Abstract
Background:
Few investigation has focused on the patients with lupus nephritis (LN) and neuropsychiatric systemic lupus erythematosus (NPSLE). This study was aimed to investigate the clinical features, mortality, and the predictors for mortality of this group of patients.
Materials and Methods:
Medical records were retrospectively reviewed in Sun Yat-sen Memorial Hospital from 1996 to 2012. Data of demographic information, clinical manifestations, laboratory tests, SLE disease activity index 2000 (SLEDAI-2K) score, diagnosis, complications, treatment, and mortality was collected.
Results:
A total of 124 patients were included in our study. Thirty-five (29.1%) patients had glomerular filtration rate <60 ml/min/1.73 m2, while 24 (19.4%) experienced acute kidney injury (AKI). Thirteen of the 19 American College of Rheumatology defined NPSLE syndromes were identified. The most frequent manifestation was seizure disorder (56/124, 45.2%), followed by psychosis (37/124, 29.8%) and cerebrovascular disease (35/124, 28.2%). One hundred and five (84.7%) patients had SLEDAI-2K scores ≥15, the mean of which was 21.5 ± 6.2. The mortality during hospitalization was 12.9% (16/124) with NP involvement itself being the leading cause of death (7/16, 43.8%). Multivariate logistic regression confirmed that age <14 years at onset of NPSLE (odds ratios [OR]: 9.95, 95% confidence intervals [CI]: 1.43-69.36, P = 0.020), AKI (OR: 10.40, 95% CI: 2.33-46.48, P = 0.002) and pneumonia (OR: 4.52, 95% CI: 1.14-17.96, P = 0.032) were risk factors for mortality, while cyclophosphamide (CYC) treatment (OR: 0.09, 95% CI: 0.02-0.54, P = 0.008) was a protective factor.
Conclusion:
Most of SLE patients with LN and new-onset NPSLE are in an active disease state. NP manifestation itself was the leading cause of death during hospitalization. Childhood-onset NPSLE, AKI and pneumonia might be predictors of mortality, whereas CYC treatment might improve the prognosis.
Keywords: Lupus nephritis, mortality, multivariate logistic regression, neuropsychiatric systemic lupus erythematosus
INTRODUCTION
Systemic lupus erythematosus (SLE) is a complicated disease that has different outcomes mainly depends on the manifestations and treatments. The kidney and the nervous system are often involved in SLE, which named lupus nephritis (LN) and neuropsychiatric SLE (NPSLE) respectively. It has been reported that 3.5-27.8% of LN patients accompanied with NP manifestations,[1,2] while 40-80% of NPSLE patients had nephritis.[3,4,5] Both of these two complications are associated with increased morbidity and mortality.[6,7,8] Until date, few investigation has focused on the population of patients both having LN and NPSLE. In our study, we retrospectively reviewed the data of SLE patients with LN and new-onset NPSLE in our center, aiming to investigate the clinical features, mortality during hospitalization and the predictors for outcome.
PATIENTS AND METHODS
Patients
This was a retrospective study. Medical records were reviewed of all SLE patients with LN and new-onset NP manifestations who were admitted to Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Guangzhou, China, from April 1996 to September 2012. Besides the clinical features, we particularly focused on the mortality during hospitalization and the associated factors.
Systemic lupus erythematosus was defined by 1997 revised American College of Rheumatology (ACR) classification criteria.[9] Disease activity at onset of NPSLE was evaluated using the SLE disease activity index 2000 (SLEDAI-2K).[10] The scores were evaluated before treatment.
