody to total TDP-43 show scattered neurons with displacement of this protein from the nucleus to the cytoplasm, forming rounded or tangle like inclusion marked by black arrows. (a): patient IV-10, Dentate fascia of the hippocampus, bar 100 um. (b): patient IV-9 Temporal neocortex, bar 50 um.
METHODS
Blocks from the formalin-fixed brain were embedded in paraffin, and 5 µm thick sections were processed for immunohistochemistry (IHC) using the Ventana BenchMarkXT automated staining system (Ventana, Tuscon, AZ), developed with diaminobenzime (DAB) and counter-stained with haematoxylin. The antibodies used were ubiquitin (DAKO anti-ubiquitin; 1:300, following microwave antigen retrieval (MAR) using CC1 Ventana for 30 minutes at 99 C), p62 (BD Transduction Laboratories p62 Lck ligand; 1:500 MAR), TDP-43 (ProteinTech Group anti-TARDBP; 1:400 MAR), Anti phospho TDP-43 (pS409/410-2, Cosmo Bio Co, 1:2000), FUS (Bethyl, 1:100), neurofilament (2F11, DAKO, 1:50), tau (DAKO, 1:1600), PHF-1 (Donation of Dr. Peter Davies, 1:5000) and following formic acid pretreatment, α-synuclein (Zymed, 1:300) and β amyloid (6F/3D, Dako).
POSTMORTEM
The brain from the propositus showed a tauopathy in the classic pattern of Progressive Supranuclear Palsy (PSP)2 (Figure 6). Neuronal tau deposits in the form of neurofibrillary tangles were present in the entorhinal cortex and in the CA1 section of the hippocampus and in the fusiform gyrus of the neocortex. They were also present in the thalamus, colliculi, red nucleus, substantia nigra, periaqueductal gray, pontine tegmentum, locus coeruleus, pontine nuclei, the brain stem reticular formation and olivary