Abstract
We report the detection of homocysteine over cysteine based upon characteristic differences between 5-and 6-membered heterocyclic amines formed upon reaction with aldehyde-bearing compounds. Homocysteine-derived thiazinane-4-carboxylic acids are more basic than cysteine-derived thiazolidines-4-carboxylic acids. Fluorescence enhancement in response to homocysteine is achieved by tuning pH and excitation wavelength.
Changes in the abundance of glutathione (GSH), cysteine (Cys), and homocysteine (Hcy) in human plasma are correlated with several diseases.1–7 Variations in GSH levels are associated with chronic diseases such as cancer, neurodegenerative diseases, cystic fibrosis (CF), HIV, and aging.1, 2 Elevated Cys levels have been linked to neurotoxicity.3 The improper metabolism of Hcy results in elevated plasma levels,4 that have been associated with Alzheimer’s,5 cancer,6 and cardiovascular diseases.7 Thus, detecting these amino thiols in human plasma is of great interest. Several techniques such as high-performance liquid chromatography (HPLC),8, 9 liquid chromatography/mass spectrometry (LC-MS),10 electrochemical,11 and optical methods12–17 have been developed for the detection of amino thiols. However, many of these methods involve labour intensive steps or complex instrumentation.
Optical methods have received a great deal of attention due to their sensitivity, relative ease, and low cost; however, to date there have been relatively few optical methods reported which can reliably distinguish GSH,18, 19 Cys,20–22 and Hcy23–25 over related compounds. For example, we recently developed a resorufin acrylate-based probe with selectivity for GSH over related analytes.18 A monochlorinated BODIPY-based GSH sensor has also been reported.19 Probes reported to show preferential responses toward Cys include a styryl BODIPY-based20 and a seminaphthofluoroscein acrylate-based Cys probe.21 We have also developed colorimetric assays for the selective detection of Hcy using viologens.23, 24 Yoon et al. recently reported pyrene aldehyde fluorophores to detect Hcy.25 However, the vast majority of reported optical thiol probes respond to classes of analytes rather than individual species.15–17 Thus, the development of new and improved probes is an active area of research.
Aldehyde-functionalized probes12–14, 25–31 have received a great deal of attention for the optical detection of amino thiols and amino acids. Interestingly, this class of probes has been reported to respond to their respective analytes through either fluorescence quenching,12, 13, 27 or fluorescence enhancement25, 26, 28 on a case by case basis. In an early example, Glass et al. reported detection of amino acids using aldehyde bearing coumarin probes, wherein reaction of the aldehyde with amino acids resulted in enhancement of the fluorescence.26 Our group popularized the visible detection of amino thiols with aldehyde bearing fluorophores12, 13 based upon the well known reaction of amino thiols to form heterocycilic thiazolidines32 and thiazinanes. Fluorescence quenching of fluorescein aldehyde probes following the formation of thiazolidine/thiazinane heterocycles occurred upon reaction of Cys and Hcy under the conditions invenstigated.12, 13 Others have subsequently used this concept for the detection of Cys and Hcy. For example, Kim et al., observed fluorescence quenching of an aldehyde bearing amino coumarin in response to Cys/Hcy.27 Hoang et al., reported an aldehyde bearing hydroxy coumarin28 in which addition of Cys/Hcy resulted in fluorescence enhancement. It is therefore of utility to develop a general understanding of the mechanisms behind differing modes of signal transduction in the related aldehyde-bearing probes. Such knowledge will lead to improved probes with increased sensitivity for selected amino thiols of interests, specifically for Hcy over Cys.