Lupus nephritis was defined as persistent proteinuria more than 0.5 g/day or proteinuria more than 3+ if quantitation not performed, or cellular casts-might be red cell, hemoglobin, granular, tubular, or mixed.[9] Urine analysis, 24-h proteinuria, serum creatinine, serum albumin, glomerular filtration rate (GFR), presence of hypertension and presence of acute kidney injury (AKI) were collected to evaluate the degree of renal injury. GFR was estimated for the purpose of evaluating the renal function by the Modification of Diet in Renal Disease method.[11] Hypertension was defined as values ≥140 mmHg systolic blood pressure (SBP) and/or ≥90 mmHg diastolic blood pressure (DBP) in adults[12] and SBP and/or DBP persistently 95th percentile or more measured at least three separate occasions with the auscultatory method in children and adolescents.[13] AKI was defined by the Kidney Disease Improving Global Outcomes guideline.[14] Specific investigations for LN such as renal biopsy were carried out in part of the patients. Histopathologic lesions of LN were categorized according to the classification revised by International Society of Nephrology (ISN) and the Renal Pathology Society (RPS) in 2003.[15]
Neuropsychiatric systemic lupus erythematosus was defined by the ACR nomenclature and case definitions published in 1999,[16] excluding the comorbid conditions and concomitant factors of offending drugs, central nervous system infection, tumor and known metabolic derangements; e.g., hypoglycemia, ketoacidosis, hypoxemia, uremia, or electrolyte imbalance. Many of the patients underwent neurological investigations such as magnetic resonance imaging (MRI) of nervous system, computerized tomographic scanning of the head, electroencephalograms and cerebrospinal fluid (CSF) examination as part of the diagnostic evaluation of nervous system involvement. Abnormal CSF examinations were defined as described elsewhere.[17] The identification and classification of NPSLE was confirmed by at least two neurologists or psychiatrists independently.
All patients received oral corticosteroids, which consisted of prednisone 1 mg/kg/day or methylprednisolone (MP) 0.8 mg/kg/day for 8 weeks followed by tapering dose as indicated. Besides, after the onset of NPSLE, most of them had received additional one or more immunosuppressive strategies as follows: (1) Pulse intravenous MP (IVMP) therapy consisted of IVMP 0.5~1.0 g/day for 3~5 days as a cycle, with or without other cycles 1~4 weeks later depending on the severity of disease and the effect of this treatment; (2) oral or IV cyclophosphamide (CYC) 0.2 mg/kg/day, or a single dose of IV CYC 0.5~1.0 g/1.73 m2 monthly; (3) other systemic immunosuppressive therapy included oral mycophenolate mofetil (MMF) 0.5~1.5 g/day, oral azathioprine (AZA) 2~2.5 mg/kg/day, oral ciclosporin (CsA) 3~5 mg/kg/day or oral tacrolimus 0.08~0.1 mg/kg/day; (4) intrathecal injection (IT) of methotrexate (MTX) 5 or 10 mg plus dexamethasone (DXM) 5 or 10 mg weekly, with or without repeated injection depending on the severity and the response to this treatment, which could be combined with other systemic immunosuppressive agents.
We analyzed the data of demographic information, clinical manifestations, laboratory tests, SLEDAI-2K scores, diagnosis, complications, treatment, and mortality during hospitalization.
Ethical consideration
This study was approved by the Ethics Committee in Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Guangzhou, China.
Statistical analysis
Results with continuous data were presented as mean ± standard deviation, while the data did not follow a symmetric distribution presented with median (range). Categorical data were presented as the absolute count and percentage. Associated factors of mortality during hospitalization were analyzed by univariate analysis and multivariate logistic regression. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Statistical analysis was performed using SPSS 19.0 (IBM® SPSS® Statistics). P < 0.05 was considered to be statistically significant.
RESULTS
Demographic information
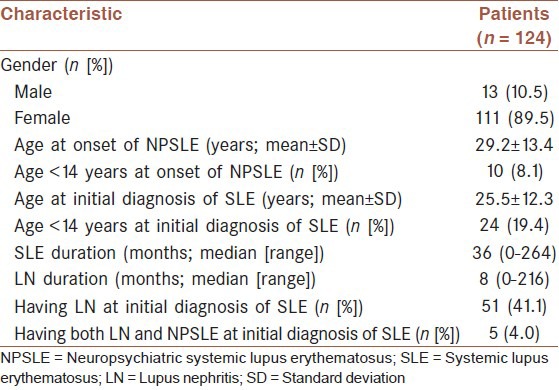
Our study examined the medical records of 124 hospitalized SLE patients with both kidney involvement and new-onset NP manifestations. There were 111 females and 13 males, with a ratio 8.5-1. The mean age at onset of NPSLE was 29.2 ± 13.4 years (range: 10-72 years). 10 (8.1%) were under 14 years old at the onset of NPSLE. The median duration of SLE was 36 months (range: 0-264 months), while the median duration of LN was 8 months (range: 0-216 months). Fifty-one patients (41.1%) had nephritic manifestations at the time of initial diagnosis of SLE, while only 5 (4.0%) had both nephritic and NP manifestations at the onset of SLE [Table 1].