nuclei. Tufted astrocytes had a different distribution, representing the main location of tau in the cerebral neocortex and neostriatum and being intermingled with the neuronal deposits in the thalamus and the brainstem. Tau deposits were common in the form of coiled bodies in the internal capsule and the cerebellar white matter. There were numerous neuropil threads in the affected areas. The cerebellar cortex showed tau deposits in the synaptic complexes of the granular cell layer, and in glial cells in the white matter. Immunostains for p62 did not demonstrate any deposits additional to those shown by tau immunostains. TDP-43 and FUS immunostains showed the normal nuclear distribution, i.e. absence of inclusions. Antibodies to phosphorylated TDP-43 confirmed the absence of abnormalities, contrasting with the staining of inclusions in cases of known TDP-43 associated FTD (Figure 7).
Figure 6.
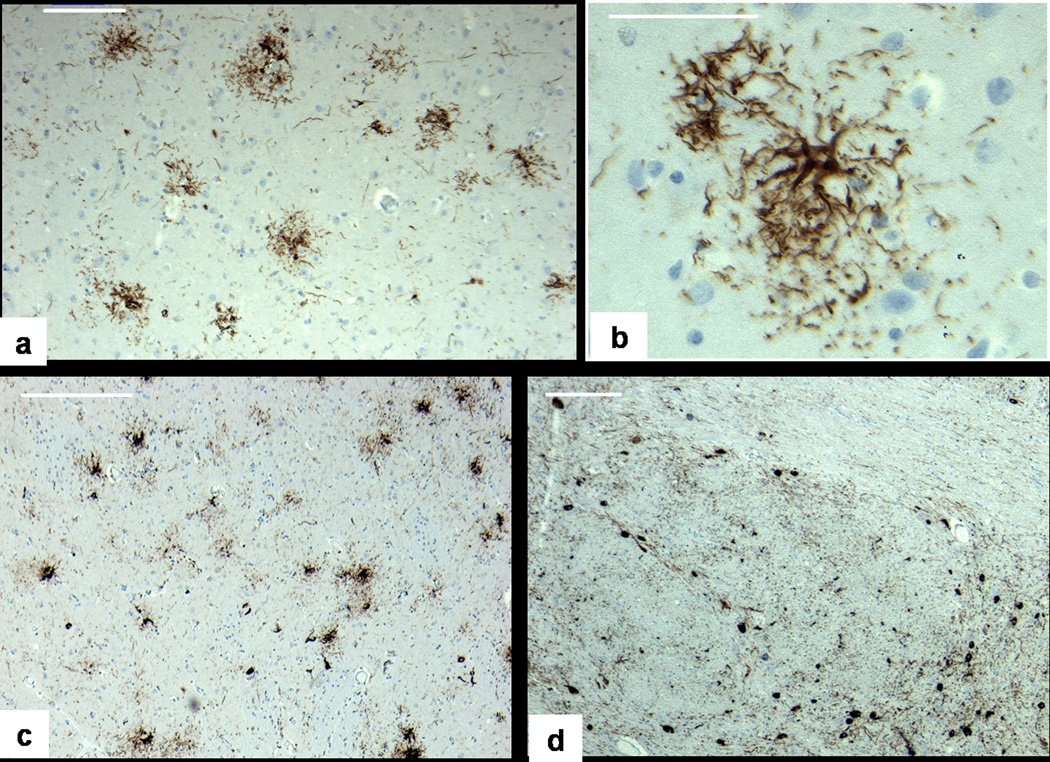
Brain histology, tau immunostain. a:Frontal cortex, showing numerous tufted astrocytes but no neurofibrillary tangles, bar 100 um. b: higher magnification, bar 50 um. c: Putamen, showing both neurofibrillary tangles (black arrow) and tufted astrocytes (white arrow), bar 200 um d:. Basis pontis, with numerous neurofibrillary tangles (black arrow), bar 200 um
Figure 7.
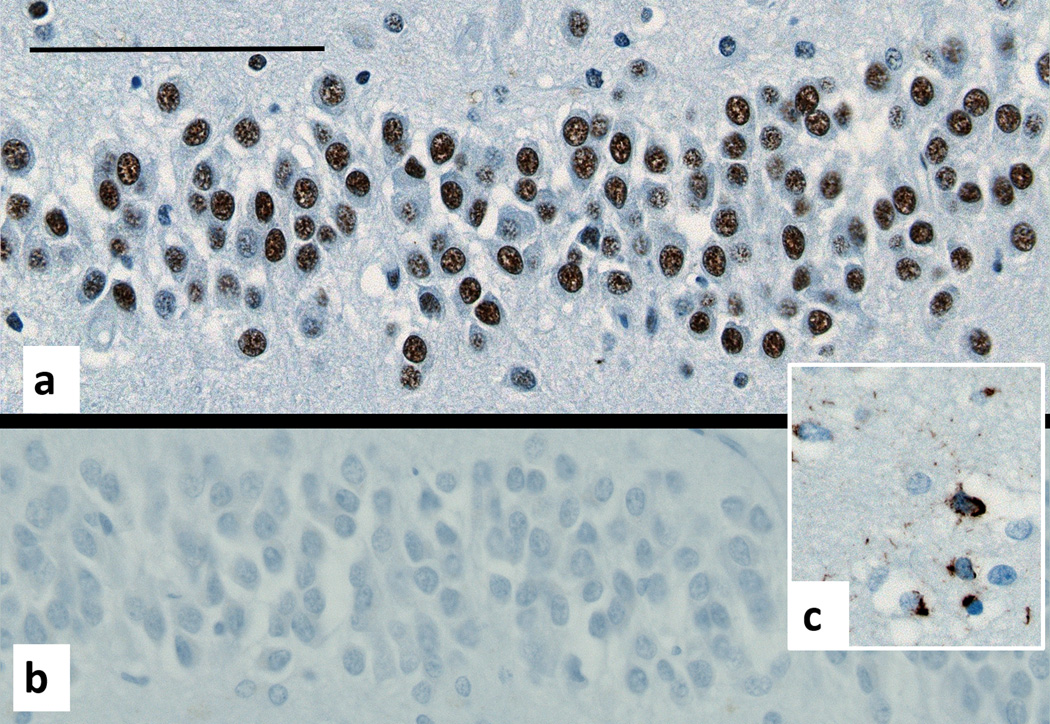
Dentate fascia of the hippocampus. a:Antibodies to total TDP-43 show its normal location in neuronal nuclei. b: Antibodies to phosphorylated TDP-43 show the absence of abnormal inclusions. c: The same antibody stains the inclusions in the cortex of a positive control (TDP-43 FTD brain), bar 50 um.