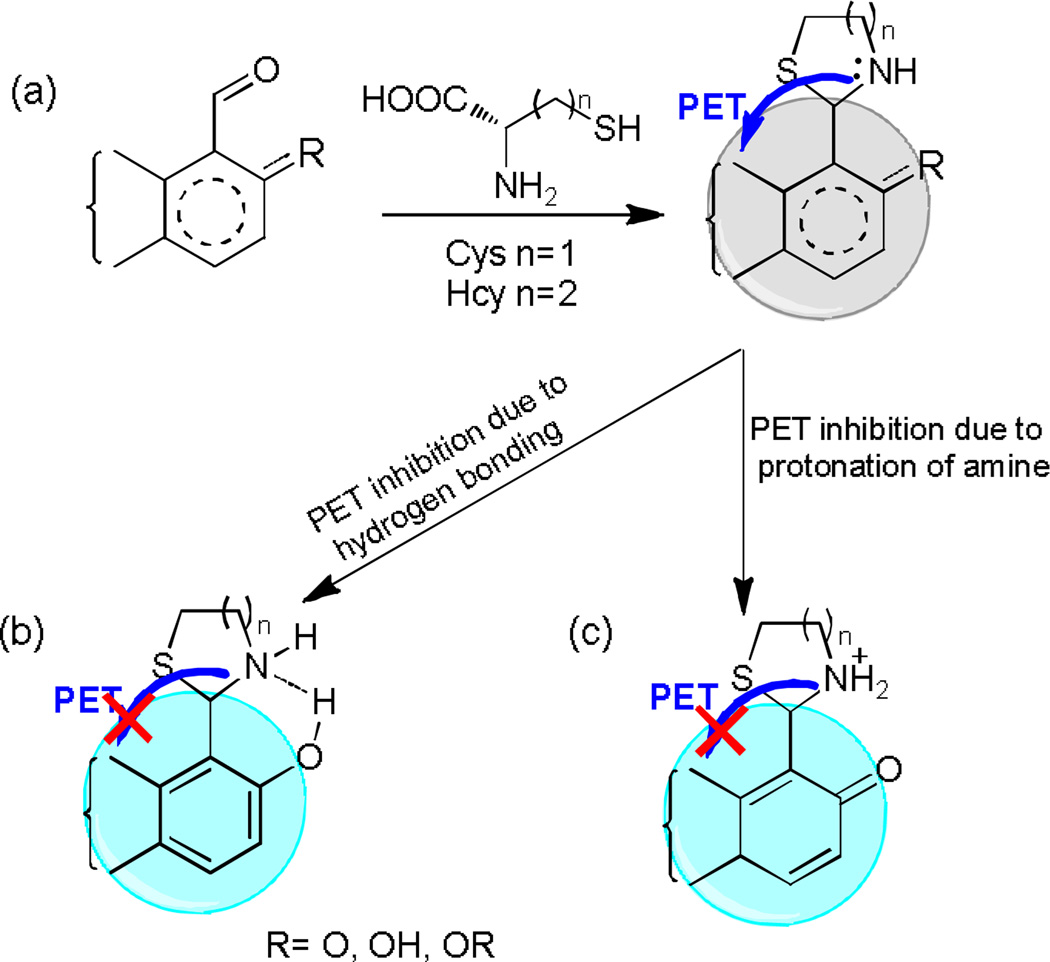
Herein we further investigate the mechanism of signal transduction in fluorescein aldehyde probes upon reaction with amino thiols. Unprecedented control over signal transduction is attained such that the probes can be tuned to respond to Cys/Hcy through either quenching or enhancement. More importantly, the response can be controlled in such a manner that Hcy is detected over Cys. There are several phenomena that impact the fluorescence response of aldehyde-bearing probes. It is important to note the differences in how aldehyde-bearing probes react with amino thiols compared to amino acids. Amino acids react to form Schiff bases26 whereas amino thiols such as Cys and Hcy react to form heterocycilic thiazolidines and thiazinanes respectively.12 These differences result in unique optical responses towards amino thiols. Fig.1 depicts the reaction of Cys/Hcy with the aldehyde group of a generic aldehyde-bearing probe. It is well established that the free electrons of the amino group in the heterocycle can quench fluorescence of the probe (Fig. 1a) through photo induced electron transfer (PET).27 PET-induced responses have been modulated through tuning the functionality of the fluorescent probes.25,28 For example, hydrogen bonding in aqueous media between the amine-containing heterocycles and adjacent groups (Fig. 1b) has been reported to inhibit PET leading to fluorescence enhancement upon reaction with both Cys and Hcy with a coumarin aldehyde.28 Conversely, in this work we demonstrate PET inhibition leading to selective fluorescence enhancement for Hcy via a mechanism involving protonation of the amino group of the heterocycle (Fig. 1c)
Figure 1.
