Abstract
Acupuncture has been demonstrated to improve menstrual frequency and to decrease circulating testosterone in women with polycystic ovary syndrome (PCOS). Our aim was to investigate whether acupuncture affects ovulation frequency and to understand the underlying mechanisms of any such effect by analyzing LH and sex steroid secretion in women with PCOS. This prospective, randomized, controlled clinical trial was conducted between June 2009 and September 2010. Thirty-two women with PCOS were randomized to receive either acupuncture with manual and low-frequency electrical stimulation or to meetings with a physical therapist twice a week for 10–13 wk. Main outcome measures were changes in LH secretion patterns from baseline to after 10–13 wk of treatment and ovulation frequency during the treatment period. Secondary outcomes were changes in the secretion of sex steroids, anti-Müllerian hormone, inhibin B, and serum cortisol. Ovulation frequency during treatment was higher in the acupuncture group than in the control group. After 10–13 wk of intervention, circulating levels of estrone, estrone sulfate, estradiol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, androstenedione, testosterone, free testosterone, dihydrotestosterone, androsterone glucuronide, androstane-3α,17β-diol-3-glucuronide, and androstane-3α,17β-diol-17-glucuronide decreased within the acupuncture group and were significantly lower than in the control group for all of these except androstenedione. We conclude that repeated acupuncture treatments resulted in higher ovulation frequency in lean/overweight women with PCOS and were more effective than just meeting with the therapist. Ovarian and adrenal sex steroid serum levels were reduced with no effect on LH secretion.
Keywords: polycystic ovary syndrome, acupuncture, ovulation, lh pulsatility, sex steroids
the main characteristics of polycystic ovary syndrome (PCOS) are polycystic ovaries (34), oligo/anovulation, and elevated serum levels of sex steroid precursors, estrogens, androgens, and glucuronidated androgen metabolites (38). PCOS is related to hyperinsulinemia and insulin resistance and is exacerbated by obesity (7). Numerous studies have reported hypersecretion of luteinizing hormone (LH) in women with PCOS (3). Together with an exaggerated ovarian response, hypersecretion of LH drives excessive ovarian androgen production and causes anovulation (13). In PCOS, altered sex steroid production, metabolic dysfunction, and obesity all contribute to changes in LH secretion patterns and to anovulation (6, 12, 29, 30).
Clomiphene citrate, exogenous gonadotropin therapy, and laparoscopic ovarian drilling are commonly used to induce ovulation in women with PCOS (1). These treatments often have negative side effects, thus indicating the importance of evaluating alternative treatments such as acupuncture. Acupuncture is used worldwide to achieve fertility, but its efficacy is supported by only limited scientific evidence. In a randomized controlled trial (RCT), we previously demonstrated that acupuncture with manual and low-frequency electrical needle stimulation was superior to both exercise and no treatment for improving menstrual frequency and total testosterone levels (15). The effect of acupuncture was, at least in part, mediated by reduction in sympathetic nerve activity (39). The main limitations of that study were the inability to confirm ovulation and to control for the increased attention associated with the therapeutic meeting and needle insertion. In another RCT, true acupuncture was compared with sham acupuncture using “placebo needles” (31). The ovulation frequency did not differ between the groups, and both groups showed improved LH-to-follicle-stimulating hormone (FSH) ratios. The lack of a difference between true and sham acupuncture in that study is in line with previous studies on different pain conditions and nausea caused by chemotherapy that demonstrated that true acupuncture is not more effective than sham acupuncture. However, all of these trials found a significant effect when groups were compared with a nonintervention group (8, 22, 28). These results indicate that sham acupuncture is not an inert method and highlight the methodological difficulties in the design of acupuncture trials.
In the present study, we tested the hypothesis that frequent acupuncture treatment could improve ovulation frequency, LH secretion and pulsatility, and sex steroid secretion in lean/overweight PCOS patients. To compensate for the attention that women treated by acupuncture would receive, a control group was randomized to meet with a therapist, because sham acupuncture has been shown not to be an inert control. The primary outcome measures were changes in LH pulsatility from baseline to the end of treatment and differences in ovulation frequency during the study.
MATERIALS AND METHODS
Study Population
PCOS patients were recruited by advertisements in local newspapers between June 2009 and September 2010. PCOS was diagnosed according to the Rotterdam criteria: ultrasound-verified (Voluson E8/E8 Expert; GE healthcare, Giles, UK) polycystic ovaries (≥12 follicles of 2–9 mm and/or ovarian volume ≥10 ml in one or both ovaries), oligo/amenorrhea, or clinical signs of hyperandrogenism (hirsutism or acne) (34). A self-reported Ferriman-Gallwey (FG) score of ≥8 defined the presence of hirsutism, and the presence of acne was defined by an affirmative answer to being asked if they had excessive acne. An intermenstrual interval of >35 days or <6 menstrual bleedings in the previous year was used as the definition for oligomenorrhea, and amenorrhea was defined as a total absence of menstrual bleeding in the previous 90 days. Patients were excluded if they were younger than 18 or older than 38 yr of age, had a body mass index (BMI) over 30, had taken any pharmacological treatments in the previous 3 mo, or had breastfed or received acupuncture during the 24 wk prior to inclusion in the study. Other exclusion criteria were cardiovascular disease, diabetes mellitus, and other endocrine disorders such as congenital adrenal hyperplasia, Cushing's syndrome, or androgen-secreting tumors. All participants gave oral and written consent before inclusion.
Study Design
The study was conducted at the Sahlgrenska Academy at the University of Göteborg, Gothenburg, Sweden, in accordance with the Declaration of Helsinki and was approved by the Regional Ethics Review Board of the University of Göteborg. The study was registered at ClinicalTrials.gov (NTC00921492), and is reported according to the CONSORT and STRICTA guidelines (24, 35). Participants recorded menstrual bleeding patterns, and ovulation in all women was confirmed by weekly progesterone measurements throughout the study. Participants were randomized before baseline assessments to either receiving acupuncture or to receiving similar attention by meeting with a therapist. Randomization was computer generated (http://www.randomization.com), and investigators were blinded until statistical analyses. After randomization, each participant underwent a 10- to 13-wk intervention period after which the baseline measurements were repeated. Baseline assessments were made at the Endocrine and Metabolic Research Centre at Sahlgrenska during an overnight stay.
Power Calculation and Sample Size
Power calculation and sample size were determined on the basis of changes in the 12-h LH pulse frequency in women with PCOS from baseline to end of treatment. We expected a 10% change in the number of pulses with a mean change (Δ) of −0.8 and standard deviation (SD) of 1.81 within the groups (32). A total of 14 patients per group was required for a statistical power of 80% at a 5% significance level. We enrolled 16 subjects per group to account for dropouts.
Interventions
Acupuncture.
