Abstract
Background:
Colorectal tumor is one of the main causes of death in our country. The aim of the present study was to determine the clinicopathological aspects of tumor and the presence of hepatic micrometastasis in patients with colorectal cancer (CRC).
Materials and Methods:
Forty two patients with CRC were evaluated in the study surgical treatment was performed and liver biopsy was taken for the evaluation of micrometastasis by immunohistochemistry and polymerase chain reaction. The variables that have been evaluated were: Patient's gender, patients age at the time of diagnosis, size and location of tumor, tumor-node-metastasis stage and grade of the primary tumor, lymph node involvement, lymphovascular and neural invasion, presence of macrometastasis and carcinoembryonic antigen level prior to surgery. After 1 year patients were called and asked to come back to the clinic for elective colonoscopy to evaluate the surgical site for recurrence of tumor and survival. All variables were compared between patients in whom liver micrometastasis were present in comparison with patients without liver micrometastasis.
Results:
Of the studied patients (6 with positive micrometatsis and 36 without micrometstasis), 38 were alive after 1 year (6 with positive micrometatsis and 32 without micrometstasis) and the difference was not significant between groups with or without micrometastasis (P = 0.52). In four of survived patients colonoscopy was abnormal, however this difference was not also significant between groups (P = 0.59).
Conclusion:
Clinicopathologic aspect of tumor was not different in CRC patients with and without hepatic micrometastasis.
Keywords: Colorectal cancer, immunohistochemistry, micrometastasis, polymerase chain reaction
INTRODUCTION
Colorectal cancer (CRC) is one of the main causes of death in western countries.[1,2] In our country CRC is the fourth most common cancer in women and the third in men respectively[3] and accounting for 6% of annual cancer death in Iran.[4] According to recent studies incidence of CRC has been increased in our society during recent 30 years.[5] However, the mortality rate has been decreased due to improvements of new methods in early detection and treatment of CRC.[6]
The treatment of choice for CRC patients depends on tumor location and stage at the time of diagnosis. Surgical treatment is commonly for early stages and chemotherapy with or without radiation therapy is often given to patients with late stages of disease.[7] The probability of recurrence is directly related to the stage of the cancer at the time of diagnosis. In stage I, 15% of patients will have cancer recurrence. This recurrence rate increases to 25% in stage II and 65% in stage III. Recurrence of cancer in patients without identified metastasis is thought to be due to residual cancer in a microscopic stage that called micrometastasis.[8] The liver is the most common site of tumor metastasis. Two-third of patients develop metastasis during the course of disease and 25% are being diagnosed with liver metastasis.[9] Based on the American Joint Committee on Cancer (AJCC) viewpoint, micrometastasis are lesions with the diameter between 0.2 and 2 mm.[10] Now-a-days neoadjuvant chemotherapy is used to remove micrometastatic tumor cells before they develop clinical disease. Hence early detection of micrometastasis could find the patients who derived more benefits from neoadjuvant therapy.[11] Currently, immunohistochemistry (IHC) and polymerase chain reaction (PCR) techniques are used for the detection of micrometastasis.[12,13,14,15,16] These techniques mainly focus on the detection of cytokeratins as specific marker of epithelial cells that have been evaluated in previous studies.[17,18] Today, there is an increasing trend in the use of neoadjuvant therapy in CRC patients for treatment of occult metastasis.[19,20,21] Therefore case selection for this purpose seems to be important. One of the points that can help the surgeon for selecting patients for neoadjuvant therapy is clinicopathological aspects of tumor. However, there is a controversy about the relationship between clinicopathological aspects of CRC and the presence of hepatic micrometastasis, and most of them were retrospective studies,[22,23] thus, we deigned this study with a prospective direction to determine the relationship between clinicopathological aspects of tumor and the presence of hepatic micrometastasis in CRC patients.
MATERIALS AND METHODS
Participants and setting
This study was a cohort study which was performed at Alzahra Hospital, a tertiary referral center in Isfahan, Iran. During a period of 8 months from April 2011 to January 2012, a total number of 44 consecutive patients who met the inclusion criteria were enrolled in the study. Eligible patients were individuals with a diagnosis of CRC who admitted for surgical intervention in the surgery ward of Alzahra Hospital. Patients were excluded if there was any synchronous cancer or administration of neoadjuvant chemotherapy for CRC prior to hospital admission.