GENETIC ANALYSIS
Three patients including the propositus were included in the repeat genetic analysis (Fig. 1, IV-1, IV-9 and IV-11). Informed consent was obtained before the samples were collected. For all patients genomic DNA was isolated from frozen blood and used in direct sequence analysis of exonic and surrounding intronic regions of MAPT (exons 1, 7, 9–13), GRN (all exons) and TARDBP (exon 5) 4,5 No mutations were found. Patients IV-1 and IV-9 were H1H1 for the MAPT haplotype, whereas patient IV-11 was H1H2. In addition, the C9ORF72 hexanucleotide repeat was PCR amplified in the same 3 patients using one fluorescently labeled primer followed by fragment length analysis on an ABI3730 DNA-analyzer6. All patients were heterozygous showing two alleles within the normal range.
DISCUSSION
The pathological findings in the propositus are indistinguishable from PSP and the clinico-pathological correlation fits the pattern commonly seen in this condition: the florid early behavioural manifestations can be attributed to the predominantly astrocytic tauopathy in the neocortex, while truncal, axial, and ocular motor signs can be related to the striatal and brainstem predominantly neuronal tauopathy. The clinical pattern of CBD/PSP following behavioral FTD has been described and well recognized for some time1,7
One other member of her family, a maternal uncle, also had axial rigidity and gaze palsy, but unfortunately an autopsy was not done (III-7) However his brother, (III-6), had tau negative pathology, although no further studies were done at the time and the blocks are not available. Three of his children (propositus’ second cousins) (IV-9, IV-10, IV-11) had bvFTD and two (IV-9 and IV-10) had autopsy with TDP-43 immunoreactive, ubiquitin positive, cytoplasmic inclusions (Figure 5).
The clinical syndromes of CBD and PSP, traditionally associated with tauopathies, are now known to also occur with TDP-43 proteinopathy substrates 7, 8 in some cases due to GRN mutations,9. A unique case with the p.K263E mutation in TDP-43 developed FTD, PSP, and chorea, but no signs of motor neuron disease. Neuropathologic examination revealed neuronal and glial TDP-43-immunoreactive deposits, predominantly in subcortical nuclei and brainstem5.
The concurrence of TDP-43 and PSP pathology in a family with dominantly inherited FTD has not yet been reported. Our patient differs from her family in that she had classical PSP pathology, but the rest of the family had FTLD-U pathology, some with well defined TDP-43 deposits. Neither MAPT nor GRN mutations have been demonstrated in 3 members of this family, including the propositus. C9ORF72 hexanucleotide expansions were also excluded in this patient and in two of her cousins (IV-9 and IV-11) and a pathogenic repeat expansion can be further excluded by the absence of the typical p62 deposits in the cerebellar cortex, a hallmark of this condition10.
It is of course possible that the propositus is a sporadic case of PSP, and not a carrier of the mutation responsible for the hereditary disease in the family. If the association is not a coincidence, the family raises the question of the relationship between tau and TDP-43 pathologies. TDP-43 NCI, neurites, and even NII have been reported in a subset (up to 34%) of cases with Alzheimer’s disease, where they are associated with hippocampal sclerosis11 TDP-43 pathology has been described in 2 other sporadic taupathies, Guam’s Parkinson-Dementia Complex12, 13 and argyrophilic grain disease14 Despite early statements to the contrary, a subset of PSP (26%) and CBD (17%) cases demonstrate TDP-43 immunoreactive NCI, neurites sometimes accompanied by NII, and this pathology is again associated with hippocampal sclerosis8.
Two different mutations in the GRN result in dual tau and TDP-43 pathologies in the same brain, comparable to the sporadic cases discussed above. The pathology of one (c.709-2A>G) has been reported in multiple individuals in 2 families15, whereas the other (c.1414-16_1590del) has only been described in one subject16. This pattern is different from that observed in our family in which only one of the two alternative pathologies is observed in each member. The closest parallel to our family is provided by mutations in the leucine rich repeat kinase (LRRK-2) gene, associated with dominantly inherited Parkinson’s disease. Intrafamilial variability has been reported for the p.R1441C mutation. At autopsy, all patients show neuronal loss in the substantia nigra, but in some this is accompanied by alpha-synuclein inclusions, whereas in others the main component of the NCI is either TDP-43 or tau17.18. It has been suggested that LRRK-2 acts upstream from other proteins implicated in neurodegeneration19 possibly through phosphorylation20. Our family also suggests a pathway that can lead to deposition of either abnormal tau or TDP-43 when the relevant yet unidentified gene is mutated.
Acknowledgements
Funding for this study included grants from NIH R01 NS065782, R01 NS080882 and P50 NS072187. We thank Dr Albrecht Steffen for performing the autopsy on the propositus.