Women in the acupuncture group were treated for 30 min twice weekly for 10–13 wk by two therapists educated in Western medical acupuncture. Subjects rested and listened to relaxing music during treatment. The acupuncture protocol was based on a previous study of acupuncture for ovulation induction in PCOS (15), an experimental study (37), and clinical experience. Sterile stainless steel needles (Hegu Xeno, Hegu Svenska; length 30 or 50 mm, diameter 0.30 mm) were inserted to a depth of 15–35 mm at acupuncture points located in abdominal and leg muscles that have innervations corresponding to the ovaries and in acupuncture points that do not innervate the ovaries. Two sets of 11 and 13 acupuncture points were alternated every other treatment due to the intensity of the treatment (Table 1). All needles were rotated manually to evoke needle sensation (de qi) when inserted. Needles in the leg and abdominal muscles were then connected to an electrical stimulator (CEFAR ACUS 4; Cefar-Compex Scandinavia, Landsbro, Sweden) and stimulated with low-frequency (2 Hz) bursts. The intensity was adjusted to produce local muscle contractions without pain or discomfort. Needles not connected to the electrical stimulator were stimulated by manual rotations every 10 min to evoke de qi.
Table 1.
Two sets of acupuncture points and their anatomic positions, needle stimulations, and muscle innervations
| Points | Location | Stimulation | Innervation | Muscle Location |
|---|---|---|---|---|
| Set 1 | ||||
| CV3 | Midline | EA, 2Hz | L1 | Fibrous tissue, linea alba |
| CV6 | Th11 | |||
| ST29 | Bilateral | EA, 2Hz | Th6-12 | M. rectus abdominis |
| SP6 | Bilateral | EA, 2Hz | L4-5, S1-2 | Mm. flexor digitorum longus, tibialis posterior |
| SP9 | S1-2 | M. gastrochnemius | ||
| LI4 | Bilateral | Manual | C8, Th1 | Mm. interosseus dorsalis I, lumbricalis II, adductor pollicis |
| GV20 | Midline | Manual | C2-3 | Aponeurosis epicranii |
| Set 2 | ||||
| CV3 | Midline | Manual | L1 | Fibrous tissue, linea alba |
| CV6 | Th11 | |||
| ST25 | Bilateral | EA, 2Hz | Th6-12 | M. rectus abdominis |
| ST29 | ||||
| SP6 | Bilateral | EA, 2Hz | L4-5, S1-2 | Mm. flexor digitorum longus, tibialis posterior |
| LR3 | S2-3 | M. interosseus dorsalis I | ||
| PC6 | Bilateral | Manual | C8, Th1 | M. flexor digitorum superficialis |
| GV20 | Midline | Manual | C2-3 | Aponeurosis epicranii |
Set 1 and Set 2 were alternated in every other treatment due to the intensity of the treatment and were the same for all women in the acupuncture group CV, conception vessel; EA, electro-acupuncture; GV, governor vessel; LI, large intestine; LR, liver; M., muscle; Mm., muscles; PC, pericardium; SP, spleen; ST, stomach.
Attention control.
Women in the control group visited the same physical therapists. The therapeutic meeting was held for an equal amount of time (twice weekly for 10–13 wk) to control for the attention involved in meeting with the therapists. Time was spent resting and listening to relaxing music, with the therapist entering the room every 10 min ensuring comfort and well-being in a manner similar to the acupuncture group.
Outcome Measurements
All measurements were taken at baseline and were repeated within 1 wk after the last treatment (referred to as end of treatment in results). Measurements were taken at menstrual cycle days 8–10 if ovulation had occurred. If no ovulation or bleeding had occurred during the period between screening for the study and the taking of the baseline measurements, the measurements were taken on an arbitrary day of the cycle.
Outcome measurements.
Anthropometrics included measurements of height, weight, waist and hip circumferences, sagittal diameter, and calculations of waist-to-hip ratio (WHR) and BMI (kg/m2). A foot-to-foot bioelectrical impedance system (Tanita, Middlesex, UK) was used to measure the percent body fat. Hirsutism was assessed by the FG score. Ovarian volume, endometrial thickness, and antral follicle (<9 mm) count were measured by ultrasound. Bleeding and progesterone measurements determined the number of ovulations per month that occurred during the study. Each 12-h sampling period started at 1930 with a sample taken to measure the level of sex hormone-binding globulin (SHBG). Samples were then drawn every 10 min for measurement of LH and cortisol, every hour for measurement of FSH, and every 4th hour for measurement of sex steroids. The LH:FSH ratio was calculated and expressed as a mean of the 12-h sampling period. The final blood sample at 0730 allowed measurements of fasting glucose, insulin, anti-Müllerian hormone (AMH), inhibin B, and cholesterol [total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL)], and calculation of the homeostatic model assessment (HOMA) index.
Immunoassay
LH, FSH, AMH, inhibin B, glucose, cholesterol (total, HDL, and LDL), insulin, progesterone, and SHBG were analyzed at an accredited laboratory at the Department of Clinical Chemistry, Sahlgrenska University Hospital. Plasma glucose [detection limit (DL) = 0.11 mmol/l], cholesterol (DL = 0.10 mmol/l), HDL-cholesterol (DL = 0.08 mmol/l), and LDL-cholesterol (DL = 0.10 mmol/l) were measured at 37°C with enzymatic photometric methods. Insulin (DL = 0.2 mU/l) was measured with immunometric methods (two-step sandwich) and chemiluminescence technology, and cortisol (DL = 0.5 nmol/l) was measured with competitive methods and chemiluminescence technology. All of these were measured with kits from Roche Diagnostics (Mannheim, Germany) on a Cobas instrument. SHBG (DL = 0.33 nmol/l) was measured using the Access Immunoassay System (Beckman-Coulter, Brea, CA), and FSH (DL = 0.05 IU/l), LH (DL = 0.07 IU/l), and progesterone (DL 0.5 nmol/l) were measured by chemiluminescence on an Architect instrument (Abbott Laboratories, Wiesbaden, Germany) with kits from the same company. AMH (DL = 0.2 μg/ml) was measured with an enzymatic amplified double-epitope immunoassay, and inhibin B (DL = 4 ng/ml) was measured with a sandwich immunoassay, both from Beckman-Coulter and measured on a multiscan flow cytometry apparatus (Thermo Scientific Oy, Vantaa, Finland).
Mass Spectrometry
Circulating concentrations of sex steroids, androgen precursors, and glucuronidated androgen metabolites were analyzed by mass spectrometry. Analyses were performed by the Endoceutics Bioanalytical Laboratory (Quebec, Canada) essentially as described (18, 19). Free testosterone (free T) was calculated with the spreadsheet developed by Mazer et al. (27) where cortisol and cortisol-binding globulin were set to zero. The area under the curve (AUC) for all sex steroid concentrations, measured every 4th hour from 0 to 12 h, was calculated using the linear trapezoidal method.