Table 1.
Demographic information
Nephritic characteristics
Nephritic characteristics of 124 patients at the onset of NP manifestations were presented as follows: One hundred and fifteen patients (92.7%) had proteinuria, of which 45 (36.3%) had 24-h proteinuria ≥3.5 g. Nearly, half of the patients (54/124, 43.5%) presented with hypertension. Thirty-five (29.1%) patients had impaired renal function with GFR <60 ml/min/1.73 m2, while 24 (19.4%) experienced AKI. Only 22 (17.7%) patients had performed percutaneous renal biopsy during the onset period of NP manifestation or before, ISN/RPS Class II LN was the most frequent pathologic type (9/22, 40.9%), followed by Class I (4/22, 18.2%), Class III (3/22, 13.6%), Class IV (3/22, 13.6%) and Class V (3/22,13.6%). Only one patient had totally normal urine and renal function during the onset of NPSLE, who had been proven ISN/RPS Class I LN by renal biopsy 5 years before.
Neuropsychiatric characteristics
A total of 165 NP events occurred in 124 patients. Thirteen of the 19 ACR syndromes were identified in our study [Table 2]. 80 patients (64.5%) presented with one set of NP symptoms, while 44 (35.5%) had more than one with a maximum of three. Central nervous system involvement accounted for 98.4% (122/165) with only 1.6% (2/165) of peripheral nervous system. The most frequent manifestation was seizure disorder, followed by psychosis, cerebrovascular disease, headache and mood disorder [Table 2].
Table 2.
NP characteristics
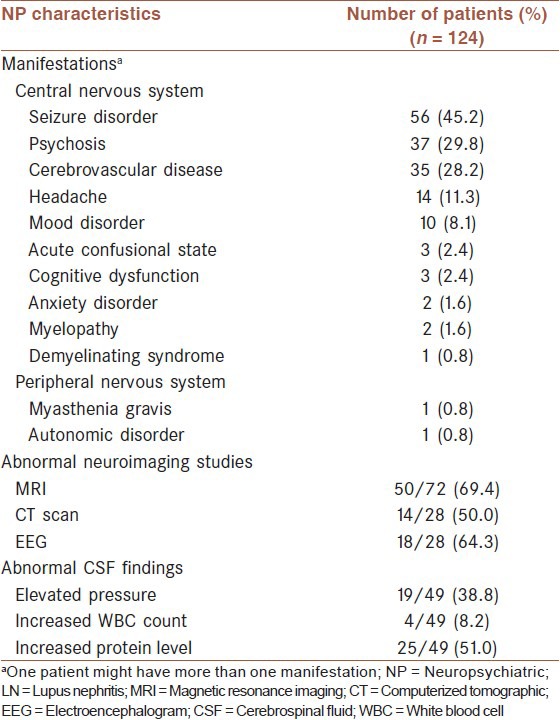
Magnetic resonance imaging of the nervous system was the most widely used investigation in NPSLE patients, with 50 out of 72 (69.4%) patients showing abnormal findings. 49 (39.5%) patients had performed lumbar puncture, and increased protein level was the most frequent abnormal manifestations of CSF [Table 2].
SLE disease activity and other characteristics
One hundred and five (84.7%) patients had SLEDAI-2K scores ≥15, the mean of which was 21.5 ± 6.2, with the highest of 35. One hundred and eighteen patients (95.2%) had positive anti-nuclear antibodies, 111 (89.5%) had elevated anti-dsDNA antibodies, 99 (79.8%) had decreased serum complement C3 level, 72 (58.1%) had decreased serum complement C4 level, 112 (90.3%) had accelerated erythrocyte sedimentation rate. With regard to the complication, 35 (28.2%) patients were accompanied by pneumonia.