Mechanism of signal transduction in generic aldehyde-bearing amino thiol probes. (a) PET-based fluorescence quenching following reaction of Cys/Hcy with the aldehyde group. (b) PET inhibition through hydrogen bonding between the amine-containing heterocycle and adjacent groups. (c) PET inhibition through protonation of the amine-containing heterocycle.
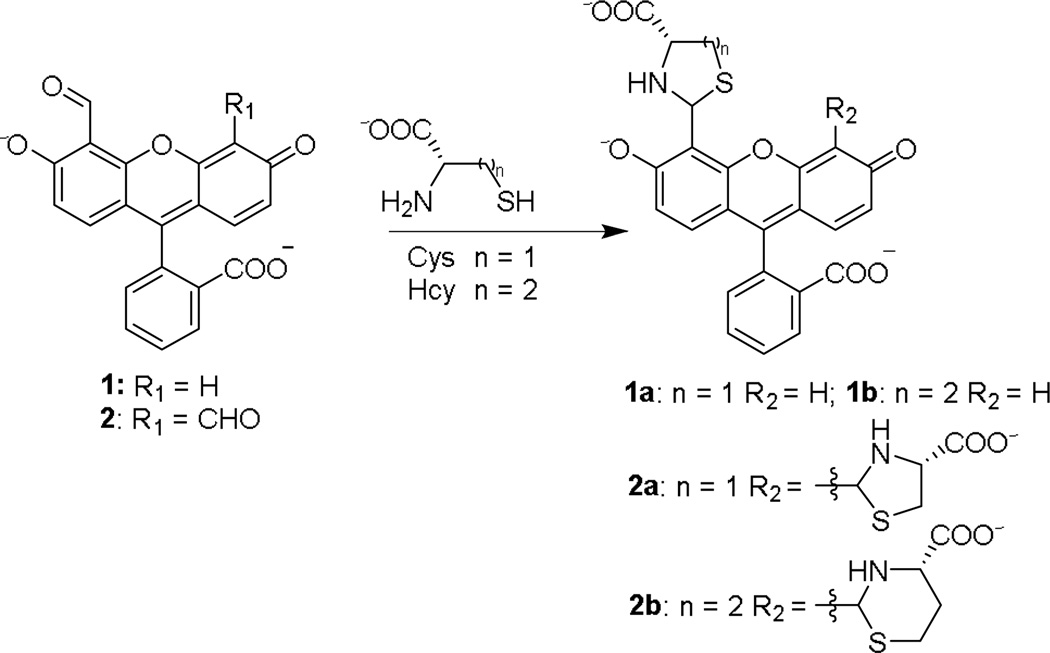
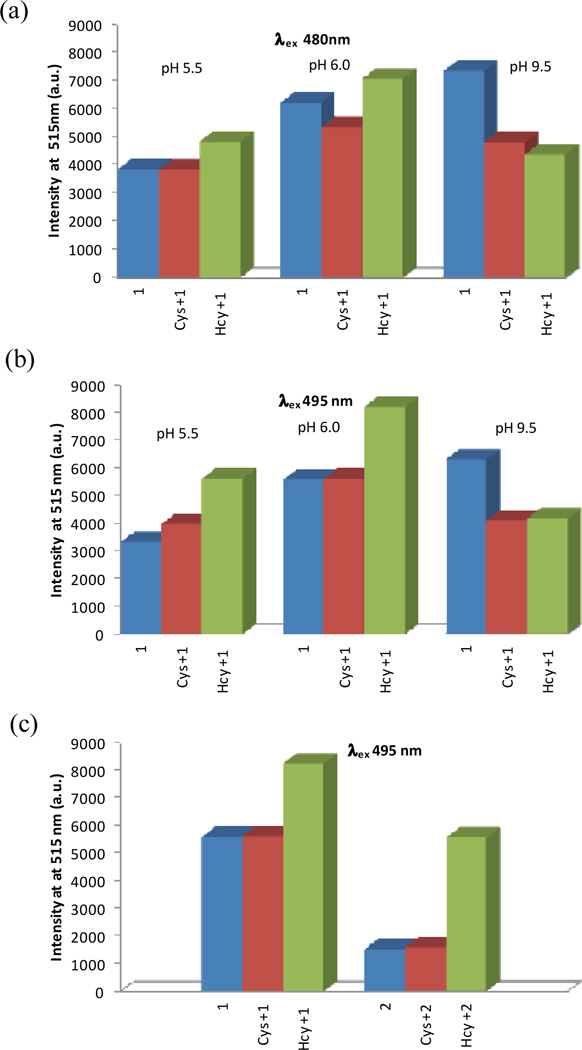
Previously we have investigated the reaction of Cys/Hcy with fluorescein mono aldehyde 1 and fluorescein dialdehyde 2 at pH 9.5 sodium bicarbonate buffer (Scheme 1). Reaction with Cys/Hcy resulted in a yellow-to-orange colour change with a shift in absorbance maxima from near 480 nm to ca. 500 nm. In addition to the colour change, a quenching-based response toward both analytes was reported.12, 13 At high pH, excitation near either peak yields a quenching-based response (Fig. 2a-b, pH 9.5). Under basic conditions, the lone pair electrons on the nitrogen of the heterocyclic moiety are free to quench fluorescence through PET (Fig. 1a).
Scheme 1.
Reaction of 1 and 2 with Cys and Hcy
Figure 2.
Modulating the signal transduction through changing the pH and excitation wavelength. (a) Fluorescence emission upon excitation at 480 nm for 1 and 1 + Cys or Hcy at various pH values. (b) Fluorescence emission upon excitation at 495 nm for 1 and 1 + Cys or Hcy at various pH values. (c) Comparison of 1 and 2 response upon excitation at 495 nm at pH 6.0. All solutions are composed of 4 µM of 1 or 2 with 1 mM of analyte in 100 mM phosphate or carbonate buffer:DMSO 99:1 at 20 °C after 2 hr, λem = 515 nm.