Gas Chromatography with Detection by Tandem Mass Spectrometry
Total deuterated estradiol (E2), total testosterone (T), androstenedione (4-DIONE), dehydroepiandrosterone (DHEA), total estrone (E1), and total dihydrotestosterone (DHT) were added as internal standards (CDN Isotopes, Essex, UK) to 0.5-ml serum samples to enable quantification of their respective free steroids. The standards and serum samples were extracted twice by liquid-liquid and solid-phase extraction on a 500 mg/ml silica column (Phenomenex, Torrance, CA) and underwent two derivatization procedures to improve selectivity and specificity. Samples were then separated by GC-MS/MS using negative ion chemical ionization.
Ultraperformance Liquid Chromatography Tandem Mass Spectrometry
Deuterated androstane-3α,17β-diol-3-glucuronide (AD3G), androstane-3α,17β-diol-17-glucuronide (AD17G), and androsterone glucuronide (ADTG) were added as internal standards to 400-μl serum samples to enable quantification of their respective glucuronidated steroids. The standards and serum samples were extracted on a 500 mg/ml silica column and reconstituted in a methanol-H2O mixture. Deuterated DHEA sulfate (DHEA-S) and E1 sulfate (E1-S) were added as internal standards to 75-μl serum samples to enable quantification of their respective sulfated steroids and were extracted as above. Detection and quantification were performed by UPLC-MS/MS (API 5000; AB Sciex, Framingham, MA) in negative mode. The limits of quantitation for each analyte were as follows: DHEA, 500 pg/ml; 4-DIONE, 100 pg/ml; T, 50 pg/ml; DHT, 10 pg/ml; E1, 4.0 pg/ml; E2, 1 pg/ml; AD3G, 100 pg/ml; AD17G, 100 pg/ml; ADTG, 4 ng/ml; DHEA-S, 100 ng/ml; and E1-S, 50pg/ml.
Data Analysis
Hormone pulsatility.
The concentrations of LH and cortisol in blood samples taken every 10 min for 12 h were analyzed with the Autodecon pulse detection algorithm (17) in the Pulse XP software package to detect and quantify secretory events. Three truncated LH data series, due to missing blood samples, were recalculated to 12 h for the time-dependent variables before statistical analysis. This recalculation was applied to one baseline series in the acupuncture group and to one end-of-treatment series in both the acupuncture and control groups. Two truncated cortisol data series in the acupuncture group, one baseline, and one end of treatment, were recalculated to 12 h for the time-dependent variables.
Quantification of irregularity.
To quantify the regularity or randomness of the LH data series, we analyzed the time courses with the Approximate Entropy (ApEn) application in the Pulse XP software package. ApEn is a statistical method that, in contrast to conventional pulse-detection procedures, evaluates both dominant and subdominant patterns in time series that are not reflected in peak occurrences or amplitudes. The parameters m (window length) and r (tolerance parameter) must be specified to measure the logarithmic likelihood that m contiguous observations that are similar (within distance r) remain close to the next incremental comparison (for m = 1, when two points have approximately equal values, within r, the next point is also approximately equal). Larger values represent greater randomness or irregularity, and smaller numbers represent more recognizable patterns (44). Here, we used a window length of m = 1 and a tolerance parameter of r = 20% of the individual subject's average standard deviation in the hormone time series (ApEn: 1, 20%). One thousand Monte Carlo simulations were used to calculate the standard deviation of approximate entropy in each series.
Statistical Analyses
Between-group differences for baseline values and the change between baseline and end of treatment (Δ values) were analyzed with the Mann-Whitney U-test. The χ2 test was used to assess differences in acne and menstrual cycle pattern (categorical variables) between groups at baseline.
Data were analyzed according to the intention-to-treat (ITT) principle. In cases where the end-of-treatment assessments were missing due to dropouts after baseline assessments, the baseline observations were used as the end-of-treatment observations according to the baseline observation carried forward approach. Dropouts occurring before the measurements of baseline values were excluded from the ITT analysis due to lack of data. Variables displaying between-group differences at baseline were further analyzed by Spearman's rank correlation to identify possible correlations between baseline and Δ values. If correlations were found, a logistic regression including both Δ values and baseline data was performed to correct for the difference at baseline and to determine whether between-group changes were due to a true treatment effect or to the difference at baseline. Within-group differences between baseline and end of treatment were analyzed with the Wilcoxon rank sum test. Bonferroni corrections were made for Δ changes between groups for measurements of sex steroids, androgen precursors, and glucuronidated androgen metabolites. Associations between ovulation frequency and all end-of-treatment variables for the entire study population (not groupwise) were estimated by Spearman's rank correlation. Data were analyzed with the Prediction Application Software package (v. 19.0 for Windows; SPSS, Chicago, IL).
RESULTS
Participant flow through the study is summarized in Fig. 1. Thirty-two women met the inclusion criteria and were randomized to the acupuncture group (n = 16) or attention control group (n = 16). There were four dropouts in the attention control group before baseline assessments and three dropouts, one in the attention control group and two in the acupuncture group, between baseline and end-of-treatment assessments. The ITT population consisted of 28 women who had baseline measurements taken and were included in the analyses: 12 in the attention control group and 16 in the acupuncture group. In the attention control group, six subjects were identified as oligoamennorheic and six as amennorheic, whereas in the acupuncture group six subjects were identified as oligoamennorheic and 10 as amennorheic. Before start of study, 10 of 12 in the attention control group and 10 of 16 in the acupuncture group had an ovulation where baseline measurements were then performed at days 8–10. Number of treatments in the acupuncture group was 19.1 ± 4.4 (mean ± SD) and 20.2 ± 3.0 in the attention control group.
Fig. 1.
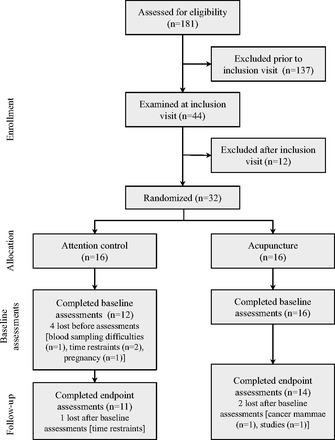
Flow of participants through the trial. All participants assigned to acupuncture or attention control treatment and who underwent baseline assessments were included in the intention-to-treat analysis.
Between-Group Comparisons at Baseline
Phenotypic characterization of all participants is presented in Table 2. All participants were oligo/amenorrheic before the start of treatment, and the menstrual cycle pattern did not differ between the acupuncture and the attention control groups (Table 2). Serum LH levels (P = 0.026), LH:FSH ratios (P = 0.004), LH secretion area (P = 0.037), ovarian volumes (P = 0.041), and 4-DIONE levels (P < 0.001) differed between the two groups at baseline (Tables 2, 3, and 4).
Table 2.
