Abstract
There is a continuous debate about the extent and prognostic value of retroperitoneal lymphadenectomy in endometrial cancer. Systematic pelvic and para-aortic lymphadenectomy in endometrial cancer provides a more accurate assessment of neoplastic spread and may help in better individualization of patients for adjuvant therapy. To evaluate the risk and pattern of retroperitoneal lymph nodes metastasis in patients with endometrial cancers having intermediate and high risk factors for nodal metastasis and recurrence. We conducted a prospective nonrandomized study of 62 cases of high risk endometrial cancers examined and treated at our regional cancer institute between the years 2008 and 2012. The inclusion criteria: The intermediate risk; all patients having grade 3 or undifferentiated adenocarcinomas with less than half MI and the grade 1, 2 tumors having more than half MI with tumor size >2 cm. The high risk group; all the patients having grade 3 or undifferentiated adenocarcinomas with more than half MI, the grade 1, 2 tumors with lymph vascular space invasion (LVSI) or cervical stromal invasion as depicted by pre-operative MRI. The type 2 histology uterine papillary serous, clear cell and squamous cell carcinomas. The patients staging was carried out according to the classification established by the FIGO for endometrial cancer in 2009. The Chi-square test was used to analyze the correlation between tumor grade, myometrial invasion, size of the lesion and lymph nodes metastasis and Fisher’s correction done whenever the frequency distribution was less than five. The patients mean age was 58.3 (range 31 to 76 years). A total of 118 endometrial cancer patients were treated during the study period. The 56 (47.5 %) patients belonged to low risk and 62 (52.5 %) patients belonged to high risk endometrial cancers. The 52 of 62 cases were eligible for the analysis. The 10 patients’ were excluded from further analysis as the post operative specimens final histopathologic examinations in nine cases revealed carcinosarcoma uterus and one case with yolk sac tumor of endometrium. The total 17(32.7 %) of 52 cases had retroperitoneal nodes metastasis; nine of 17 (52.9 %) in this group had both pelvic and para-aortic lymph nodal metastasis and one of 17 (5.9 %) had isolated para-aortic lymph nodal metastasis. The high grade tumors (grade 3) revealed 41.4 % pelvic and 20.7 % para-aortic lymph nodes metastasis and there was statistically significant higher nodal metastasis in both pelvic and para-aortic lymph nodes with increasing depth of myometrial invasion (P = 0.0119 and P = 0.0001) and increasing size of the lesion. (P = 0.04 and P = 0.0501). The intermediate and high risk endometrial cancer is associated with greater degree of lymph node metastasis. A complete surgical staging which involves extrafascial hysterectomy or a type 3 radical hysterectomy when there is a cervical involvement, along with bilateral salphingo-oophorectomy, pelvic, para-aortic lymphadenectomy and an omentectomy when indicated as in the present study, is a valuable modality of treatment in intermediate and high risk cases of endometrial cancers for determining the prognosis and appropriate categorization of these women for adjuvant therapy. It is also possible to achieve a complete surgical staging in these groups of women with acceptable morbidity when performed by a trained gynaecologic oncologist.
Keywords: Endometrial cancer, Intermediate and high risk, Retroperitoneal lymph nodal metastasis
Introduction
Endometrial cancer encompasses a group of tumors affecting the endometrium. World wide it is the sixth most common cancer in women [1]. Global incidences vary due to differentiation in the risk factors, especially use of unopposed estrogens such as hormone replacement therapy (HRT), prevalence of polycystic ovarian disease and Obesity. The age standardized incidence is 13 per 100,000 women per year in developed countries compared with 5.9 per 1,000,000 per year in developing countries [1].
To date, the therapeutic role of lymphadenectomy in presumed stage I endometrial cancer is not well established, but its ability to modify adjuvant therapy is being increasingly accepted [2]. The systematic reviews reveal there is no proven benefit and proven harms to systematic pelvic lymph nodes dissection [2]. Currently, routine pelvic and para aortic lymph node dissection is not the standard of practice and it varies from center to center and country to country.