Footnotes
Authors contributions and conflict or interest declarations.
Dr Kertesz has examined and followed all the members of this family for decades, conceived and wrote the first draft of the article. He has no disclosures
Dr Finger continued to follow members of this family and collected the genetic material on some, and contributed to the manuscript. She has no disclosures.
Dr Murrell explored the genetics in the propositus and contributed to the manuscript. No disclosures
Dr Ang contributed to the preparation and neuropathologic examination of several members of the family, Dr Ang has no disclosures.
Dr Chertkow has examined investigated and contributed to the case report of the propositus, no disclosures
Dr. Matt Baker: Acquisition of data, Analysis or interpretation of data. No disclosures
Dr. Thomas Ravenscroft Acquisition of data, Analysis or interpretation of data. No disclosures
Dr. Rademakers: Acquisition of data, Analysis or interpretation of data, revising the manuscript for content, including writing for content, obtaining funding. She further received honoraria for lectures or educational activities not funded by industry; holds a patent on methods to screen for the hexanucleotide repeat expansion in the C9ORF72 gene.
Dr Munoz has examined the neuropathology on several members of this family, prepared the histological illustrations and rewrote the first draft substantially. Dr. Munoz has received speaker fees from Janssen and Novartis
References
- 1.Kertesz A, Blair M, Davidson W, McMonagle P, Munoz DG. The Evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Kertesz A, Kawarai T, Rogaeva E, et al. Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology. 2000;54:818–827. doi: 10.1212/wnl.54.4.818. [DOI] [PubMed] [Google Scholar]
- 4.Spina S, Murrell JR, Huey ED, et al. Corticobasal syndrome associated with the A9D Progranulin mutation. J Neuropathol Exp Neurol. 2007 Oct;66(10):892–900. doi: 10.1097/nen.0b013e3181567873. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs GG, Murrell JR, Horvath S, et al. TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord. 2009 Sep 15;24(12):1843–1847. doi: 10.1002/mds.22697. [DOI] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011 Oct 20;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paviour DC, Lees AJ, Josephs KA, et al. Frontotemporal lobar degeneration with ubiquitin-only-immunoreactive neuronal changes: broadening the clinical picture to include progressive supranuclear palsy. Brain/ 2004 Nov;127(Pt 11):2441–2451. doi: 10.1093/brain/awh265. Epub 2004 Sep 30. [DOI] [PubMed] [Google Scholar]
- 8.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 2010 Jul;120(1):55–66. doi: 10.1007/s00401-010-0702-1. Epub 2010 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masellis M, Momeni P, Meschino W, et al. Novel splicing mutation in the progranulin gene causing familial corticobasal syndrome. Brain. 2006;129:3115–3123. doi: 10.1093/brain/awl276. [DOI] [PubMed] [Google Scholar]
- 10.Hsiung GY, Dejesus-Hernandez M, Feldman HH, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007 May;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa M, Arai T, Akiyama H, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007 May;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 13.Geser F, Winton MJ, Kwong LK, et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008 Jan;115:133–145. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 14.Fujishiro H, Uchikado H, Arai T, et al. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol. 2009 Feb;117:151–158. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 15.Leverenz JB, Yu CE, Montine TJ, et al. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain. 2007;130(pt 5):1360–1374. doi: 10.1093/brain/awm069. [DOI] [PubMed] [Google Scholar]
- 16.Yu CE, Bird TD, Bekris LM, et al. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch Neurol. 2010 Feb;67:161–170. doi: 10.1001/archneurol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimprich A, Muller-Myhsok B, Farrer M, et al. The PARK8 locus in autosomal dominant parkinsonism: confirmation of linkage and further delineation of the disease-containing interval. Am J Hum Genet. 2004 Jan;74:11–19. doi: 10.1086/380647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross OA, Whittle AJ, Cobb SA. Lrrk2 R1441 substitution and progressive supranuclear palsy. Neuropathol Appl Neurobiol/ 2006 Feb;32(1):23–25. doi: 10.1111/j.1365-2990.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 19.Wider C, Dickson DW, Wszolek ZK. Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener Dis. 2010;7(1–3):175–179. doi: 10.1159/000289232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagalwar S, Berry RW, Binder LI. Relation of hippocampal phospho-SAPK/JNK granules in Alzheimer's disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol. 2007 Jan;113(1):63–73. doi: 10.1007/s00401-006-0159-4. [DOI] [PubMed] [Google Scholar]