We hypothesize that at sufficiently low pH, the heterocycles formed upon reaction with the probe must be protonated; thus inhibiting PET and leading to fluorescence enhancement. PET inhibition and a wavelength-dependent fluorescence response are observed upon lowering solution pH (Fig. 2a-b). In acidic media (pH 5.5) longer wavelength excitations near the absorption maxima of the heterocyclic reaction products (495 nm) result in fluorescence enhancement (Fig. 2b), unlike the quenching observed under basic conditions. (Fig. 2b, pH 9.5)
We further hypothesize that under certain conditions, the response can be tuned to detect Hcy over Cys due to pKa differences caused by the differing C-N-C bond angles in 5-vs. 6-membered ring heterocycles. In the case of a 5-memebered Cys-derived thiazolidine ring the C-N-C bond angle is smaller than in a 6-membered Hcy-derived thiazinane ring. This results in greater s-character of the N-H bond in the thiazolidines, rendering them less basic than the thiazinanes.33–35
We observe that on adjusting the pH from 5.5 to 6.0, the signal is modulated such that Hcy enhances the fluorescence of 1 while there is minimal change upon reaction with Cys (Fig. 2b). Dialdehyde 2 was more sensitive than monoaldehyde 1 at high pH.12, 13 This trend in sensitivity is also observed under these new conditions with a >5 fold fluorescence enhancement of 2 observed in response to Hcy (Fig. 2c). The response of both 1 and 2 towards Hcy is easily observed in as little as 15 min and plateaus near 45 min (Fig. S12 and S13, ESI†). For the purpose of investigating the mechanism responsible for this unique selectivity, we focused first on the simpler monoaldehyde 1.