Treatment
Pulse IVMP was the most frequent used therapeutic method (62/124, 50.0%) followed by oral or IV CYC (61/124, 49.2%). 34 (27.4%) patients had received combined treatment of pulse IVMP plus oral or IV CYC. Other immunosuppressive drugs such as MMF, AZA, CsA and tacrolimus were administered in 13.7% (17/124), 9.7% (12/124), 5.6% (7/124) and 4.0% (5/124) of patients respectively. At least one IT injection with MTX plus CYC was administered to 25 (20.2%) patients.
Mortality during hospitalization
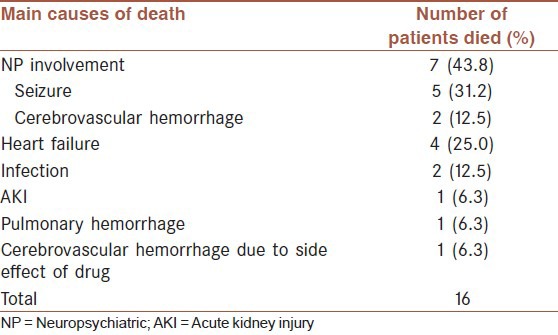
The mortality during hospitalization was 12.9% (16/124) in this group of patients. NP involvement itself being the leading cause of death, other causes of death included heart failure, infection, AKI and hemorrhage [Table 3]. One patient died of cerebrovascular hemorrhage due to bone marrow suppression induced by IT injection of MTX at the 2nd time.
Table 3.
Main causes of death during hospitalization
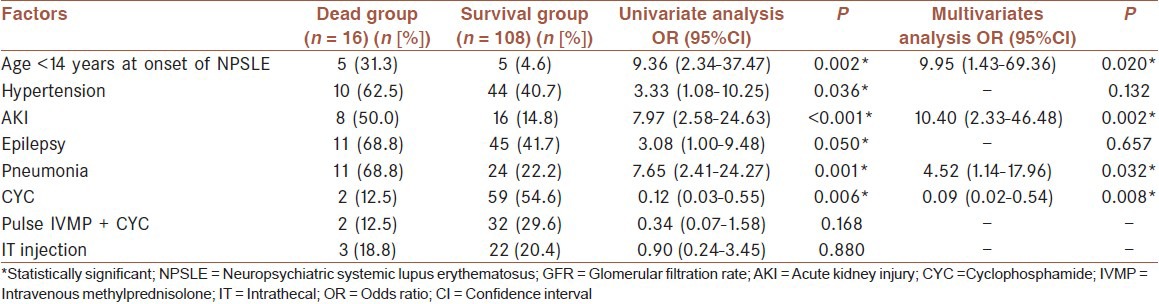
Correlative factors of mortality during hospitalization
Univariate analysis showed that age <14 years at onset of NPSLE (P = 0.002), hypertension (P = 0.036), AKI (P < 0.001), epilepsy (P = 0.050), pneumonia (P = 0.001), CYC treatment (P = 0.006) were the correlative factors of mortality during hospitalization. Multivariate logistic regression further confirmed that age <14 years at onset of NPSLE (OR: 9.95, 95% CI: 1.43-69.36, P = 0.020), AKI (OR: 10.40, 95% CI: 2.33-46.48, P = 0.002) and pneumonia (OR: 4.52, 95% CI: 1.14-17.96, P = 0.032) were risk factors for mortality during hospitalization, while CYC treatment (OR: 0.09, 95% CI: 0.02-0.54, P = 0.008) was a protective factor for survival [Table 4].
Table 4.
Correlative factors of mortality during hospitalization
DISCUSSION
In our study, we investigated the main clinical features of SLE patients with LN and new-onset NPSLE, estimated the mortality during hospitalization and analyzed the correlative factors for mortality.