There is a continuous debate about the extent and prognostic value of retroperitoneal lymphadenectomy in endometrial cancer. Many authors have opined in their retrospective studies, the role of systematic pelvic and para-aortic lymphadenectomy as therapeutic and had improved overall survival in intermediate and high risk for nodal metastasis in endometrial cancers [3–7]. The author’s previous retrospective study revealed high-grade endometroid adenocarcinomas, deep myometrial tumor invasion, were associated with significantly higher incidence of retroperitoneal nodal metastases in comparison with low grade tumors, and superficial or less than half of myometrial tumor invasion [8]. Therefore, we undertook a prospective nonrandomized study which aimed to analyze the risk factors and pattern of pelvic and para-aortic lymph nodes metastasis in women with intermediate and high risk endometrial cancer.
Material and Methods
The present non randomized and prospective study was conducted in the Department of Gynaecologic Oncology. Sixty two women with intermediate and high risk endometrial cancers were prospectively examined and treated at our regional cancer institute between the years 2008 and 2012. The study was approved by the Departmental Review board.
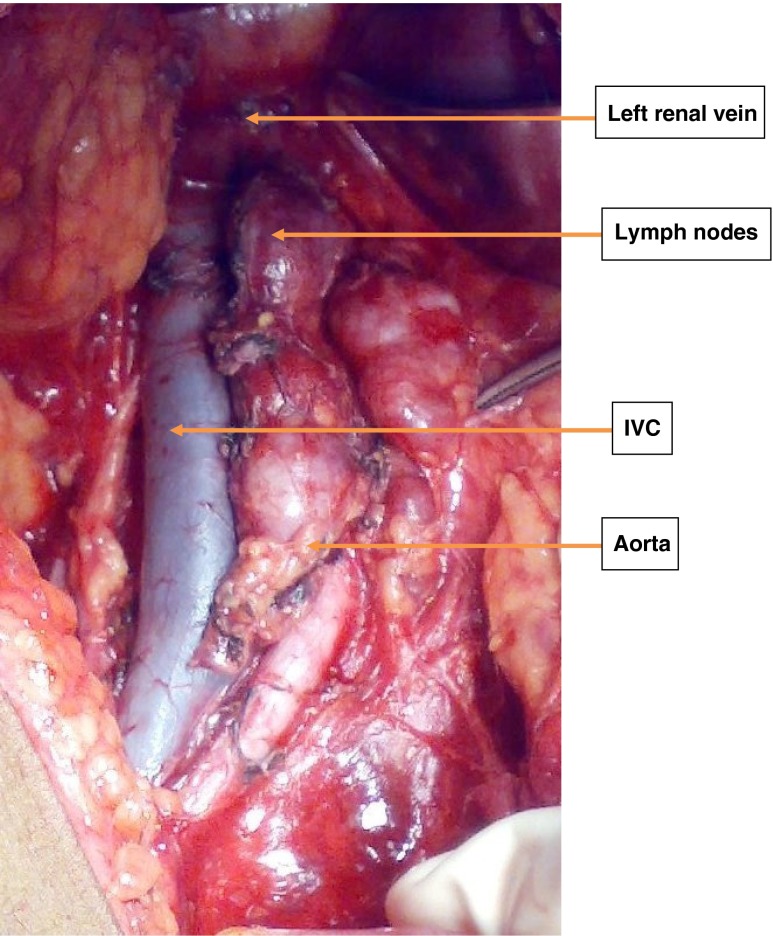
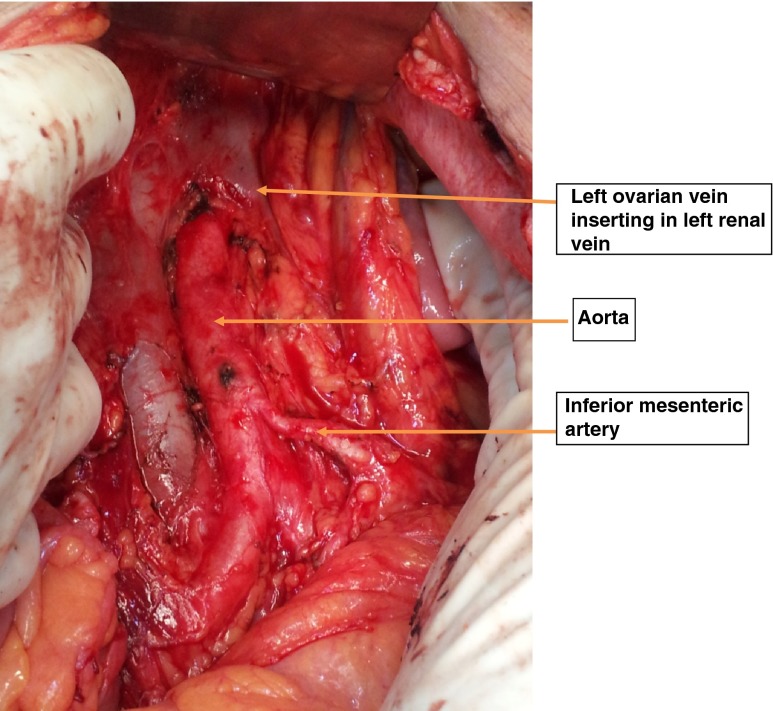
As a departmental policy, all women underwent an extrafascial hysterectomy with bilateral salpingo-opherectomy, pelvic and para-aortic lymph nodes dissection up to renal vessels (Figs. 1 and 2). Omentectomy was performed in women with an endometrial histopathology of an endometroid adenocarcinoma having a uterine serosal invasion, uterine papillary serous carcinoma (UPSC) and clear cell carcinoma. A type 3 radical hysterectomy was performed in women with suspected cervical extension. All the surgical specimens were reviewed and reported by gynecologic oncopathologists. They followed the histopathologic criteria defined by WHO [9].