To demonstrate the mechanism behind this pH dependent response and Hcy-derived enhancement, a series of 5-and 6-membered 2-substituted thiazolidine/thiazinanes-4-carboxylic acids were synthesized (see ESI†) as model compounds of 1a-b. Cys-and Hcy-derived heterocycles of fluorescein aldehydes are stable under the conditions previously investigated12 as well as under the present system (Fig. S14, ESI†). However, they are extremely polar and we were unable to isolate 1a-b.
To confirm the hypothesis that the pH dependent Hcy enhancement was related to the differing basicity of 5-and 6-membered heterocyclic amines, we measured the pKa of the Cys-and Hcy-derived analogues (Table 1) (Fig. S16, ESI†). Cys-derived 3a–5a are known and their pKa values previously published36 are in general agreement with those in Table 1. We report and measure the pKa of Hcy-derived 3b–5b for the first time. As seen in Table 1, the amine pKa of 6-membered thiazinanes is higher than that of 5-membered thiazolidines by at least one unit. Therefore based on the pKa of these analogues, we expect the amine pKa of Hcy-derived 1b to be at least 1 unit greater (~6.7) than Cys-derived 1a (~5.7).
Table 1.
pKa values of the amine moiety of 2-substituted thiazolidine/thiazinane-4-carboxylic acids.
| Compound | pKa exp, (pKa lit) | Compound | pKa exp |
|---|---|---|---|
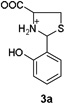 |
NA,a (5. 6736) | 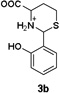 |
6.67b |
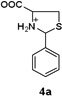 |
5.50,a (5.3136) | 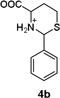 |
6.85b |
| 6.44, (6.19) | 7.76 |
3a and 4a were found to be relatively unstable showing signs of decomposition in aqueous solution during the course of the titration (Fig. S15 and S16, ESI†).
3b and 4b showed no indication of decomposition (Fig. S15 and S16, ESI†)
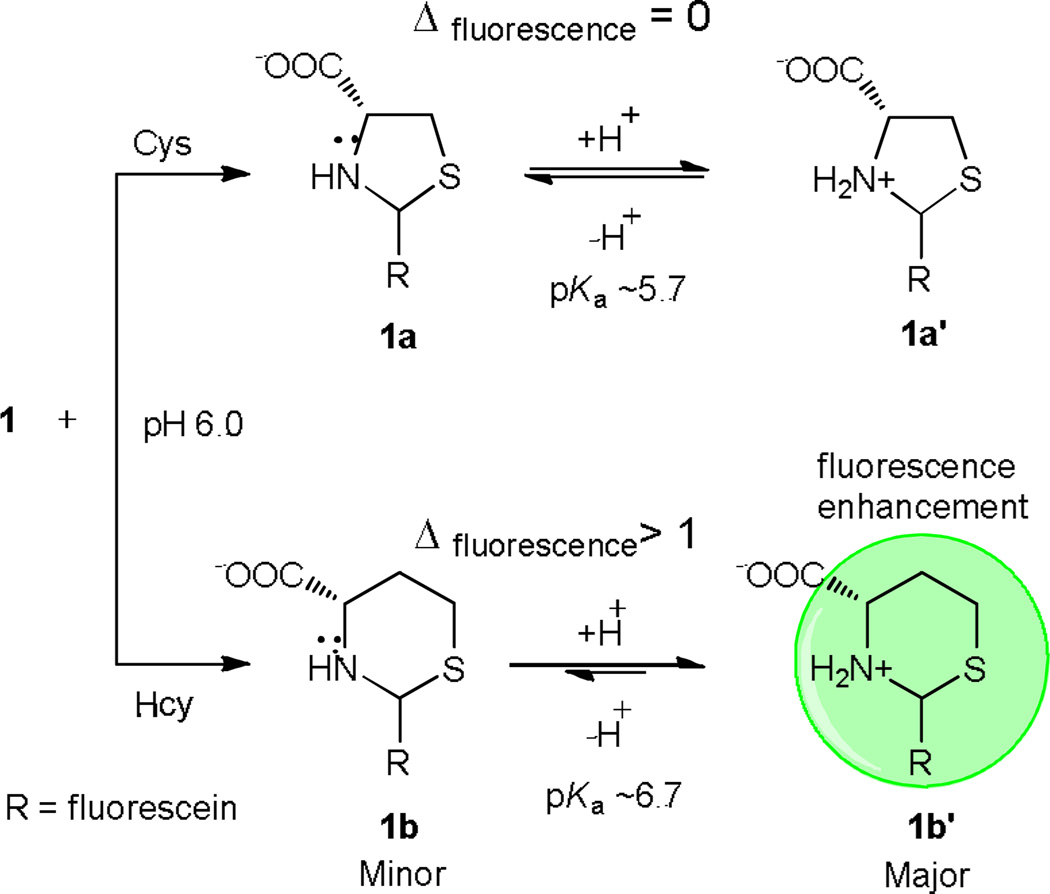