Clinical features of LN at the onset of NPSLE were investigated in our study. We found that proteinuria (92.7%) presented as the most common manifestation, which is consistent with that of general LN patients as previously reported in western countries.[18] The frequency of nephrotic syndrome is 36.3% in our patients with the similarity of 35-65% reported in western and Asian LN patients before.[1,18,19] And what's more, the frequency of hypertension (43.5%) is similar to previous studies, which reported that hypertension appeared in around 15-50% LN patients.[18] A previous study showed that AKI occurred in 20.5% LN patients,[20] which is consistent with the result of 19.4% in our investigation. The incidence of impaired renal function with a GFR <60 ml/min/1.73 m2 was 29.1% in our study, which is higher than 10-20% of other studies in LN patients.[1,21,22] The reason might be that the population in our study was the LN patients with new-onset NP manifestations, of which the mean SLEDAI-2K score was 21.5 ± 6.2 and 84.7% patients had SLEDAI-2K scores ≥15, indicating that the disease might be more severe and more active in our patients. In our study, ISN/APS Class II LN (40.9%) was the predominant pathologic type, whereas Class IV only accounted for 13.6%, which is different from current LN studies that Class IV represents the most common pathologic type no matter in western or Asian countries.[19,20,21,22] It may be explained that renal biopsy had been performed on only 17.7% of our patients with NPSLE, who might be in a less severe disease state so that could endure the performance of renal biopsy. And the selecting bias of the patients to perform renal biopsy leads to the less severity in renal pathological results.
With regard to the NP features, 13 of 19 ACR defined NP manifestations occurred in our study. The most frequent NP manifestation of LN patients was seizure disorder (45.2%), followed by psychosis (29.8%) and cerebrovascular disease (28.2%). The distribution of NP manifestations is different in different SLE studies.[5,23,24,25] It was found that seizure occurred related to nephritis and cumulative organ damage in SLE,[26,27] which might contribute to the high prevalence of seizure in our study. Among the examinations for the diagnosis of NPSLE, MRI of the nervous system was the most widely used neuroimaging technique with a positive rate of 69.4% in our study, being consistent with 60-78% of previous studies on Chinese or other ethnic NPSLE patients.[23,25,28] CSF analysis found that increased protein level in 51.0% of patients was the most frequent abnormal findings, which is similar to 53% of Yu's study.[5]
The mortality during hospitalization of LN patients with NP manifestation was 12.9%, which is similar to 10.8% reported in NPSLE patients.[23] The main reason for death was NP involvement itself which is consistent with other NPSLE studies.[23,29] Taking the factors including age at onset of NPSLE, hypertension, AKI, NP manifestations, pneumonia and treatments into account, multivariate logistic regression found that age <14 years at onset of NPSLE, AKI and pneumonia were associated with death, while CYC treatment contributed to survival.
It has been reported that approximately 15-20% of patients with SLE are diagnosed during childhood,[30] which is similar to our study with the result of 19.4%. Moreover, we also found that the proportion of children under 14 years old in the deaths was significantly higher than that in the survivals (31.5% vs. 4.6%). Childhood-onset NPSLE was found to be an independent risk factor for mortality in our investigation, which might be explained by the previous studies showing that childhood-onset SLE has a more aggressive course, higher rates of renal and NP involvement and higher mortality rate compared with adult-onset SLE.[31,32]
In SLE, a considerable proportion of patients present with AKI as reported by many previous studies. In our study, AKI appeared in 19.4% of patients, which appeared more frequently in the deaths than in the survivals (50.0% vs. 14.8%) as being a predictor of mortality. Looking into a previous AKI study, the authors found that SLE patients with AKI had significantly higher proportions of neurologic disorder and higher SLEDAI-2K scores, what's more, AKI was an independent risk factor for renal outcome.[20] SLE with AKI seems to be more active and more severe, by which our result might be partially explained.