Clinical characteristics at baseline and changes from baseline after 10–13 wk of treatment
| Attention Control (n = 12) | Acupuncture (n = 16) | Attention Control (n = 12) | Acupuncture (n = 16) | |||
|---|---|---|---|---|---|---|
| Variable |
Baseline |
†P |
Δ (Endpoint-baseline) |
†P | ||
| Anthropometry | ||||||
| Age, yr | 27.9 ± 3.2 | 28.4 ± 3.1 | NS | n/a | n/a | n/a |
| Height, cm | 166.9 ± 7.8 | 165.8 ± 6.1 | NS | n/a | n/a | n/a |
| Weight, kg | 68.5 ± 7.6 | 63.9 ± 8.6 | NS | −0.93 ± 1.5* | −0.41 ± 1.2 | NS |
| BMI, kg/m2 | 24.7 ± 3.1 | 23.3 ± 3.6 | NS | −0.3 ± 0.5* | −0.1 ± 0.5 | NS |
| Waist, cma | 83.8 ± 9.3 | 80.4 ± 8.5 | NS | −4.4 ± 5.2* | −2.2 ± 3.7* | NS |
| Hip, cm | 100.5 ± 5.9 | 98.2 ± 7.6 | NS | 1.2 ± 5.7 | −0.6 ± 2.1 | NS |
| WHRa | 0.83 ± 0.1 | 0.82 ± 0.1 | NS | −0.05 ± 0.1* | −0.02 ± 0.0 | NS |
| Sagittal ⌀, cm | 17.2 ± 1.7 | 16.9 ± 2.2 | NS | n/a | n/a | n/a |
| Fat, % | 30.8 ± 4.47 | 27.7 ± 7.1 | NS | −0.8 ± 1.8 | −0.05 ± 1.5 | NS |
| Clinical hyperandrogenism | ||||||
| FG score | 11.2 ± 8.1 | 13.0 ± 8.8 | NS | 0.3 ± 4.2 | −1.3 ± 2.9 | NS |
| Acne (yes) | 0.58 ± 0.5 | 0.56 ± 0.5 | NS | n/a | n/a | n/a |
| Menstrual cycle pattern | ||||||
| Oligomenorrhea, n, % | 6 (50.0) | 6 (37.5) | NS | n/a | n/a | n/a |
| Amenorrhea, n, % | 6 (50.0) | 10 (62.5) | NS | n/a | n/a | n/a |
| Regular, n, % | 0 (0) | 0 (0) | NS | n/a | n/a | n/a |
| Ovarian function | ||||||
| Antral follicle, ≤9 mm (n) | 18.5 ± 6.3 | 21.8 ± 5.7 | NS | 1.5 ± 4.9 | −1.5 ± 4.0 | NS |
| Ovarian volume, cm3b | 6.62 ± 2.5 | 8.90 ± 3.0 | 0.041 | 0.3 ± 1.5 | −0.5 ± 3.1 | NS |
| Endometrial thickness, mm | 7.6 ± 2.7 | 7.1 ± 2.0 | NS | 0.3 ± 3.3 | −0.4 ± 2.1 | NS |
| AMH, μg/lc | 4.41 ± 3.4 | 5.49 ± 3.0 | NS | 0.045 ± 1.15 | −0.70 ± 1.76 | NS |
| Inhibin B, ng/l | 70.8 ± 39.8 | 88.9 ± 45.7 | NS | −5.82 ± 52.26 | −23.8 ± 34.0* | NS |
| Metabolism | ||||||
| P-glucose | 4.69 ± 0.32 | 4.63 ± 0.31 | NS | −0.15 ± 0.27 | 0.069 ± 0.24 | 0.023 |
| Cholesterol | 3.92 ± 0.66 | 4.22 ± 0.50 | NS | −0.23 ± 0.33* | 0.025 ± 0.49 | NS |
| LDL-cholesterol | 2.03 ± 0.78 | 2.37 ± 0.48 | NS | 0.04 ± 0.51 | 0.14 ± 0.33 | NS |
| HDL-cholesterol | 1.46 ± 0.31 | 1.47 ± 0.35 | NS | 0.033 ± 0.13 | −0.022 ± 0.21 | NS |
| Triglycerides | 0.90 ± 0.29 | 1.06 ± 0.61 | NS | −0.20 ± 0.18* | 0.055 ± 0.75 | NS |
| Insulin | 6.61 ± 3.01 | 6.46 ± 2.51 | NS | −1.68 ± 2.57 | −0.22 ± 2.03 | NS |
| HOMA | 1.38 ± 0.67 | 1.35 ± 0.60 | NS | −0.37 ± 0.60 | −0.01 ± 0.41 | 0.041 |
Values are means ± SD. AMH, anti-Müllerian hormone; BMI, body mass index; FG, Ferriman-Gallwey; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; L, liter; LDL, low-density lipoprotein; n/a, not applicable; NS, not significant; WHR, waist-to-hip ratio; ⌀, diameter.
Attention control, n = 10.
Ovarian volume is 0.523 × length × width × height.
Attention control, n = 11; Acupuncture, n = 14.
Between-group differences were determined with the Mann-Whitney U-test.
P < 0.05 versus baseline for within-group differences determined by the Wilcoxon rank-sum test.
Table 3.
Baseline LH, FSH, and LH pulsatility and changes from baseline after 10–13 wk of treatment
| Attention Control (n = 12) | Acupuncture (n = 16) | Attention Control (n = 12) | Acupuncture (n = 16) | |||
|---|---|---|---|---|---|---|
| Variable |
Baseline |
†P |
Δ (endpoint-baseline) |
†P | ||
| LH, IU/l | 3.19 ± 1.73 | 6.03 ± 3.59 | 0.026 | 1.11 ± 2.32 | −0.35 ± 3.04 | NS |
| FSH, IU/l | 4.20 ± 1.70 | 4.37 ± 1.88 | NS | −0.19 ± 1.42 | 0.61 ± 1.28 | NS |
| LH/FSH | 0.94 ± 0.89 | 1.36 ± 0.60 | 0.004 | 0.28 ± 0.70 | −0.24 ± 0.55 | NSa |
| LH pulsatility | ||||||
| Half-duration, min | 7.77 ± 4.12 | 6.69 ± 3.12 | NS | −2.19 ± 4.9 | −0.92 ± 3.24 | NS |
| Half-life, min | 69.60 ± 21.98 | 64.42 ± 16.29 | NS | 3.08 ± 29.5 | −1.51 ± 16.9 | NS |
| Secretion events, frequency/12 h | 8.92 ± 3.73 | 8.93 ± 3.83 | NS | 0.54 ± 4.5 | 0.41 ± 2.9 | NS |
| Total pulsatile production, IU/l/12 h | 21.01 ± 19.98 | 30.30 ± 16.45 | NS | 0.30 ± 22.6 | −0.9 ± 14.1 | NS |
| Interpulse interval, min | 72.93 ± 21.63 | 108.39 ± 114.12 | NS | 4.6 ± 43.8 | −32.3 ± 112.9 | NS |
| Lg ApEn | 0.91 ± 0.35 | 0.87 ± 0.40 | NS | 0.09 ± 0.4 | 0.13 ± 0.2* | NS |
| Secretion area, IU/l | 1.78 ± 1.50 | 2.80 ± 1.38 | 0.037 | 0.30 ± 1.57 | −0.27 ± 1.75 | NS |
Values are means ± SD. FSH, follicle-stimulating hormone; Lg ApEn, log10 approximate entropy; LH, luteinizing hormone.