Fig. 1.
The enlarged para aortic lymph nodes. The exposed retroperitoneal area showing enlarged chain of para aortic lymph nodes in one of the high risk case of endometrial cancer
Fig. 2.
The para aortic area after the lymph adenectomy. The retroperitoneal area after the para aortic lymphadenectomy showing right ovarian vein, inferior mesenteric artery and aorta
The European Society for Medical Oncology (ESMO) and French consensus risk stratification criteria was used to classify as low, intermediate and high risk endometrial cancers [10]. The patients belonging to intermediate and high risk groups are selected for the study. The preoperative parameters, endometrial biopsy histopathology revealing type and grade, radiological study by MRI or Ultrasonography revealing the preoperative size of lesion, myometrial invasion (MI) and cervical invasion (CI) were used for initial enrollment.
The inclusion criteria: The intermediate risk; all patients having grade 3 or undifferentiated adenocarcinomas with less than half MI and the grade 1, 2 tumors having more than half MI with tumor size >2 cm. The high risk group; all the patients having grade 3 or undifferentiated adenocarcinomas with more than half MI, the grade 1, 2 tumors with lymph vascular space invasion (LVSI) or cervical stromal invasion as depicted by pre-operative MRI. The type 2 histology uterine papillary serous, clear cell, and squamous cell carcinomas.
Exclusion criteria: low risk cases; grade 1 and 2 tumors having less half MI with small sized lesion ≤2 cm, carcinosarcomas, endodermal stromal tumors. The carcinosarcomas are classified under uterine sarcomas [11–13]. Final stage allocation was done according to the FIGO staging for endometrial cancer in 2009[14].
Statistical Software
SPSS 15.0, Stata 8.0, MedCalc 9.0.1 and Systat 11.0 were used forthe analysis of the data. The Chi-square test was used to analyze the correlation between tumor grade, myometrial invasion, size of the lesion and lymph nodes metastasis and Fisher’s correction done whenever the frequency distribution was less than five [15, 16].
Results
The mean age of the women was 58.3 (range 31 to 76 years). A total of 118 endometrial cancer patients were treated during the study period. The 56 (47.5 %) patients belonged to low risk and 62 (52.5 %) patients belonged to high risk endometrial cancers. The 52 of 62 women were eligible for the analysis. Ten women were excluded from further analysis as the final histopathology revealed 9 of them with carcinosarcoma and one among them as yolk sac tumor of endometrium (Table 1). The 28 of 52 (53.8 %) patients were intermediate risk and 24 of 52 (46.2 %) were high risk during the study period. Histopathologic variations in the grades, type of tumour and FIGO stage are shown in Tables 1 and 2. The mean pelvic and para-aortic lymph nodes retrieved were 14 nodes (ranged from 12 to 22 nodes) and 6 nodes (ranged from 4 to 11 nodes) respectively. The average operative time was 2 h (ranged from 1 h 30 min to 3 h 15 min). A total of 17of 52 (32.7 %) of the women had retroperitoneal nodal metastasis; nine of 17 (52.9 %) in this group had both pelvic and para-aortic lymph nodal metastasis, one of 17 (5.9 %) had isolated para-aortic lymph nodal metastasis and remaining seven of 17 (41.2 %) had only pelvic nodal metastasis. Table 3 denotes the correlation between the grades of tumor, types of tumor, myometrial invasion (MI) and the size of lesion with the pelvic and para-aortic lymph nodes metastasis. There was statistically significant higher nodal metastasis in both pelvic and para-aortic lymph nodes with increasing depth of myometrial invasion (p = 0.0119 and p = 0.0001) and increasing size of the lesion. (p = 0.04 and p = 0.0501). Totally 13 women had additional omentectomy due to grossly visible uterine serosal invasion found intra-operatively in 07, UPSC in 03 and clear cell carcinoma in 03 women respectively. Further analysis revealed 3 of 13 (15.4 %) women had omental metastasis (Table 4).