Surgical and pathological procedures
All patients who enrolled in the study underwent laparotomy. Prior to surgery blood sample was taken for the determination of carcinoembryonic antigen (CEA) level. Before any intervention in the tumor site, liver has evaluated for macrometastasis and a 1 cm × 1 cm biopsy were taken from an area which was free of macrometastasis. Biopsies were taken from normal appearing tissue of liver segment 5 of right lobe for the tumors locating in right colon and transverse colon and for the tumors of rectum, sigmoid and left colon biopsies were taken from segment 3 of left lobe that sent for the further examinations.[24]
Standard surgical treatments were performed for each patient and the tumor resected. After resection of the tumor, lymph nodes were dissected from pericolic fat with care given to avoid contact with the primary tumor. Resected tissues including tumoral tissue and lymph node were sent to pathology laboratory.
Liver biopsies were evaluated for micrometastasis using IHC and PCR techniques for to examine the expression of cytokeratin 20 (CK20) by a single expert pathologist. For the PCR procedure specimens cut into 10-20 μm slides with cryosection machine, then ribonucleic acid (RNA) was extracted using modified single-step RNA isolation method and the integrity was checked using gel electrophoresis. In each circulation, there were one positive and negative control. More details about this technique is described in the previous studies that have been performed in this field.[25] Lymph node involvement, neuronal invasion and lymphovascular invasion were also evaluated by light microscopy.
Tumor grade and stage were also determined by the study pathologist. Grading was performed using the conventional grading system and staging was done tumor-node-metastasis (TNM) classification by the AJCC.[26]
Factors influencing hepatic micrometastasis
In this study, we evaluated eleven clinicopathologic variables and their relationship with the presence of hepatic micrometastasis. These variables were: Patient's gender and age at the time of diagnosis, size and location of tumor, TNM stage and grade of the primary tumor, lymph node involvement, lymphovascular and neural invasion, presence of macrometastasis and CEA level prior to surgery.
After 1 year, patients were called and asked to come back to the clinic for elective colonoscopy to evaluate the surgical site for recurrence of tumor and survival.
Sample size and statistical analyze
Sample size for this projects were calculated using statistical formula, considering α = 0.0.5 and β = 0.2. Statistical analysis was performed using the statistical software (SPSS, Inc., Chicago, IL, USA) version 16. Statistical difference considered as significant if P was <0.05.
Written informed consents were obtained from all patients for authorize use of their medical records for research purposes. The protocol of study was approved by Ethical Committee of our university (Project Number 30151).
RESULTS
During the period of study 44 patients were enrolled in the study, two of them were excluded due to administration of neoadjuvant chemotherapy before admission to our hospital. In the remaining 42 patients, 19 (45.23%) were males and 23 (54.77%) were females, aged ranges between 28 and 88 (61.97 ± 13.89). Micrometastasis was found in 6 (14.28%) patients considering IHC and PCR. IHC results were positive in 6 patients and PCR results also were positive in this 6 patients and another 36 were negative for IHC and PCR.
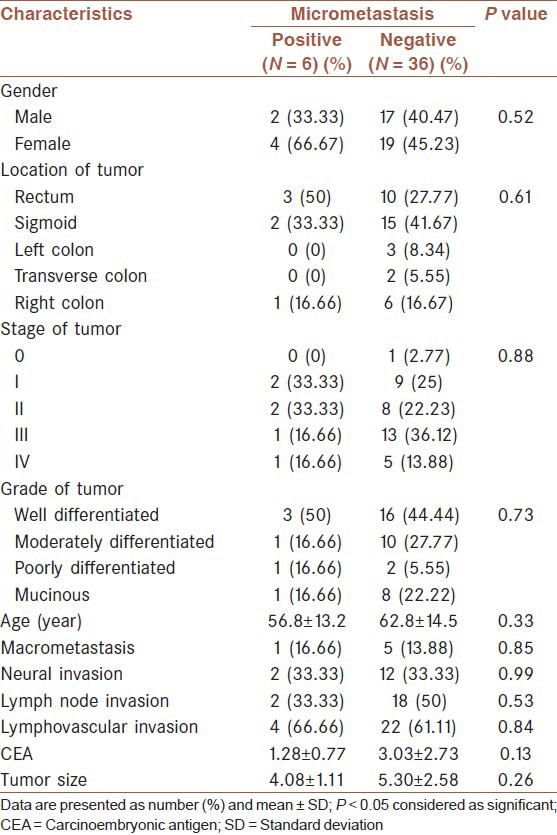
Data analyses showed that none of the study variables had a significant relationship with the presence of hepatic micrometastasis. Detailed data are shown in Table 1.