Between-group differences were determined with the Mann-Whitney U-test.
P < 0.05 vs. baseline for within-group differences determined by the Wilcoxon rank-sum test.
After logistic regression with adjustment for baseline differences, NS.
Table 4.
Baseline sex steroids, precursors, and glucuronidated metabolites and changes from baseline after 10–13 wk of treatment
| Attention Control (n = 11) | Acupuncture (n = 15) | Attention Control (n = 11) | Acupuncture (n = 15) | ||||
|---|---|---|---|---|---|---|---|
| Variable |
Baseline |
†P |
Δ (endpoint-baseline) |
†P | Bonferroni correction | ||
| SHBG, nmol/la | 49.67 ± 25.5 | 40.40 ± 13.7 | NS | −6.0 ± 17.4 | 4.3 ± 16.4 | NS | NS |
| E1, pg/ml | 59.90 ± 34.1 | 57.41 ± 19.9 | NS | 2.87 ± 39.5 | −12.63 ± 15.7* | 0.008 | NS |
| E1-S, ng/ml | 0.96 ± 0.5 | 1.10 ± 0.9 | NS | 0.40 ± 0.5* | −0.40 ± 0.7* | 0.001 | 0.012 |
| E2, pg/ml | 42.84 ± 23.1 | 49.43 ± 24.9 | NS | 20.15 ± 30.0* | −21.38 ± 27.20** | 0.001 | 0.012 |
| DHEA, ng/ml | 4.75 ± 1.8 | 6.03 ± 2.5 | NS | 1.30 ± 1.6* | −1.74 ± 1.6** | <0.001 | 0.002 |
| DHEA-S, μg/ml | 1.72 ± 0.7 | 1.86 ± 0.7 | NS | 0.32 ± 0.6 | −0.35 ± 0.7* | 0.008 | NS |
| 4-DIONE, ng/ml | 1.07 ± 0.3 | 1.75 ± 0.5 | <0.001 | 0.27 ± 0.3* | −0.24 ± 0.4* | NSb | NS |
| T, ng/ml | 0.28 ± 0.04 | 0.39 ± 0.2 | NS | 0.26 ± 0.1 | −0.09 ± 0.2* | 0.018 | NS |
| Free T, pg/ml | 4.34 ± 1.47 | 6.83 ± 6.0 | NS | 1.05 ± 1.5* | −2.22 ± 6.4* | 0.004 | 0.048 |
| DHT, pg/ml | 91.40 ± 45.8 | 108.1 ± 47.0 | NS | 21.48 ± 38.5* | −14.64 ± 47.8 | 0.027 | NS |
| ADT-G, ng/ml | 72.43 ± 42.8 | 56.16 ± 22.5 | NS | 10.49 ± 12.3* | −8.23 ± 15.2 | 0.001 | 0.012 |
| AD3G, ng/ml | 0.85 ± 0.5 | 0.83 ± 0.5 | NS | 0.16 ± 0.3* | −0.14 ± 0.3 | 0.012 | NS |
| AD17G, ng/ml | 1.78 ± 1.7 | 1.82 ± 1.9 | NS | 0.10 ± 0.9 | −0.29 ± 0.5 | 0.040 | NS |
Values are means ± SD. AD3G, androstane-3α,17β-diol-3-glucuronide; AD17G, androstane-3α,17β-diol-17-glucuronide; ADT-G, androsterone glucuronide; DHEA, dehydroepiandrosterone; DHEA-S, DHEA sulfate; DHT, 5α-dihydrotestosterone; E1, estrone; E1-S, E1 sulfate; E2, estradiol; SHBG, sex hormone-binding globulin; T, testosterone; 4-DIONE, androstenedione.
Between-group differences were determined with the Mann-Whitney U-test.
P and
P, < 0.05 and < 0.01, respectively, vs. baseline for within-group differences determined by the Wilcoxon rank-sum test.
Attention control, n = 12; Acupuncture, n = 16.
After logistic regression with adjustment for baseline differences, NS.
Effect of the Intervention
During the study period, ovulation frequency was higher in the acupuncture group than in the attention control group (0.76 ± 0.27 vs. 0.41 ± 0.28 ovulations per month, P = 0.002; Fig. 2). There were no changes in LH or cortisol pulsatility after treatment, except for an increase in LH Log10 ApEn within the acupuncture group (Tables 3 and 5). However, there were no between-group differences in the Δ changes in any of the LH and cortisol variables between baseline and end of treatment assessments.
Fig. 2.
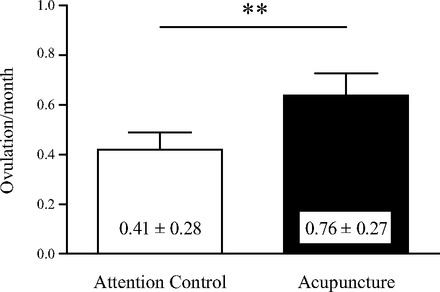
Ovulation frequency during treatment. Frequency is expressed as ovulations per month ± SD in women with polycystic ovary syndrome (PCOS) during 10–13 wk of acupuncture or attention control treatment. **P < 0.01 vs. attention control.
Table 5.