Infection is a common complication of corticosteroids and other immunosuppressive drugs, which is severe and not easy to control in some cases. Pneumonia is the most common type of infection in the SLE patients,[33] and it is associated with death as previous study reported.[34] In our study, we found that the incidence of pneumonia in the deaths was significantly higher than that in the survivals (68.8% vs. 22.2%), which was found to be a risk factor for mortality. Our result is consistent with the previous result as mentioned above.[34]
Various studies about treatment of LN have been reported, which have shown the effectiveness of CYC, MMF, CsA, tacrolimus and AZA on LN.[35,36,37] But as so far, study about treatment of NPSLE is not that abundant. The only one controlled clinical trial in NPSLE is the study comparing the efficacy of IV CYC and IVMP. The result showed that IV CYC was more effective than IVMP in the treatment of acute, severe NPSLE.[38] Except for that, several retrospective studies have also shown the effectiveness of CYC in NPSLE,[39,40] which is consistent with our result that CYC treatment was a protective factor for survival. Meanwhile, a small trial has found that IVMP plus IV CYC made favorable outcome in severe NLSLE children.[41] In our study, although it seemed that pulse IVMP plus CYC treatment was prescribed more frequently in the survivals than in the deaths (29.6% vs. 12.5%), but the difference was not significant (P = 0.168), which might be due to only a small number of patients had received this combined treatment. IT injection of MTX plus DXM was reported to be effective in NPSLE,[42] but in our series the number of patients that received this treatment was too small to have favorable result. The effects of MMF, AZA, CsA and tacrolimus in NPSLE are uncertain, which were only reported sporadically.[43,44,45,46] In our study, only a little part of patients had been prescribed these immunosuppressive drugs.
In summary, most SLE patients with both LN and new-onset NPSLE are in an active disease state. NP manifestation itself is the leading cause of death during hospitalization in this group of patients. Childhood-onset NPSLE, AKI and pneumonia might be predictors of mortality, whereas CYC treatment might improve the prognosis. However, this is only a retrospective investigation with some limitations that may bias the results. A prospective follow-up study with a large number of patients needs to be conducted for the purpose of confirming the results.
AUTHORS’ CONTRIBUTIONS
MF carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. JL, SF, BL coordinated and carried out all the experiments. YT, XW, PFL, YCZ, JGL, YYL, XML, and APX provided assistance for all experiments. All authors have read and approved the content of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jing Hou (School of Public Health and Primary Care, The Chinese University of Hong Kong) for her help with statistical consultation.
Footnotes
Source of Support: This work was supported by grant from Guangdong Natural Science Foundation (S2012010009667) and Guangdong Science and Technology Planning Project (2011B031800122)
Conflict of Interest: None declared.
REFERENCES
- 1.Zheng ZH, Zhang LJ, Liu WX, Lei YS, Xing GL, Zhang JJ, et al. Predictors of survival in Chinese patients with lupus nephritis. Lupus. 2012;21:1049–56. doi: 10.1177/0961203312445230. [DOI] [PubMed] [Google Scholar]
- 2.Vyas S, Hidalgo G, Baqi N, Von Gizyki H, Singh A. Outcome in African-American children of neuropsychiatric lupus and lupus nephritis. Pediatr Nephrol. 2002;17:45–9. doi: 10.1007/s004670200008. [DOI] [PubMed] [Google Scholar]
- 3.Garin EH, Donnelly WH, Fennell RS, 3 rd, Richard GA. Nephritis in systemic lupus erythematosus in children. J Pediatr. 1976;89:366–71. doi: 10.1016/s0022-3476(76)80529-x. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JS. Lupus nephritis in childhood and adolescence. Pediatr Nephrol. 1994;8:230–49. doi: 10.1007/BF00865490. [DOI] [PubMed] [Google Scholar]