This difference in basicity results in the selectivity for Hcy over Cys (Fig. 2c). We investigated the response of 1 towards Hcy and Cys over a range of pH (Fig. S17, ESI†) values. At the appropriate pH the ratio of ionization states of the amine moiety in thiazolidine 1a would be such that fluorescence quenching by the lone pair electrons is cancelled by PET inhibition from the ammonium species 1a' (Scheme 2). At this pH the amine group in thiazinane 1b exists predominately as the more fluorescent ammonium 1b' (Scheme 2) because the pKa of 1b' is greater than that of 1a'. At pH = 6 the reaction between 1 and Cys does not result in a change in fluorescence while reaction with Hcy results in fluorescence enhancement.
Scheme 2.
Different ionization states of the the amine moieties in Cys-and Hcy-derived heterocycles at pH 6.0.
Thus pH 6.0 is optimal for the detection of Hcy. Raising the pH increases the ratio of amine to ammonium which leads to quenching, while decreasing the pH leads to enhancement (Fig. S17, ESI†). We also investigated the response of 1 towards GSH and various amino acids at pH 6.0 (Fig. S18, ESI†). Only Hcy promotes fluorescence enhancement.
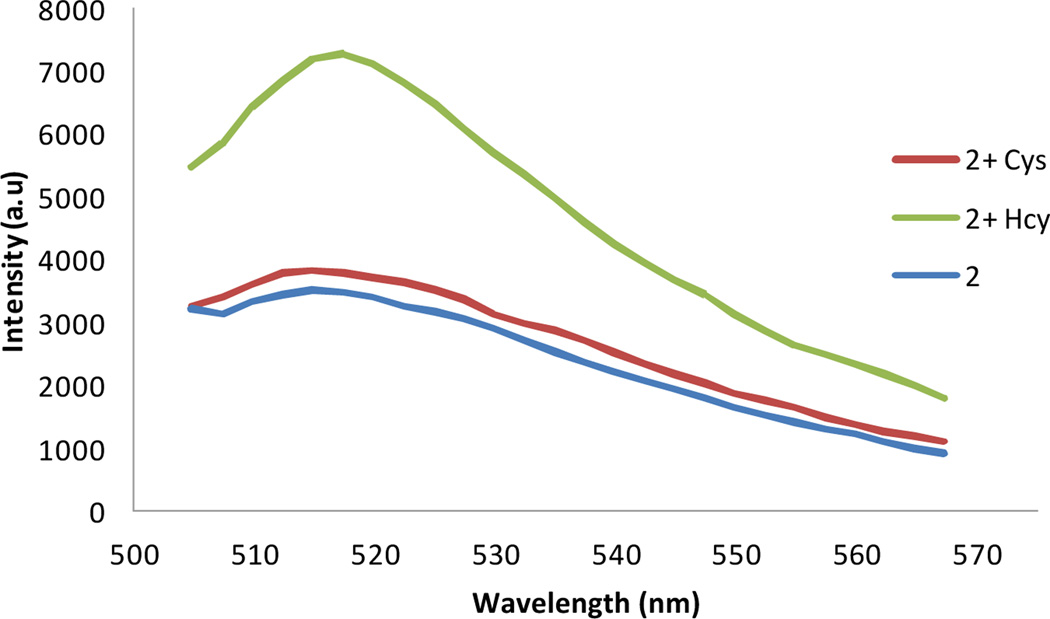
Having identified the optimal conditions for the discrimination of Hcy over Cys, we investigated the response of the more sensitive dialdehyde 2 towards normal healthy concentrations of amino thiols in pH 6.0 buffer. The fluorescence response of 2 towards Hcy increased linearly with increasing Hcy concentration and the limit of detection was found to be as low as 2.5 µM (Fig. S19, ESI†) No response was observed toward 250 µM Cys, while 15 µM Hcy resulted in a ~10% increase in fluorescence (Fig. S20, ESI†). Importantly, 2 was also shown to function similarly in deproteinized bovine plasma detecting an elevated level of Hcy (Fig. 3 and S21, ESI†).