Cortisol pulsatility in women with PCOS at baseline and the changes from baseline after 10–13 wk of attention control or acupuncture treatment
| Attention Control (n = 12) | Acupuncture (n = 16) | Attention Control (n = 12) | Acupuncture (n = 16) | |||
|---|---|---|---|---|---|---|
| Variable | Baseline | †P | Δ (endpoint-baseline) | †P | ||
| Cortisol | 162.9 ± 51.4 | 150.4 ± 53.6 | NS | −10.09 ± 46.8 | 3.21 ± 47.3 | NS |
| Cortisol pulsatility | ||||||
| Half-duration, min | 7.85 ± 5.2 | 7.21 ± 4.92 | NS | −0.98 ± 4.9 | 0.27 ± 5.9 | NS |
| Half-life, min | 59.25 ± 17.2 | 52.60 ± 16.3 | NS | −2.91 ± 18.4 | 0.34 ± 23.1 | NS |
| Secretion events, frequency/12 h | 9.58 ± 1.93 | 9.44 ± 3.1 | NS | −0.17 ± 2.04 | 0.45 ± 4.5 | NS |
| Total production, nmol/l/12 h | 1790.1 ± 695.0 | 1810.4 ± 1029.4 | NS | 20.6 ± 723.7 | 89.2 ± 1020.5 | NS |
| Total pulsatile production, nmol/l/12 h | 1622.4 ± 592.5 | 1543.6 ± 874.1 | NS | −57.6 ± 495.0 | 12.6 ± 767.7 | NS |
| Interpulse interval, min | 53.58 ± 13.5 | 56.51 ± 13.8 | NS | 0.92 ± 13.1 | −0.94 ± 20.1 | NS |
| Lg ApEn | 0.49 ± 0.11 | 0.47 ± 0.12 | NS | 0.04 ± 0.2 | 0.01 ± 0.1 | NS |
| Secretion area, nmol/l | 112.6 ± 36.4 | 100.6 ± 47.1 | NS | 5.24 ± 61.8 | 6.32 ± 52.5 | NS |
Values are means ± SD.
Between-group differences were determined with the Mann-Whitney U-test.
Significant changes in plasma glucose levels and HOMA from baseline to end of treatment occurred between groups, but no significant differences were seen within the groups (Table 2). There were no anthropometric differences between the groups, but weight, BMI, waist circumference, and WHR decreased in the attention control group, and waist circumference decreased in the acupuncture group (Table 2).
Means and AUCs of calculated free T and all measured serum sex steroids, their precursors, and their metabolites decreased from baseline to end of treatment in the acupuncture group and differed significantly between the groups. The only exceptions were mean SHGB, the mean and AUC for 4-DIONE, and the AUC for AD17G (Table 4 and Fig. 3). After correction for multiple comparisons, differences remained for the means of E1-S, E2, DHEA, free T, and ADT-G (Table 4) and for the AUCs of E1-S, E2, DHEA, and ADT-G (Fig. 3). Within the acupuncture group, means and AUCs for E1, E1-S, E2, DHEA, DHEA-S, 4-DIONE, T, and free T decreased from baseline to end of treatment. Within the attention control group, means and AUCs for E1-S, E2, DHEA, 4-DIONE, free T, DHT, ADT-G, and AD3G, and the AUC of DHEA-S, increased from baseline to end of treatment (Table 4 and Fig. 3). Acupuncture reduced circulating inhibin B from baseline to end of treatment, but the between-group difference was not significant (P = 0.075) (Table 2).
Fig. 3.
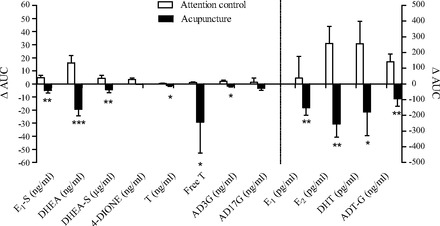
Changes in the area under the curve from baseline to end of treatment for sex steroids, sex steroid precursors, and glucuronidated androgen metabolites in women with PCOS after 10–13 wk of acupuncture or attention control treatment. Values are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. attention control before Bonferroni correction.
Correlations Between Ovulation Frequency During the Study and All End-Of-Treatment Variables
Spearman's rank correlation analyses were carried out for all variables (not groupwise), with ovulation frequency as the dependent variable and end-of-treatment measurements as the independent variables. E1, E1-S, E2, free T, and AD3G were all negatively correlated with ovulation frequency (Table 6).
Table 6.
Correlation between ovulation frequency and endpoint values of measured variables in the entire study group to explain ovulation
| Variable | Rs | P |
|---|---|---|
| E1 mean, pg/ml | −0.578 | 0.002 |
| E1 AUC | −0.580 | 0.002 |
| E1-S mean, ng/ml | −0.467 | 0.019 |
| E1-S AUC | −0.456 | 0.022 |
| E2 mean, pg/ml | −0.454 | 0.023 |
| E2 AUC | −0.452 | 0.023 |
| AD3G mean, ng/ml | −0.408 | 0.043 |
| Free T mean, pg/ml | −0.447 | 0.025 |
| Free T AUC | −0.445 | 0.026 |
Correlations were determined by Spearman's rank correlation. P < 0.05 was considered significant.
DISCUSSION
In the present study, we demonstrate that repeated acupuncture treatments with manual and electrical stimulation in lean/overweight women with PCOS results in a higher ovulation frequency during the treatment period than in the control group. This effect can be separated from the attention involved in meeting with a therapist for the same amount of time, but it is not associated with a decrease in the LH pulsatility pattern. Instead, the effect on ovulation frequency appears to be related mainly to a decrease in circulating sex steroids, their precursors, and their glucuronidated androgen metabolites but also to a decrease in inhibin B. These results are novel and extend previous RCTs on the effect of acupuncture with manual and electrical stimulation on reproductive function in women with PCOS (15, 31). The major difference between the present study and earlier RCTs is the increased treatment frequency and the attention control that does not include sham acupuncture.
The higher ovulation frequency during the treatment period in the acupuncture group confirms our previous data that showed increased menstrual frequency after 14 acupuncture treatments (15). Although we have no data on ovulation frequencies before the start of the study, there was no difference between the groups in terms of menstrual cycle pattern prior to the start of the study. This clearly indicates that the higher ovulation frequency in the acupuncture group can be attributed to a treatment effect. Importantly, ovulation frequency in the control group was also high (41%) and was comparable to clomiphene citrate stimulation and to the frequency seen in the study by Pastore et al. that used sham needles and fewer treatments (21, 31). This observation suggests a specific treatment effect by acupuncture that is separate from the less pronounced effect of meeting with a therapist. Even though ovulation frequency was here established during a treatment period of 10–13 wk, it is possible that the long-term effect is similar to our previous study, where menstrual frequency remained increased 16 wk after treatment ended (15). We previously reported that increased menstrual frequency is associated with a decrease in total T. In this study, we explored whether central gonadotropin release or changes in ovarian responsiveness are involved in the underlying mechanism behind the observed increase in menstrual frequency/ovulation with acupuncture therapy. Previous studies have indicated a role for LH in ovarian hyperandrogenism, and downregulation of LH secretion correlates with increased ovulation frequency and reduced levels of total T and 4-DIONE (4, 36). In the recent RCT by Pastore et al, they found that circulating LH levels and the LH:FSH ratio decreased, but these decreases were the same in both the true and sham acupuncture groups (31). Repeated acupuncture treatments with combined manual and electrical stimulation in our present study, however, did not affect LH levels or pulsatility pattern. However, it is possible that the increased ovulation frequency induced by acupuncture did lead to a slowing of the LH pulsatility frequency during the luteal and early follicular phases followed by a normal increase in pulsatility in the late follicular phase preceding the next ovulation (33). Our timing of measurements may have been to late to discriminate possible differences in LH pulsatility between groups. Furthermore, although there were no within-group changes, we found a between-group difference in Δchange of glucose metabolism that could be due to the concurrent improvement in anthropometrics (weight, BMI, waist circumference, and WHR) within the attention control group.