Table 1.
hepatic micrometastasis and clinicopathologic variables between two groups
After 1 year, patients were called. Of the 42 patients who enrolled in the study, 38 (90.47%) were alive and 4 (9.53%) patients were dying due to their tumor. All of four patients were belonged to micrometastasis negative group, however the difference was not statistically significant between two groups (P = 0.52).
Survived patients were asked to come to our clinic for elective colonoscopy. Of the 38 colonoscopy four was abnormal. One of them had hepatic micrometastasis and another three did not. The difference was not statistically significant (P = 0.59).
DISCUSSION
The aim of the present study was to determine the relation between some of the clinicopathologic aspects of colorectal tumor with the presence of micrometastasis. The presence of micrometastasis in this study was evaluated by IHC and PCR for the expression of CK20. CK20 is a member of cytokeratin family, which was first described by Moll et al., to be present in the vast majority of colorectal tumors.[27] Other studies found CK20 appears frequently on colonic tumors; however, it is absent in the normal hepatic parenchyma[27,28,29] and due to this matter CK20 became an accurate marker for discerning hepatic micrometastasis from the normal liver tissue. Our study results mainly showed that none of clinicopathologic variables had a significant relation with the presence of hepatic micrometastasis.
There is some evidence that neoadjuvant chemotherapy may reduce hepatic micrometastasis and cancer recurrence.[30] Wakai et al. in their study noted that neoadjuvant chemotherapy can reduce hepatic micrometastasis and cancer recurrence.[30] However, their study was a retrospective, and a question remained unanswered that which patients derive benefit from neoadjuvant chemotherapy. To answer this question tumor characteristics and its correlation with hepatic micrometastasis should be considered.
There are few studies, which evaluated the relation between clinicopathologic aspects of tumor and the presence of hepatic micrometastasis. In Yokoyama et al. study some of these variables such as gender, age, location of tumor and Duke stage have been evaluated.[31] In line with our results, they also revealed that none of these variables had a significant relation with the presence of micrometastasis.
Our data showed that the 1 year survival did not differ significantly between patients with or without micrometastasis; however, previous studies in this filed with longer follow-up time showed that the 10 year survival rate is significantly higher in patients without hepatic micrometastasis in comparison with patients with hepatic micrometastasis.[31]
Our study also had some limitations on the sample size and the time of follow-up, we suggest to perform following researches in this field with larger sample sizes to find clinicopathologic characteristic which correlate with hepatic micrometastasis.
CONCLUSION
Clinicopathologic aspect of tumor was not different in CRC patients with and without hepatic micrometastasis.
Footnotes
Source of Support: This study was a residency thesis which performed by the financial support from vice chancellery for Research of Isfahan University of Medical Sciences (Grant Number: 30151)
Conflict of Interest: None declared.
REFERENCES
- 1.Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS One. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 4.Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6:359–63. [PubMed] [Google Scholar]
- 5.Yazdizadeh B, Jarrahi AM, Mortazavi H, Mohagheghi MA, Tahmasebi S, Nahvijo A. Time trends in the occurrence of major GI cancers in Iran. Asian Pac J Cancer Prev. 2005;6:130–4. [PubMed] [Google Scholar]
- 6.Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 8.Ho SB, Hyslop A, Albrecht R, Jacobson A, Spencer M, Rothenberger DA, et al. Quantification of colorectal cancer micrometastases in lymph nodes by nested and real-time reverse transcriptase-PCR analysis for carcinoembryonic antigen. Clin Cancer Res. 2004;10:5777–84. doi: 10.1158/1078-0432.CCR-03-0507. [DOI] [PubMed] [Google Scholar]