Table 1.
Clinical features, pre and post-operative histopathologic variations
| Total number of endometrial cancers treated during study | 118 | |
| Low risk patients | 56 (47.5 %) | |
| High risk patients (analyzed) | 62 (52.5 %) | |
| Mean age (range) years | 58.4 (36–74) | |
| Nulliparous | 09 (17.3 %) | |
| Multiparous | 43 (82.7 %) | |
| Grades and histopathology types | Pre-operative | Post-operative |
| Grade 1 adenocarcinoma | 08a | 02 |
| Grade 2 adenocarcinoma | 12 | 13 |
| Grade 3 adenocarcinoma | 30 | 29 |
| Undifferentiated Carcinoma | 04 | 01 |
| Uterine Papillary Serous Carcinoma | 04 | 03 |
| Clear Cell Carcinoma | 04 | 03 |
| Squamous Cell Carcinoma of endometrium | – | 01 |
| Carcinosarcomas uterus | – | 09b |
| Yolk Sac tumor endometrium | – | 01b |
| Total | 62 | 62 |
aFour cases upgraded to grade 2, and 2 other cases upgraded to grade 3 and carcinosarcomas each
bTen patients were further excluded and a total of 52 patients were eligible for the final analysis
Table 2.
FIGO Stage
| I | 28/52 (53.8 %) |
| IA | 15 |
| IB | 13 |
| II | 05/52 (9.6 %) |
| III | 19/52 (36.5 %) |
| IIIA | 1 |
| IIIB | 1 |
| IIIC1 | 7 |
| IIIC2 | 10 |
Table 3.
Correlations of grades, types, myometrial invasion (MI), size of lesion and lymph nodes metastasis
| Risk factors | No. of patients | Pelvic nodes mets | Para-aortic nodes mets | |||
|---|---|---|---|---|---|---|
| Grade 1 | >1/2 MI | 02 | – | – | ||
| Total | 02 | – | – | |||
| Grade 2 | <1/2 MI | 00 | – | – | ||
| >1/2 MI | 13 | 2 (15.4%) | 1 (7.7%) | |||
| Serosa | 00 | – | – | |||
| Total | 13 | 2 (15.4%) | 1 (7.7%) | |||
| Grade 3 | <1/2 MI | 15 | 2 (13.3%) | – | ||
| >1/2 MI | 09 | 5 (55.5%) | 1 (11.1%) | |||
| Serosa | 05 | 5 (100%) | 5 (100%) | |||
| Total | 29 | 12 (41.4%) | 6 (20.7%) | |||
| Undifferentiated grade | 01 | – | – | |||
| Uterine papillary serous carcinoma | 03 | 1 (33.3%) | 2(66.7%) | |||
| clear cell carcinoma | 03 | 1(33.3%) | 1 (33.3%) | |||
| Squamous cell carcinoma endometrium | 01 | – | – | |||
| Myometrial invasion (MI) | ||||||
| <1/2 | 18 | 02 (11.1%) | P = 0.0119 | 00 | P = 0.0001 | |
| >1/2 | 27 | 09 (33.3%) | 04 (14.8%) | |||
| Serosa | 07 | 05 (71.4%) | 06 (85.7%) | |||
| Size of lesion | ||||||
| <2cma | 13 | 01 (7.7%) | P = 0.04 | 00 | P = 0.0501 | |
| >2cm | 39 | 15 (38.5%) | 10 (25.6%) | |||
aThe patients having lesion size <2cm had > ½ MI & some had grade 3 lesions, i.e. intermediate risk group
Table 4.