Our results suggest that the effects of acupuncture are mediated at the peripheral level. This is based on the observed reductions in most circulating estrogens, androgens, and glucuronidated androgen metabolites after correction for multiple comparisons. These changes involve steroids of mainly ovarian but also of adrenal origin and, along with a lack of effect on LH pulsatility, were all accompanied by a higher ovulation frequency in the acupuncture group. However, we cannot exclude that, due to the timing of the measurements discussed above, we missed an effect on LH pulsatility earlier in the follicular phase that could explain the reduction in the ovarian part of sex steroids. The higher ovulation frequency in the acupuncture group is probably not explained by improved anthropometrics, although waist circumference was decreased. Additionally, when associating ovulation frequency with the endpoint values measured in all of the participants regardless of treatment group, we found an association with a reduction in the levels of estrogens, free T, and AD3G. This is an indication that reductions in sex steroids are important for the induction of ovulation.
Other factors that may affect ovarian function and sex steroid secretion are ovarian AMH and inhibin B. ΔChanges in AMH and inhibin B did not differ between the groups despite a significant reduction in inhibin B within the acupuncture group. Inhibin B is associated with FSH levels, correlates to total follicle number, and may be a marker of follicle health (5, 43). The decrease in circulating sex steroids and the reduction of inhibin B within the acupuncture group suggests that the effect of acupuncture is of ovarian origin even though no changes were seen in ovarian morphology. Furthermore, adrenal sex steroid production was decreased by acupuncture, but we saw no changes in cortisol pulsatility. This suggests a peripheral adrenal effect, although a suppression of the hypothalamic-pituitary-adrenal axis cannot be excluded because we did not measure adrenocorticotropic hormone levels. Thus, the effect of acupuncture at both the ovarian and adrenal levels suggests a central underlying control.
Women with PCOS have generally high muscular sympathetic nerve activity that is associated with high circulating T (42). This is supported by data showing dense ovarian innervation by catecholaminergic nerve fibers in women with PCOS (14, 20, 42). We suggest that the effect of acupuncture is at least in part mediated via modulation of both general and ovarian (peripheral) sympathetic activity (37, 39, 40). The acupuncture needles in the present study were placed in the same somatic innervation as the sympathetic innervation of the ovaries. This placement was similar to that used in previous RCTs (15, 31), where we demonstrated a decrease in sympathetic nerve activity (39). Data obtained in rats also show that acupuncture with low-frequency electrical stimulation decreases ovarian expression of nerve growth factor (40, 41) and increases ovarian blood flow. These effects are mediated via ovarian sympathetic nerves (37) and controlled via the central nervous system (37). Additional acupuncture studies have demonstrated a reduction of hypothalamic androgen receptor, gonadotropin-releasing hormone, steroid receptor, and opioid receptor expression together with restored estrous cycles (10, 11). Thus, although we did not observe an effect on LH pulsatility or secretion pattern, it remains possible that the effect on sex steroid secretion could be mediated via the brain.
In PCOS, both central and peripheral opioid activities seem to be altered, although the data are conflicting (9). Experimental and clinical data indicate involvement of the central opioid system in the regulation of ovulation and sex steroid secretion by acupuncture in PCOS, although not included in this study (2, 9–11). Acupuncture with low-frequency electrical stimulation implies a more general effect on the sympathetic nervous system, including a decrease in muscle sympathetic nerve activity and circulating levels of β-endorphins (39, 45).
In rats, the effect of electro-acupuncture seems to be dose related (10, 16, 25, 26). In the current study, we increased the number of treatments to almost twice those in our previous RCT and the study by Pastore et al. (15, 31), and this may explain the high ovulation frequency and the strong effect on circulating sex steroids.
In the present study, we did not use placebo or sham needles or minimal acupuncture, because studies suggest that that these are not inert treatments (8, 22, 23, 28). Instead, our research question aimed to elucidate whether acupuncture is superior to the time and attention of meeting with the therapist.
In summary, we have shown that repeated acupuncture treatments result in a higher ovulation frequency in lean/overweight women with PCOS and are more effective than the attention and time involved in the meeting with a therapist. The reduction of both ovarian and adrenal serum sex steroids with no concomitant effect on LH and cortisol pulsatility or secretion pattern, together with the within-group reduction of inhibin B levels, indicate that the effect of acupuncture is mainly of peripheral origin. Acupuncture may represent an alternative or complementary therapy to standard pharmacological or surgical treatments, but clinical trials comparing acupuncture with these approaches need to be performed to determine the efficacy of such treatment.
GRANTS
This study (ClinicalTrials.gov Identifier: NCT00921492) was financed by grants from the Swedish Research Council (Project No. K2012–55X-15276-08-3), the Jane and Dan Olsson Foundations, the Novo Nordisk Foundation, The Hjalmar Svensson Foundation, the Adlerbert Research Foundation, Wilhelm and Martina Lundgrens's Science Fund, the Swedish federal government under the LUA/ALF agreement (ALFGBG-136481), and the Regional Research and Development Agreement (VGFOUREG-153221, -74721, and -17611). L. M. R is supported by NIH funding (R00 HD-060762).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.J., L.R., G.H., G.J., and E.S.-V. conception and design of research; J.J., A.S., and E.S.-V. performed experiments; J.J., P.P.V., F.L., and E.S.-V. analyzed data; J.J., P.P.V., G.J., and E.S.-V. interpreted results of experiments; J.J. and E.S.-V. prepared figures; J.J. and E.S.-V. drafted manuscript; J.J., L.R., P.P.V., A.S., F.L., G.H., G.J., and E.S.-V. edited and revised manuscript; J.J., L.R., P.P.V., A.S., F.L., G.H., G.J., and E.S.-V. approved final version of manuscript.
ACKNOWLEDGMENT
We thank Carola Gustafsson, Anna-Lena Jönsson, and Ingrid Hansson for technical assistance and Anders Odén for statistical advice. We acknowledge the use of the Pulse XP software (mljohnson.pharm.virginia.edu) and Norman A. Mazer, Boston University School of Medicine, Boston, MA, for the use of the spreadsheet for calculating free T (27).