Figure 3.
Optical sensing behavior of 2 towards Cys and Hcy in deproteinized bovine plasma at pH 6.0. Solutions are composed of 25 µM of 2 with 250 µM of Cys and 100 µM of Hcy in phosphate buffer (100 mM, pH 6.0):DMSO 99:1 at 20 °C after 2 hr, λex = 495 nm.
In conclusion, we report a new approach for the selective detection of Hcy using fluorescein aldehyde-based probes. This method is based on intrinsic differences between the pKa of 5-and 6-membered thiazolidines and thiazinanes formed upon reaction with the aldehyde-bearing probes. Selective Hcy probes based on the mechanism described herein with improved sensitivity are currently being developed in our laboratory.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R15EB016870).
Footnotes
† Electronic supplementary information (ESI) available: Detailed experimental procedures and additional spectral data. See DOI: 10.1039/b000000x/.
References
- 1.Townsend DM, Tew KD, Tapiero H. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu GY, Fang YZ, Yang S, Lupton JR, Turner ND. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 3.Wang XF, Cynader MS. J. Neurosci. 2001;21:3322–3331. doi: 10.1523/JNEUROSCI.21-10-03322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmel R, Jacobsen DW, editors. Homocysteine in health and disease. Cambridge University Press; 2001. [Google Scholar]
- 5.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PWF, Wolf PA. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 6.Ozkan Y, Yardim-Akaydin S, Firat H, Caliskan-Can E, Ardic S, Simsek B. Anticancer Res. 2007;27:1185–1189. [PubMed] [Google Scholar]
- 7.Bostom AG, Silbershatz H, Rosenberg IH, Selhub J, D'Agostino RB, Wolf PA, Jacques PF, Wilson PW. Arch. Intern. Med. 1999;159:1077–1080. doi: 10.1001/archinte.159.10.1077. [DOI] [PubMed] [Google Scholar]
- 8.Nolin TD, McMenamin ME, Himmelfarb J. J. Chromatogr. B. 2007;852:554–561. doi: 10.1016/j.jchromb.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMenamin ME, Himmelfarb J, Nolin TD. J. Chromatogr. B. 2009;877:3274–3281. doi: 10.1016/j.jchromb.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Guan XM, Hoffman B, Dwivedi C, Matthees DP. J. Pharm. Biomed. Anal. 2003;31:251–261. doi: 10.1016/s0731-7085(02)00594-0. [DOI] [PubMed] [Google Scholar]
- 11.Baron M, Sochor J. Int. J. Electrochem. Sci. 2013;8:11072–11086. [Google Scholar]
- 12.Rusin O, St Luce NN, Agbaria RA, Escobedo JO, Jiang S, Warner IM, Dawan FB, Lian K, Strongin RM. J. Am. Chem. Soc. 2004;126:438–439. doi: 10.1021/ja036297t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WH, Rusin O, Xu XY, Kim KK, Escobedo JO, Fakayode SO, Fletcher KA, Lowry M, Schowalter CM, Lawrence CM, Fronczek FR, Warner IM, Strongin RM. J. Am. Chem. Soc. 2005;127:15949–15958. doi: 10.1021/ja054962n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S, Escobedo JO, Lowry M, Xu X, Strongin R. Chem. Commun. 2010;46:5707–5709. doi: 10.1039/c0cc01398f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobedo JO, Rusin O, Wang W, Alpturk O, Kim KK, Xu X, Strongin RM. In: Reviews in Fluorescence 2006. Geddes CD, Lakowicz JR, editors. US: Springer; 2006. pp. 139–162. [Google Scholar]