- 5.Yu HH, Lee JH, Wang LC, Yang YH, Chiang BL. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus: A 20-year study. Lupus. 2006;15:651–7. doi: 10.1177/0961203306070990. [DOI] [PubMed] [Google Scholar]
- 6.Ward MM, Pyun E, Studenski S. Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus. Arch Intern Med. 1996;156:1337–44. [PubMed] [Google Scholar]
- 7.Rubin LA, Urowitz MB, Gladman DD. Mortality in systemic lupus erythematosus: The bimodal pattern revisited. Q J Med. 1985;55:87–98. [PubMed] [Google Scholar]
- 8.Gibson T, Myers AR. Nervous system involvement in systemic lupus erythematosus. Ann Rheum Dis. 1975;35:398–406. doi: 10.1136/ard.35.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–91. [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–38. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 13.Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, et al. Management of high blood pressure in children and adolescents: Recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–42. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA, Lameire N, Aspelin P. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 15.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 16.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.William WC. 7th ed. Vol. 737. China: Lippincott Williams and Wilkins; 2013. Dejong's the Neurologic Examination; p. 44. [Google Scholar]
- 18.Cameron JS. Lupus nephritis. J Am Soc Nephrol. 1999;10:413–24. doi: 10.1681/ASN.V102413. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama H, Okuyama H, Yamaya H. Clinicopathological insights into lupus glomerulonephritis in Japanese and Asians. Clin Exp Nephrol. 2011;15:321–30. doi: 10.1007/s10157-011-0434-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhu D, Qu Z, Tan Y, Yu F, Zhao MH. Acute kidney injury in Chinese patients with lupus nephritis: A large cohort study from a single center. Lupus. 2011;20:1557–65. doi: 10.1177/0961203311417035. [DOI] [PubMed] [Google Scholar]
- 21.Martins L, Rocha G, Rodrigues A, Santos J, Vasconcelos C, Correia J, et al. Lupus nephritis: A retrospective review of 78 cases from a single center. Clin Nephrol. 2002;57:114–9. doi: 10.5414/cnp57114. [DOI] [PubMed] [Google Scholar]
- 22.Uthman IW, Muffarij AA, Mudawar WA, Nasr FW, Masri AF. Lupus nephritis in Lebanon. Lupus. 2001;10:378–81. doi: 10.1191/096120301670808045. [DOI] [PubMed] [Google Scholar]
- 23.Zhou HQ, Zhang FC, Tian XP, Leng XM, Lu JJ, Zhao Y, et al. Clinical features and outcome of neuropsychiatric lupus in Chinese: Analysis of 240 hospitalized patients. Lupus. 2008;17:93–9. doi: 10.1177/0961203307085671. [DOI] [PubMed] [Google Scholar]
- 24.Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. 2014 Feb 11;10:338–47. doi: 10.1038/nrrheum.2014.15. doi:10.1038/nrrheum.2014.15. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Sattar AB, Goda T, Negm MG. Neuropsychiatric manifestations in a consecutive cohort of systemic lupus erythematosus; a single center study. Int J Rheum Dis. 2013;16:715–23. doi: 10.1111/1756-185X.12213. [DOI] [PubMed] [Google Scholar]
- 26.Appenzeller S, Cendes F, Costallat LT. Epileptic seizures in systemic lupus erythematosus. Neurology. 2004;63:1808–12. doi: 10.1212/01.wnl.0000144178.32208.4f. [DOI] [PubMed] [Google Scholar]
- 27.Hanly JG, Urowitz MB, Su L, Gordon C, Bae SC, Sanchez-Guerrero J, et al. Seizure disorders in systemic lupus erythematosus results from an international, prospective, inception cohort study. Ann Rheum Dis. 2012;71:1502–9. doi: 10.1136/annrheumdis-2011-201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steup-Beekman GM, Zirkzee EJ, Cohen D, Gahrmann BM, Emmer BJ, Steens SC, et al. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: Epidemiology and radiology pointing to an immune-mediated cause. Ann Rheum Dis. 2013;72(Suppl 2):ii76–9. doi: 10.1136/annrheumdis-2012-202369. [DOI] [PubMed] [Google Scholar]