REFERENCES
- 1. Anonymous. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 23: 462–477, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod 23: 2564–2569, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab 82: 3728–3733, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Chang RJ, Laufer LR, Meldrum DR, DeFazio J, Lu JK, Vale WW, Rivier JE, Judd HL. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab 56: 897–903, 1983 [DOI] [PubMed] [Google Scholar]
- 5. Chu MC, Carmina E, Wang J, Lobo RA. Mullerian-inhibiting substance reflects ovarian findings in women with polycystic ovary syndrome better than does inhibin B. Fertil Steril 84: 1685–1688, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab 82: 4179–4183, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38: 1165–1174, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Enblom A, Lekander M, Hammar M, Johnsson A, Onelov E, Ingvar M, Steineck G, Borjeson S. Getting the grip on nonspecific treatment effects: emesis in patients randomized to acupuncture or sham compared to patients receiving standard care. PLoS One 6: e14766, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eyvazzadeh AD, Pennington KP, Pop-Busui R, Sowers M, Zubieta JK, Smith YR. The role of the endogenous opioid system in polycystic ovary syndrome. Fertil Steril 92: 1–12, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Feng Y, Johansson J, Shao R, Manneras-Holm L, Billig H, Stener-Victorin E. Electrical and manual acupuncture stimulation affects estrous cyclicity and neuroendocrine function in a DHT-induced rat polycystic ovary syndrome model. Exp Physiol 97: 651–662, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Feng Y, Johansson J, Shao R, Manneras L, Fernandez-Rodriguez J, Billig H, Stener-Victorin E. Hypothalamic neuroendocrine functions in rats with dihydrotestosterone-induced polycystic ovary syndrome: effects of low-frequency electro-acupuncture. PLoS One 4: e6638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genazzani AD, Strucchi C, Luisi M, Casarosa E, Lanzoni C, Baraldi E, Ricchieri F, Mehmeti H, Genazzani AR. Metformin administration modulates neurosteroids secretion in non-obese amenorrhoic patients with polycystic ovary syndrome. Gynecol Endocrinol 22: 36–43, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 47: 93–99, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Heider U, Pedal I, Spanel-Borowski K. Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertil Steril 75: 1141–1147, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Jedel E, Labrie F, Oden A, Holm G, Nilsson L, Janson PO, Lind AK, Ohlsson C, Stener-Victorin E. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 300: E37–E45, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Johansson J, Yi F, Shao R, Lonn M, Billig H, Stener-Victorin E. Intense acupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of Polycystic ovary syndrome. Am J Physiol Endocrinol Metab 299: E551–E559, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Boyd DG, Evans WS. AutoDecon, a deconvolution algorithm for identification and characterization of luteinizing hormone secretory bursts: description and validation using synthetic data. Anal Biochem 381: 8–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, Gomez J, Candas B, Castiel I, Chaussade V, Deloche C, Leclaire J. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol 99: 182–188, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Belanger A, Vandenput L, Mellstrom D, Ohlsson C. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J Steroid Biochem Mol Biol 113: 52–56, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 133: 2690–2695, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 356: 551–566, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Linde K, Streng A, Jurgens S, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes MG, Weidenhammer W, Willich SN, Melchart D. Acupuncture for patients with migraine: a randomized controlled trial. JAMA 293: 2118–2125, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lundeberg T, Lund I, Sing A, Naslund J. Is placebo acupuncture what it is intended to be? Evid-Based Compl Alt 1–5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, Moher D. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. J Evid Based Med 3: 140–155, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Manneras L, Cajander S, Lonn M, Stener-Victorin E. Acupuncture and exercise restore adipose tissue expression of sympathetic markers and improve ovarian morphology in rats with dihydrotestosterone-induced PCOS. Am J Physiol Regul Integr Comp Physiol 296: R1124–R1131, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology 149: 3559–3568, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids 74: 512–519, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Melchart D, Streng A, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes M, Hummelsberger J, Irnich D, Weidenhammer W, Willich SN, Linde K. Acupuncture in patients with tension-type headache: randomised controlled trial. Br Med J 331: 376–382, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moret M, Stettler R, Rodieux F, Gaillard RC, Waeber G, Wirthner D, Giusti V, Tappy L, Pralong FP. Insulin modulation of luteinizing hormone secretion in normal female volunteers and lean polycystic ovary syndrome patients. Neuroendocrinology 89: 131–139, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Pasquali R, Casimirri F. The impact of obesity on hyperandrogenism and polycystic ovary syndrome in premenopausal women. Clin Endocrinol Oxf 39: 1–16, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Pastore LM, Williams CD, Jenkins J, Patrie JT. True and sham acupuncture produced similar frequency of ovulation and improved LH to FSH ratios in women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab 96: 3143–3150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel K, Coffler MS, Dahan MH, Yoo RY, Lawson MA, Malcom PJ, Chang RJ. Increased luteinizing hormone secretion in women with polycystic ovary syndrome is unaltered by prolonged insulin infusion. J Clin Endocrinol Metab 88: 5456–5461, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Rossmanith WG. Ultradian and circadian patterns in luteinizing hormone secretion during reproductive life in women. Hum Reprod 8, Suppl 2: 77–83, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Rotterdam T, group EAsPcw. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19: 41–47, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 1: 100–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steingold K, De Ziegler D, Cedars M, Meldrum DR, Lu JK, Judd HL, Chang RJ. Clinical and hormonal effects of chronic gonadotropin-releasing hormone agonist treatment in polycystic ovarian disease. J Clin Endocrinol Metab 9 65: 773–778, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Stener-Victorin E, Fujisawa S, Kurosawa M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J Appl Physiol 101: 84–91, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Stener-Victorin E, Holm G, Labrie F, Nilsson L, Janson PO, Ohlsson C. Are there any sensitive and specific sex steroid markers for polycystic ovary syndrome? J Clin Endocrinol Metab 95: 810–819, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 297: R387–R395, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Stener-Victorin E, Lundeberg T, Cajander S, Aloe L, Manni L, Waldenstrom U, Janson PO. Steroid-induced polycystic ovaries in rats: effect of electro-acupuncture on concentrations of endothelin-1 and nerve growth factor (NGF), and expression of NGF mRNA in the ovaries, the adrenal glands, and the central nervous system. Reprod Biol Endocrinol 1: 33, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stener-Victorin E, Lundeberg T, Waldenstrom U, Manni L, Aloe L, Gunnarsson S, Janson PO. Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol Reprod 63: 1497–1503, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Sverrisdottir YB, Mogren T, Kataoka J, Janson PO, Stener-Victorin E. Is polycystic ovary syndrome associated with high sympathetic nerve activity and size at birth? Am J Physiol Endocrinol Metab 294: E576–E581, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Torgac M, Kokcu A, Cetinkaya MB, Alper T, Malatyalioglu E. Do basal inhibin A and inhibin B levels have value in the diagnosis of polycystic ovary syndrome? Gynecol Endocrinol 20: 322–326, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus SM. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol Regul Integr Comp Physiol 281: R1975–R1985, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Yao T, Andersson S, Thoren P. Long-lasting cardiovascular depression induced by acupuncture-like stimulation of the sciatic nerve in unanaesthetized spontaneously hypertensive rats. Brain Res 240: 77–85, 1982 [DOI] [PubMed] [Google Scholar]