- 16.Peng H, Chen W, Cheng Y, Hakuna L, Strongin R, Wang B. Sensors. 2012;12:15907–15946. doi: 10.3390/s121115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung HS, Chen X, Kim JS, Yoon J. Chem. Soc. Rev. 2013;42:6019–6031. doi: 10.1039/c3cs60024f. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Yang X, Hakuna L, Barve A, Escobedo JO, Lowry M, Strongin RM. Sensors. 2012;12:5940–5950. doi: 10.3390/s120505940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu LY, Guan YS, Chen YZ, Wu LZ, Tung CH, Yang QZ. J. Am. Chem. Soc. 2012;134:18928–18931. doi: 10.1021/ja309079f. [DOI] [PubMed] [Google Scholar]
- 20.Shao J, Guo H, Ji S, Zhao J. Biosens. Bioelectron. 2011;26:3012–3017. doi: 10.1016/j.bios.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Guo Y, Strongin RM. Org. Biomol. Chem. 2012;10:2739–2741. doi: 10.1039/c2ob25178g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Zhao N, Sun Y, Miao F, Liu Y, Liu X, Zhang Y, Ai W, Song G, Shen X, Yu X, Sun J, Wong W-Y. Chem. Commun. Vol. 48. Cambridge, UK: 2012. pp. 3442–3444. [DOI] [PubMed] [Google Scholar]
- 23.Escobedo JO, Wang W, Strongin RM. Nat. Protoc. 2006;1:2759–2762. doi: 10.1038/nprot.2006.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hakuna L, Escobedo JO, Lowry M, Barve A, McCallum N, Strongin RM. Chem. Commun. 2014;50:3071–3073. doi: 10.1039/c4cc00432a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HY, Choi YP, Kim S, Yoon T, Guo Z, Lee S, Swamy KMK, Kim G, Lee JY, Shin I, Yoon J. Chem. Commun. 2014 doi: 10.1039/c4cc00243a. [DOI] [PubMed] [Google Scholar]
- 26.Feuster EK, Glass TE. J. Am. Chem. Soc. 2003;125:16174–16175. doi: 10.1021/ja036434m. [DOI] [PubMed] [Google Scholar]
- 27.Kim T-K, Lee D-N, Kim H-J. Tetrahedron Lett. 2008;49:4879–4881. [Google Scholar]
- 28.Lee KS, Kim TK, Lee JH, Kim HJ, Hong JI. Chem. Commun. 2008:6173–6175. doi: 10.1039/b814581d. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Xiao J, Wang S, Yang B, Ba X. Supramol. Chem. 2010;22:380–386. [Google Scholar]
- 30.Mei J, Tong J, Wang J, Qin A, Sun JZ, Tang BZ. J. Mater. Chem. 2012;22:17063–17070. [Google Scholar]
- 31.Mei J, Wang Y, Tong J, Wang J, Qin A, Sun JZ, Tang BZ. Chem. -Eur. J. 2013;19:613–620. doi: 10.1002/chem.201202969. [DOI] [PubMed] [Google Scholar]
- 32.Kim T-R, Yun S-J, Park B-B. Bull. Korean Chem. Soc. 1986;7:25–29. [Google Scholar]
- 33.Yoshikawa K, Hashimoto M, Morishima I. J. Am. Chem. Soc. 1974;96:288–289. [Google Scholar]
- 34.Aue DH, Webb HM, Bowers MT. J. Am. Chem. Soc. 1972;94:4726–4728. [Google Scholar]
- 35.Ohwada T, Hirao H, Ogawa A. J. Org. Chem. 2004;69:7486–7494. doi: 10.1021/jo0486589. [DOI] [PubMed] [Google Scholar]
- 36.Butvin P, Al-Ja'Afreh J, Svetlik J, Havranek E. Chem. Pap.-Chem. Zvesti. 1999;53:315–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.