Omental metastasis
| Omentectomy (13 cases) | Endometroid adenoca with serosal invasion | UPSC | Clear cell Ca |
|---|---|---|---|
| 2/13 (15.4 %) | 1/7 (14.3 %) | 1/3 (33.3 %) | 0/3 |
The Table 5 reveals the patients with lymph node positivity and number of risk factors in each case. The 13 of 17 (76.5 %) nodes positive cases belonged to high risk group, the eight cases of these 13 (61.5 %) had both pelvic and para-aortic nodal mets, four cases had only pelvic nodal mets and one case had isolated para-aortic nodal mets. The four of 17 (23.5 %) cases belonged to intermediate risk, the three of these four cases had only pelvic and other one case had both pelvic with para-aortic nodal mets. The high risk groups tend to have more of both pelvic and para-aortic nodal metastasis in comparison with intermediate risk group which had mainly pelvic nodal metastasis.
Table 5.
The patients with lymph node positivity and risk factors in each case
| S No | Lymph node mets | Number of risk factors | |
|---|---|---|---|
| Pelvic nodes | Para-aortic nodes | ||
| Case 5 | yes | yes | Grade 3, MI up to serosa and > 2 cm size |
| Case 7 | yes | yes | Clear cell ca, > half MI, and > 2 cm size |
| Case 11a | yes | - | Grade 2, > half MI, and > 2 cm size |
| Case 13 | yes | yes | UPSC, > half MI, and > 2 cm size |
| Case 14a | yes | yes | Grade 2, > half MI and > 2 cm size |
| Case 15 | yes | – | Grade 3, > half MI, and > 2 cm size |
| Case 16 | yes | yes | Grade 3, MI up to serosa and > 2 cm size |
| Case 23 | yes | yes | Grade 3, > half MI and > 2 cm size |
| Case 26 | yes | – | Grade 3, > half MI and > 2 cm size |
| Case 29 | yes | – | Grade 3, > half MI and > 2 cm size |
| Case 30 | yes | yes | Grade 3, MI up to serosa and > 2 cm size |
| Case 34 | yes | – | Grade 3, > half MI, and < 2 cm size |
| Case 37 | yes | yes | Grade 3, MI up to serosa and > 2 cm size |
| Case 40a | yes | – | Grade 3, < half MI and > 2 cm size |
| Case 42 | yes | yes | Grade 3, MI up to serosa and > 2 cm size |
| Case 43 | – | yes | UPSC, > MI up to serosa and > 2 cm size |
| Case 46a | yes | – | Grade 3, < half MI and > 2 cm size |
aIntermediate risk group with nodal mets (4 cases)
High risk group with nodal mets (13 cases)
There were acceptable surgical morbidities; three cases with trivial vascular injuries and one case with bladder injury due to difficult hysterectomy repaired successfully. There were three events post-operatively viz., one patient had angina on 2nd day, another with abdominal wound dehiscence on 10th day recovered well and third woman discharged in good general condition on 6th day but she died of myocardial infarction on 14th day at home.
Discussion
The metastasis to lymph nodes is a major prognostic factor in patients with endometrial cancers. No preoperative diagnostic modalities can detect micro-metastases in lymph nodes. Therefore, if accurate surgical staging is to be obtained, then a complete retroperitoneal lymphadenectomy should be performed on all women having these risk factors for nodal metastasis and recurrences. Histopathologic examination provides the most accurate method of ascertaining the degree of metastatic spread to the lymph nodes and guides in planning the appropriate adjuvant therapy.
The therapeutic significance of combined pelvic and para-aortic lymphadenectomy for patients with endometrial cancer is controversial or it’s less understood [2]. The lymphadenectomy in the two large recently published randomized prospective trials; the ASTEC trial [17] and Benedetti-Panici and colleagues’ study [18] in Italy revealed no therapeutic benefit, but their study design had some limitations. In the ASTEC trial, the follow-up period was short, lymphadenectomy was selective rather than systematic, neither study included para-aortic lymphadenectomy and subsequent radiation was not tailored to the nodal status, so it is difficult to draw any definitive conclusions.