- 9.Wang CC, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol. 2012;18:25–33. doi: 10.3748/wjg.v18.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicastri DG, Doucette JT, Godfrey TE, Hughes SJ. Is occult lymph node disease in colorectal cancer patients clinically significant? A review of the relevant literature. J Mol Diagn. 2007;9:563–71. doi: 10.2353/jmoldx.2007.070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–24. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 12.Bustin SA, Siddiqi S, Ahmed S, Hands R, Dorudi S. Quantification of cytokeratin 20, carcinoembryonic antigen and guanylyl cyclase C mRNA levels in lymph nodes may not predict treatment failure in colorectal cancer patients. Int J Cancer. 2004;108:412–7. doi: 10.1002/ijc.11596. [DOI] [PubMed] [Google Scholar]
- 13.Mohajeri G, Sanei MH, Tabatabaee SA, Hashemi SM, Amjad E, Mohajeri MR, et al. Micrometastasis in non-small-cell lung cancer: Detection and staging. Ann Thorac Med. 2012;7:149–52. doi: 10.4103/1817-1737.98848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrie AE, van Rij AM, Dennett ER, Phillips LV, Yun K, McCall JL. Prognostic significance of occult metastases in colon cancer. Dis Colon Rectum. 2003;46:221–31. doi: 10.1007/s10350-004-6527-z. [DOI] [PubMed] [Google Scholar]
- 15.Kronberg U, López-Kostner F, Soto G, Zúñiga A, Wistuba I, Miranda V, et al. Detection of lymphatic micrometastases in patients with stages I and II colorectal cancer: Impact on five-year survival. Dis Colon Rectum. 2004;47:1151–7. doi: 10.1007/s10350-004-0560-9. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda K, Adachi Y, Shiraishi N, Yamaguchi K, Hirabayashi Y, Kitano S. Pattern of lymph node micrometastasis and prognosis of patients with colorectal cancer. Ann Surg Oncol. 2001;8:300–4. doi: 10.1007/s10434-001-0300-5. [DOI] [PubMed] [Google Scholar]
- 17.Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S, et al. Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol. 2001;8:158–62. doi: 10.1007/s10434-001-0158-6. [DOI] [PubMed] [Google Scholar]
- 18.Noura S, Yamamoto H, Ohnishi T, Masuda N, Matsumoto T, Takayama O, et al. Comparative detection of lymph node micrometastases of stage II colorectal cancer by reverse transcriptase polymerase chain reaction and immunohistochemistry. J Clin Oncol. 2002;20:4232–41. doi: 10.1200/JCO.2002.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385–90. doi: 10.1245/s10434-009-0492-7. [DOI] [PubMed] [Google Scholar]
- 20.Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17:492–501. doi: 10.1245/s10434-009-0781-1. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: Friend or foe? Ann Surg. 2012;255:237–47. doi: 10.1097/SLA.0b013e3182356236. [DOI] [PubMed] [Google Scholar]
- 22.Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: Analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–9. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 23.Nanko M, Shimada H, Yamaoka H, Tanaka K, Masui H, Matsuo K, et al. Micrometastatic colorectal cancer lesions in the liver. Surg Today. 1998;28:707–13. doi: 10.1007/BF02484616. [DOI] [PubMed] [Google Scholar]
- 24.Schimanski CC, Linnemann U, Berger MR. Sensitive detection of K-ras mutations augments diagnosis of colorectal cancer metastases in the liver. Cancer Res. 1999;59:5169–75. [PubMed] [Google Scholar]
- 25.Cui JH, Krueger U, Henne-Bruns D, Kremer B, Kalthoff H. Orthotopic transplantation model of human gastrointestinal cancer and detection of micrometastases. World J Gastroenterol. 2001;7:381–6. doi: 10.3748/wjg.v7.i3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 27.Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Shimonishi T, Miyazaki K, Nakanuma Y. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology. 2000;37:55–63. doi: 10.1046/j.1365-2559.2000.00932.x. [DOI] [PubMed] [Google Scholar]
- 29.Litle VR, Warren RS, Moore D, 2nd, Pallavicini MG. Molecular cytogenetic analysis of cytokeratin 20-labeled cells in primary tumors and bone marrow aspirates from colorectal carcinoma patients. Cancer. 1997;79:1664–70. [PubMed] [Google Scholar]
- 30.Wakai T, Shirai Y, Sakata J, Kameyama H, Nogami H, Iiai T, et al. Histologic evaluation of intrahepatic micrometastases in patients treated with or without neoadjuvant chemotherapy for colorectal carcinoma liver metastasis. Int J Clin Exp Pathol. 2012;5:308–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama N, Shirai Y, Ajioka Y, Nagakura S, Suda T, Hatakeyama K. Immunohistochemically detected hepatic micrometastases predict a high risk of intrahepatic recurrence after resection of colorectal carcinoma liver metastases. Cancer. 2002;94:1642–7. doi: 10.1002/cncr.10422. [DOI] [PubMed] [Google Scholar]