- 29.Zirkzee EJ, Huizinga TW, Bollen EL, van Buchem MA, Middelkoop HA, van der Wee NJ, et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE) Lupus. 2014;23:31–8. doi: 10.1177/0961203313512540. [DOI] [PubMed] [Google Scholar]
- 30.Cassidy JT, Petty RE. 5th ed. Philadelphia: Elsevier Saunders; 2005. Textbook of Pediatric Rheumatology. [Google Scholar]
- 31.Tucker LB, Uribe AG, Fernández M, Vilá LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: Results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII) Lupus. 2008;17:314–22. doi: 10.1177/0961203307087875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58:556–62. doi: 10.1002/art.23204. [DOI] [PubMed] [Google Scholar]
- 33.Goldblatt F, Chambers S, Rahman A, Isenberg DA. Serious infections in British patients with systemic lupus erythematosus: Hospitalisations and mortality. Lupus. 2009;18:682–9. doi: 10.1177/0961203308101019. [DOI] [PubMed] [Google Scholar]
- 34.Souza DC, Santo AH, Sato EI. Mortality profile related to systemic lupus erythematosus: A multiple cause-of-death analysis. J Rheumatol. 2012;39:496–503. doi: 10.3899/jrheum.110241. [DOI] [PubMed] [Google Scholar]
- 35.Lightstone L. Lupus nephritis: Where are we now? Curr Opin Rheumatol. 2010;22:252–6. doi: 10.1097/BOR.0b013e3283386512. [DOI] [PubMed] [Google Scholar]
- 36.Bomback AS, Appel GB. Updates on the treatment of lupus nephritis. J Am Soc Nephrol. 2010;21:2028–35. doi: 10.1681/ASN.2010050472. [DOI] [PubMed] [Google Scholar]
- 37.Tesar V, Hruskova Z. Treatment of proliferative lupus nephritis: A slowly changing landscape. Nat Rev Nephrol. 2011;7:96–109. doi: 10.1038/nrneph.2010.170. [DOI] [PubMed] [Google Scholar]
- 38.Barile-Fabris L, Ariza-Andraca R, Olguín-Ortega L, Jara LJ, Fraga-Mouret A, Miranda-Limón JM, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:620–5. doi: 10.1136/ard.2004.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuwelt CM, Lacks S, Kaye BR, Ellman JB, Borenstein DG. Role of intravenous cyclophosphamide in the treatment of severe neuropsychiatric systemic lupus erythematosus. Am J Med. 1995;98:32–41. doi: 10.1016/S0002-9343(99)80078-3. [DOI] [PubMed] [Google Scholar]
- 40.Sanna G, Bertolaccini ML, Mathieu A. Central nervous system lupus: A clinical approach to therapy. Lupus. 2003;12:935–42. doi: 10.1191/0961203303lu505oa. [DOI] [PubMed] [Google Scholar]
- 41.Baca V, Lavalle C, García R, Catalán T, Sauceda JM, Sánchez G, et al. Favorable response to intravenous methylprednisolone and cyclophosphamide in children with severe neuropsychiatric lupus. J Rheumatol. 1999;26:432–9. [PubMed] [Google Scholar]
- 42.Xuan Z, Yi D, Fu-Lin T, Fen-Chun Z. Central nervous system involvement in systemic lupus erythematosus in a hospital-based study of 171 cases: The possible therapeutic role of intrathecal therapy. J Clin Rheumatol. 1999;5:314–9. doi: 10.1097/00124743-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Benseler SM, Silverman ED. Neuropsychiatric involvement in pediatric systemic lupus erythematosus. Lupus. 2007;16:564–71. doi: 10.1177/0961203307078971. [DOI] [PubMed] [Google Scholar]
- 44.Popescu A, Kao AH. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol. 2011;9:449–57. doi: 10.2174/157015911796557984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mok CC. Mycophenolate mofetil for non-renal manifestations of systemic lupus erythematosus: A systematic review. Scand J Rheumatol. 2007;36:329–37. doi: 10.1080/03009740701607042. [DOI] [PubMed] [Google Scholar]
- 46.Kizu H, Dobashi H, Kameda T, Susaki K, Kawanishi M, Ishida T. Improvement of irregularity of brain vessel walls in systemic lupus erythematosus by tacrolimus. Clin Rheumatol. 2011;30:715–8. doi: 10.1007/s10067-010-1591-3. [DOI] [PubMed] [Google Scholar]