Findings from our study have shown that 32.7 % had retroperitoneal lymph nodal metastasis, more than half (52.9 %) in this group had both pelvic and para-aortic lymph nodal metastasis, and 5.9 % had isolated para-aortic lymph nodal metastasis. Therefore, the pelvic lymphadenectomy alone might have missed half the patients with para aortic nodal metastasis and it would have been a sub-optimal surgical procedure in this group of women, hence supporting the data published by Mariani A et al. [19] and Yokoyama Y et al. [20]. Mariani A et al. reported in their study, that more than half of patients with pelvic lymph node metastasis had para-aortic lymph node metastasis, and about 10 % of lymph node metastases occurred exclusively in the para-aortic region. A retrospective review of 841 women with endometrial cancer by Nomura et al. [21] reported in a multivariate analysis, the clinicopathologic factors most strongly related to para aortic nodal metastasis was pelvic lymph node metastasis; when the pelvic nodes were positive, 48 % (24 of 50 cases) also had positive para aortic nodes. These findings are also consistent with a study by Mariani A et al., from the Mayo clinic [22].
The GOG data [23] suggested that patients with positive para aortic nodes were likely to have; grade 2 or 3 lesions with outer-third myometrial invasion, grossly positive pelvic nodes, and grossly positive adnexae. Schink et al. [24] and Mariani et al. [25] reported the incidence of lymph node metastasis also correlated with tumor size <2 cm, >2 cm, entire cavity and cervical involvement was associated with about 4 %, 15 %, 35 % and 15 % risk of lymph node metastasis respectively. Disaia et al. [26] observed in their study, the extra uterine spread of diseases increases the risk of lymph node metastasis. Adnexal metastasis increases the risk for pelvic and para-aortic nodal metastasis to 32 % and 20 %, respectively. Furthermore, Niikura H et al., from their sentinel lymph node study reported, the para-aortic region has been shown to be an important site of sentinel nodes in endometrial cancer, with 47 % of para-aortic sentinel nodes located above the inferior mesenteric artery [27]. We had similar observations in our study. Grade 3 tumors revealed 41.4 % of pelvic and 20.7 % of para-aortic lymph node metastasis. In women with deep myometrial invasion, uterine serosal invasion and lesions >2 cms in size had statistically significant higher rate of retroperitoneal lymph nodes metastasis in comparison with less than half of myometrial invasion and lesions ≤ 2 cm in size. Intra-operatively seven women had grossly visible uterine serosal invasion and one (14.3 %) of the seven had omental metastasis.
Therefore we recommend, systematic pelvic and para aortic lymphadenectomy for surgical staging of endometrial cancer patients having intermediate and high risk factors for nodal metastasis and recurrences. Identification of these intrauterine and extra uterine risk factors allows for a more informed and appropriate use of postoperative adjuvant therapy. There are known risks and morbidity associated with lymphadenectomy, but these risks and morbidity decrease in the hands of trained Gynaecologic Oncologist. We believe that this approach of complete surgical staging and identification of risk factors for nodal metastasis will identify women who need tailored post operative adjuvant treatment.
Conclusion
The intermediate and high risk endometrial cancer is associated with greater degree of lymph node metastasis. A complete surgical staging which involves extrafascial hysterectomy or a type 3 radical hysterectomy when there is a cervical involvement, along with bilateral salphingo-oophorectomy, pelvic, para-aortic lymphadenectomy and an omentectomy when indicated as in the present study, is a valuable modality of treatment in intermediate and high risk cases of endometrial cancers for determining the prognosis and appropriate categorization of these women for adjuvant therapy. It is also possible to achieve a complete surgical staging in these groups of women with acceptable morbidity when performed by a trained gynaecologic Oncologist.
Acknowledgments
Conflict of interest
Authors report no conflict of interests.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v 2.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10 [Accessed on 12/01/13]
- 2.May K, Bryant A, Dickinson HO, Kehoe S, Morrison J (2010) Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev, Issue 1. Art. No.: CD007585. doi:10.1002/14651858.CD007585.pub2 [DOI] [PMC free article] [PubMed]
- 3.Chang SJ, Kim WY, Yoon JH, Yoo SC, Chang KH, Ryu HS. Para-aortic lymphadenectomy improves survival in patients with intermediate to high-risk endometrial carcinoma. Acta Obstet Gynecol Scand. 2008;87:1361–1369. doi: 10.1080/00016340802503054. [DOI] [PubMed] [Google Scholar]
- 4.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375(9721):1165–72. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 5.Mariani A, Dowdy S, Podratz K. The role of pelvic and para-aortic lymph node dissection in the surgical treatment of endometrial cancer: a view from the USA. Obstet Gynaecol. 2009;11:199–204. doi: 10.1576/toag.11.3.199.27505. [DOI] [Google Scholar]
- 6.Mariani A, Webb MJ, Galli L, Podratz KC. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol Oncol. 2000;76(3):348–56. doi: 10.1006/gyno.1999.5688. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer NL, Lurain JR. The role of lymphadenectomy in surgical staging of endometrial cancer. Int J Surg Oncol. 2011;2011:814649. doi: 10.1155/2011/814649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathod PS, Reddihalli PV, Krishnappa S, Devi UK, Bafna UD (2014) A retrospective clinicopathological study of 131 cases with endometrial cancers- is it possible to define the Role of Retroperitoneal Lymphadenectomy in Low Resourace Settings?. http://www.indianjcancer.com/aheadofprint.asp. Indian J Cancer [DOI] [PubMed]
- 9.Silverberg SG, Mutter GL, Kurman RJ, Kubik-Huch RA, Nogales F, Tavassoli FA. Tumors of the uterine corpus: epithelial tumors and related lesions. In: Tavassoli FA, Stratton MR, editors. WHO classification of tumors: pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 221–232. [Google Scholar]
- 10.Querleu D, Planchamp F, Narducci F, Morice P, Joly F, et al. Clinical practice guidelines for the management of patients with endometrial cancer in France: recommendations of the Institut National du Cancer and the Société Française d’Oncologie Gynécologique. Int J Gynecol Cancer. 2011 Jul;21(5):945–50. doi: 10.1097/IGC.0b013e31821bd473. [DOI] [PubMed] [Google Scholar]
- 11.Berek and Hacker’s Gynecologic Oncology, 5e. Chapter 10: Uterine Cancers; Uterine sarcomas. pp 429–30
- 12.DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology, 9e. Chapter 102: Cancers of the Uterine Body; Uterine Sarcomas. pp 1356–7
- 13.Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104(3):177–8. doi: 10.1016/j.ijgo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.FIGO Committee on Gynaecologic Oncology Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obst. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Rosner B (2000) Fundamentals of biostatististics, 5th edn, Duxbury
- 16.Reddy V. Statistics for mental health care research. India: NIMHANS publication; 2002. [Google Scholar]
- 17.Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study. Lancet. 2009;373(9658):125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panici PB, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 19.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama Y, Maruyama H, Sato S, Saito Y. Indispensability of pelvic and para aortic lymphadenectomy in endometrial cancers. Gynecol Oncol. 1997;64:411–17. doi: 10.1006/gyno.1996.4573. [DOI] [PubMed] [Google Scholar]
- 21.Nomura H, Aoki D, Suzuki N, Susumu N, Suzuki A, Tamada Y, et al. Analysis of clinicopathologic factors predicting para aortic lymph node metastasis in endometrial cancer. Int J Gynecol Cancer. 2006;16:799–804. doi: 10.1111/j.1525-1438.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- 22.Mariani A, Dowdy SC, Cliby WA, Haddock MG, Keeney GL, Lesnick TG, et al. Efficacy of systematic lymphadenectomy and adjuvant radiotherapy in node-positive endometrial cancer patients. Gynecol Oncol. 2006;10:200–208. doi: 10.1016/j.ygyno.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathologic riskfactors and outcome in clinical stage I and II carcinoma of theendometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-K. [DOI] [PubMed] [Google Scholar]
- 24.Schink JC, Lurain JR, Wallemark CB, Chmiel JS. Tumor size in endometrial cancer: a prognostic factor for lymph node metastasis. Obstet Gynecol. 1987;70(2):216–219. [PubMed] [Google Scholar]
- 25.Mariani A, Webb MJ, Keeney GL, Lesnick TG, Podratz KC. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. 2002;87(3):274–280. doi: 10.1006/gyno.2002.6836. [DOI] [PubMed] [Google Scholar]
- 26.Disaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in stage I endometrial cancer. Am J Obstet Gynecol. 1985;151(8):1009–1015. doi: 10.1016/0002-9378(85)90371-0. [DOI] [PubMed] [Google Scholar]
- 27.Niikura H, Okamura C, Utsunomiya H, et al. Sentinel lymph node detection in patients with endometrial cancer. Gynecol Oncol. 2004;92:669–74. doi: 10.1016/j.ygyno.2003.10.039. [DOI] [PubMed] [Google Scholar]